A Breeding Plumage in the Making: The Unique Process of Plumage Coloration in the Crested Ibis in Terms of Chemical Composition and Sex Hormones
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Samples
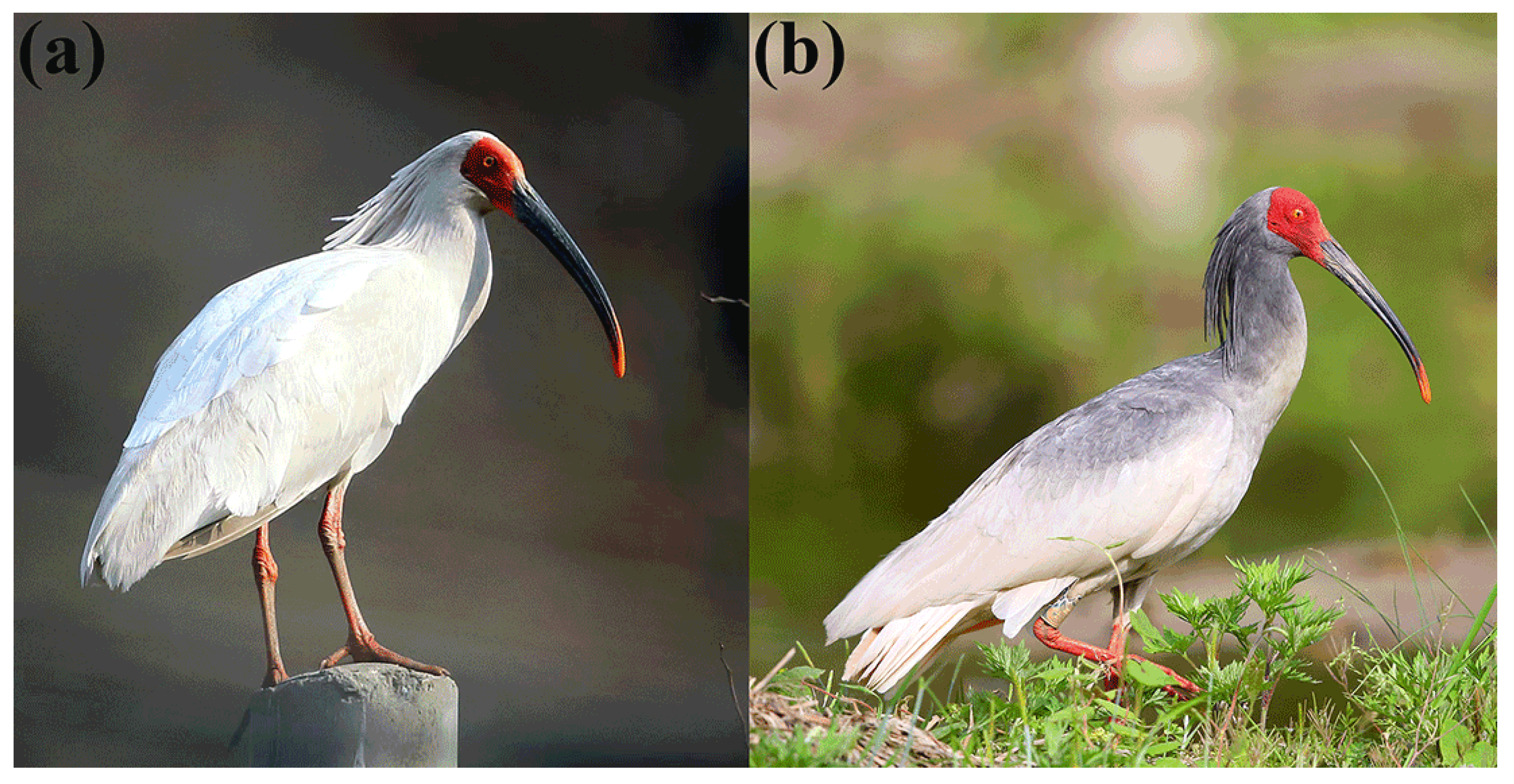
2.2. Cosmetic Behavior of Crested Ibis
2.3. Collection of Feces and Black Substance
2.4. Qualitative Analysis of the Sex Hormone Level and the Black Substance
2.5. Statistic Analysis
3. Results
3.1. Cosmetic Behavior and Sex Hormone Levels
3.2. Chemical Components of the Black Substance
4. Discussion
4.1. Sex Hormone and Plumage Coloration
4.2. Chemical Components and Plumage Coloration
4.3. Biological Significance of Avian Plumage Coloration
4.4. Implications for Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, D.; Cao, Y. Crested Ibis in China; China Forestry Publishing House: Beijing, China, 2001; pp. 106–110. [Google Scholar]
- Liu, Y.Z. Rediscovery of the Crested Ibis in the Qinling Mountains. Acta Zool. Sin. 1981, 27, 273. [Google Scholar]
- Li, X.; Li, D. Current state and the future of the Crested Ibis (Nipponia nippon): A case study by population viability analysis. Ecol. Res. 1998, 13, 323–333. [Google Scholar] [CrossRef]
- Yu, X.; Li, X.; Huo, Z. Breeding ecology and success of a reintroduced population of the endangered Crested Ibis (Nipponia nippon). Bird Conserv. Int. 2015, 25, 207–219. [Google Scholar] [CrossRef]
- Lan, H.; Zhou, T.; Wan, Q.H.; Fang, S.G. Genetic Diversity and Differentiation at Structurally Varying MHC Haplotypes and Microsatellites in Bottlenecked Populations of Endangered Crested Ibis. Cells 2019, 8, 377. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Fang, Q.; Barnett, R.; Li, C.; Han, S.; Kuhlwilm, M.; Zhou, L.; Pan, H.; Deng, Y.; Chen, G.; et al. The Genomic Footprints of the Fall and Recovery of the Crested Ibis. Curr. Biol. 2019, 29, 340–349.e7. [Google Scholar] [CrossRef]
- Zhang, B.; Fang, S.G.; Xi, Y.M. Low genetic diversity in the Endangered Crested Ibis (Nipponia nippon) and implications for conservation. Bird Conserv. Int. 2004, 14, 183–190. [Google Scholar] [CrossRef]
- Xi, Y.; Wood, C.; Lu, B.; Zhang, Y. Prevalence of a septicemia disease in the Crested Ibis (Nipponia nippon) in China. Avian Dis. 2007, 51, 614–617. [Google Scholar] [CrossRef]
- Chen, S.; Hao, H.; Liu, Q.; Wang, R.; Zhang, P.; Wang, X.; Du, E.; Yang, Z. Phylogenetic and pathogenic analyses of two virulent Newcastle disease viruses isolated from Crested Ibis (Nipponia nippon) in China. Virus genes 2013, 46, 447–453. [Google Scholar] [CrossRef]
- Li, X.; Li, D.; Li, Y.; Ma, Z.; Zhai, T. Habitat evaluation for Crested Ibis: A GIS-based approach. Ecol. Res. 2002, 17, 565–573. [Google Scholar] [CrossRef]
- Ma, L.; Li, X.; Zhai, T.; Zhang, Y.; Song, K.; Holyoak, M.; Sun, Y. Changes in the Habitat Preference of Crested Ibis (Nipponia nippon) during a Period of Rapid Population Increase. Animals 2021, 11, 2626. [Google Scholar] [CrossRef]
- Dongping, L.; Changqing, D.; Guozhong, C. Home range and habitat utilization of the Crested Ibis in the breeding period. Acta Geol. Sin. 2003, 49, 755–763. [Google Scholar]
- Ren, Y.; Ding, C.; Zhang, Y.; Qing, B.; Duan, W. Public attitudes and willingness to pay toward the conservation of Crested Ibis: Insights for management. J. Nat. Conserv. 2022, 66, 126118. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, T.; Skidmore, A.K.; Palmer, S.C.; Ye, X.; Ding, C.; Wang, Q. Predicting and understanding spatio-temporal dynamics of species recovery: Implications for Asian Crested Ibis (Nipponia nippon) conservation in China. Divers. Distrib. 2016, 22, 893–904. [Google Scholar] [CrossRef]
- Xi, Y.; Lu, B.; Ozaki, K.; Hattori, M.A. Effects of environmental stress on the parental behavior in Crested Ibis (Nipponia nippon). J. Yamashina Inst. Ornithol. 2003, 35, 30–38. [Google Scholar] [CrossRef]
- Jiang, N.; Wu, S.; Tong, Y.W.; Zhang, Y.Z.; Li, X.; Ye, X.P.; Yu, X.P. The impact of predation on population dynamics of the Crested Ibis in the Qinling Mountains, Shaanxi, central China. Restor. Ecol. 2023, 31, e13741. [Google Scholar] [CrossRef]
- Amadon, D. Avian Plumages and Molts. Condor 1966, 68, 263–278. [Google Scholar] [CrossRef]
- Stevens, M.; Merilaita, S. Animal camouflage: Current issues and new perspectives. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Hill, G.E. Plumage coloration is a sexually selected indicator of male quality. Nature 1991, 350, 337–339. [Google Scholar] [CrossRef]
- Pyle, P. Identification Guide to North American Birds: A Compendium of Information on Identifying, Ageing, and Sexing "Near-Passerines" and Passerines in the Hand; Slate Creek Press: Bolinas, CA, USA, 1997. [Google Scholar]
- Andersson, M. Sexual Selection; Princeton University Press: Princeton, NJ, USA, 1994; Volume 72. [Google Scholar]
- Yang, J.; Liu, X.; Zhang, J.; Qing, B.; Lu, B. Geneyangtics and evolution of plumage color in Crested Ibis: Analysis of the melanocortin-1 receptor (MC1R). Cell. Mol. Biol. 2015, 61, 63–69. [Google Scholar] [CrossRef]
- Enbody, E.D.; Lantz, S.M.; Karubian, J. Production of plumage ornaments among males and females of two closely related tropical passerine bird species. Ecol. Evol. 2017, 7, 4024–4034. [Google Scholar] [CrossRef]
- Lee, S.I.; Kim, M.; Choe, J.; Jablonski, P. Evolution of plumage coloration in the crow family (Corvidae) with a focus on the color-producing microstructures in the feathers: A comparison of eight species. Anim. Cells Syst. 2016, 20, 1–8. [Google Scholar] [CrossRef]
- Lozano, G. Carotenoids, Parasites, and Sexual Selection. Oikos 1994, 70, 309–311. [Google Scholar] [CrossRef]
- Olson, V.A.; Owens, I.P. Costly sexual signals: Are carotenoids rare, risky or required? Trends Ecol. Evol. 1998, 13, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Hill, G.E.; McGraw, K.J. Bird Coloration; Harvard University Press: Cambridge, MA, USA, 2006; Volume 1, pp. 177–294. [Google Scholar]
- Auber, L. The distribution of structural colours and unusual pigments in the class Aves. Ibis 1957, 99, 463–476. [Google Scholar] [CrossRef]
- Sun, L.; Zhou, T.; Wan, Q.H.; Fang, S.G. Transcriptome Comparison Reveals Key Components of Nuptial Plumage Coloration in Crested Ibis. Biomolecules 2020, 10, 905. [Google Scholar] [CrossRef] [PubMed]
- Yamashina, Y. The plight of the Japanese crested ibis. Animals 1967, 10, 275–277. [Google Scholar]
- Yasuda, K. On a Description about Color Change on Plumage of Japanese Crested Ibis (Nipponia nippon) Observed by M. Berezovsky, 1884-’85. J. Yamashina Inst. Ornithol. 1984, 16, 174–177. [Google Scholar] [CrossRef]
- Uchida, Y. On the color change in Japanese Crested Ibis. J. Yamashina Inst. Ornithol. 1970, 6, 54–72. [Google Scholar] [CrossRef]
- Delhey, K.; Peters, A.; Kempenaers, B. Cosmetic Coloration in Birds: Occurrence, Function, and Evolution. Am. Nat. 2007, 169, S145–S158. [Google Scholar] [CrossRef]
- Boersma, J.; Enbody, E.D.; Jones, J.A.; Nason, D.; Lopez-Contreras, E.; Karubian, J.; Schwabl, H. Testosterone induces plumage ornamentation followed by enhanced territoriality in a female songbird. Behav. Ecol. 2020, 31, 1233–1241. [Google Scholar] [CrossRef]
- Bókony, V.; Garamszegi, L.Z.; Hirschenhauser, K.; Liker, A. Testosterone and melanin-based black plumage coloration: A comparative study. Behav. Ecol. Sociobiol. 2008, 62, 1229–1238. [Google Scholar] [CrossRef]
- Caro, S.P.; Balthazart, J. Pheromones in birds: Myth or reality? J. Comp. Physiol. A 2010, 196, 751–766. [Google Scholar] [CrossRef] [PubMed]
- Cockrem, J.; Rounce, J. Non-invasive assessment of the annual gonadal cycle in free-living Kakapo (Strigops habroptilus) using fecal steroid measurements. Auk 1995, 112, 253–257. [Google Scholar] [CrossRef]
- Riters, L.V.; Alger, S.J. Hormonal Regulation of Avian Courtship and Mating Behaviors. In Hormones and Reproduction of Vertebrates; Norris, D.O., Lopez, K.H., Eds.; Academic Press: London, UK, 2011; pp. 153–180. [Google Scholar]
- Price-Waldman, R.; Stoddard, M.C. Avian Coloration Genetics: Recent Advances and Emerging Questions. J. Hered. 2021, 112, 395–416. [Google Scholar] [CrossRef] [PubMed]
- López-Rull, I.; Salaberría, C.; Fargallo, J.A. Plastic plumage colouration in response to experimental humidity supports Gloger’s rule. Sci. Rep. 2023, 13, 858. [Google Scholar] [CrossRef] [PubMed]
- Stoehr, A.M.; Hill, G.E. The effects of elevated testosterone on plumage hue in male House Finches. J. Avian Biol. 2001, 32, 153–158. [Google Scholar] [CrossRef]
- Ducrest, A.L.; Keller, L.; Roulin, A. Pleiotropy in the melanocortin system, coloration and behavioural syndromes. Trends Ecol. Evol. 2008, 23, 502–510. [Google Scholar] [CrossRef]
- Béziers, P.; Ducrest, A.L.; Simon, C.; Roulin, A. Circulating testosterone and feather-gene expression of receptors and metabolic enzymes in relation to melanin-based colouration in the barn owl. Gen. Comp. Endocrinol. 2017, 250, 36–45. [Google Scholar] [CrossRef]
- Badyaev, A. Stress-induced variation in evolution: From behavioural plasticity to genetic assimilation. Proc. Biol. Sci. 2005, 272, 877–886. [Google Scholar] [CrossRef]
- Lucas, A.; Stettenheim, P. Avian Anatomy: Integument; Agriculture Handbook; U.S. Agricultural Research Service: Washington, DC, USA, 1972; p. 365.
- Møller, A.P.; Erritzøe, J. Acquisition of Breeding Coloration Depends on Badge Size in Male House Sparrows (Passer domesticus). Behav. Ecol. Sociobiol. 1992, 31, 271–277. [Google Scholar] [CrossRef]
- Ernest, J.; Willoughby, M.M.; Gorton, H.L. Molt, plumage abrasion, and color change in Lawrence’s Goldfinch. Wilson Bull. 2002, 114, 380–392. [Google Scholar]
- Tökölyi, J.; Bokony, V.; Barta, Z. Seasonal colour change by moult or by the abrasion of feather tips: A comparative study. Biol. J. Linn. Soc. 2008, 94, 711–721. [Google Scholar] [CrossRef]
- Shawkey, M.D.; Pillai, S.R.; Hill, G.E.; Siefferman, L.M.; Roberts, S.R. Bacteria as an Agent for Change in Structural Plumage Color: Correlational and Experimental Evidence. Am. Nat. 2007, 169, S112–S121. [Google Scholar] [CrossRef] [PubMed]
- Burtt, E.H., Jr.; Ichida, J.M. Occurrence of Feather-Degrading Bacilli in the Plumage of Birds. Auk 1999, 116, 364–372. [Google Scholar] [CrossRef]
- Mati, K.; Anders, P.M. Sexual selection, feather breakage and parasites: The importance of white spots in the tail of the barn swallow (Hirundo rustica). Behav. Ecol. Sociobiol. 1999, 45, 430–436. [Google Scholar]
- Surmacki, A. Preen waxes do not protect carotenoid plumage from bleaching by sunlight. Ibis 2008, 150, 335–341. [Google Scholar] [CrossRef]
- Örnborg, J.; Andersson, S.; Griffith, S.C.; Sheldon, B.C. Seasonal changes in a ultraviolet structural colour signal in blue tits, Parus caeruleus. Biol. J. Linn. Soc. 2002, 76, 237–245. [Google Scholar] [CrossRef]
- Montgomerie, R. Cosmetic and adventitious colors. In Bird Coloration; Harvard University Press: Cambridge, MA, USA, 2006; Volume 1, pp. 399–428. [Google Scholar]
- Prum, R.O. Anatomy, Physics, and Evolution of Structural Colors. In Bird Coloration; Harvard University Press: Cambridge, MA, USA, 2006; Volume 1, pp. 295–353. [Google Scholar]
- Griffith, S.C.; Parker, T.H.; Olson, V.A. Melanin- versus carotenoid-based sexual signals: Is the difference really so black and red? Anim. Behav. 2006, 71, 749–763. [Google Scholar] [CrossRef]
- Galván, I.; Solano, F. Melanin Chemistry and the Ecology of Stress. Physiol. Biochem. Zool. 2015, 88, 352–355. [Google Scholar] [CrossRef]
- García-Navas, V.; Ferrer, E.S.; Sanz, J.J. Prey Choice, Provisioning Behaviour, and Effects of Early Nutrition on Nestling Phenotype of Titmice. Ecoscience 2013, 20, 9–18. [Google Scholar] [CrossRef]
- Inaba, M.; Chuong, C.M. Avian Pigment Pattern Formation: Developmental Control of Macro- (Across the Body) and Micro- (Within a Feather) Level of Pigment Patterns. Front. Cell Dev. Biol. 2020, 8, 620. [Google Scholar] [CrossRef] [PubMed]
- Brush, A.H. Avian pigmentation. Chem. Zool. 1978, 10, 141–161. [Google Scholar]
- Jeon, D.J.; Paik, S.; Ji, S.; Yeo, J.S. Melanin-based structural coloration of birds and its biomimetic applications. J. Microsc. 2021, 51, 14. [Google Scholar] [CrossRef] [PubMed]
- Stradi, R.; Celentano, G.; Boles, M.; Mercato, F. Carotenoids in bird plumage: The pattern in a series of red-pigmented Carduelinae. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1997, 117, 85–91. [Google Scholar] [CrossRef]
- Cooke, T.F.; Fischer, C.R.; Wu, P.; Jiang, T.X.; Xie, K.T.; Kuo, J.; Smith, E.A. Genetic Mapping and Biochemical Basis of Yellow Feather Pigmentation in Budgerigars. Cell 2017, 171, 427–439.e21. [Google Scholar] [CrossRef]
- Braasch, I.; Schartl, M.; Volff, J.N. Evolution of pigment synthesis pathways by gene and genome duplication in fish. BMC Evol. Biol. 2007, 7, 74. [Google Scholar] [CrossRef]
- Blanco, G.; Frías, O.; Garrido-Fernández, J.; Hornero-Méndez, D. Environmental-induced acquisition of nuptial plumage expression: A role of denaturation of feather carotenoproteins? Proc. R. Soc. B 2005, 272, 1893–1900. [Google Scholar] [CrossRef]
- Matrková, J.; Remeš, V. Environmental and genetic effects on pigment-based vs. structural component of yellow feather colouration. PLoS ONE 2012, 7, e36640. [Google Scholar] [CrossRef]
- Windsor, R.L.; Fox, G.A.; Bowman, R. Consistency of structural color across molts: The effects of environmental conditions and stress on feather ultraviolet reflectance. Auk 2019, 136, ukz019. [Google Scholar] [CrossRef]
- Endler, J.A.; Westcott, D.A.; Madden, J.R.; Robson, T. Animal visual systems and the evolution of color patterns: Sensory processing illuminates signal evolution. Evolution 2005, 59, 1795–1818. [Google Scholar]
- Krishnan, A.; Singh, A.; Tamma, K. Visual signal evolution along complementary color axes in four bird lineages. Biol. Open 2020, 9, bio052316. [Google Scholar] [CrossRef] [PubMed]
- Stoddard, M.C.; Prum, R.O. How colorful are birds? Evolution of the avian plumage color gamut. Behav. Ecol. 2011, 22, 1042–1052. [Google Scholar] [CrossRef]
- Stoddard, M.C.; Prum, R.O. Evolution of avian plumage color in a tetrahedral color space: A phylogenetic analysis of new world buntings. Am. Nat. 2008, 171, 755–776. [Google Scholar] [CrossRef] [PubMed]





| Color Plastic Ring Number | Gender | Birth -Year | Metal
Ring Number | Feeding Area | |
|---|---|---|---|---|---|
| For Hormone | 543 | ♂ | 2006 | 7060 | 5-3 |
| 491 | ♂ | 2005 | 7081 | 5-4 | |
| 461 | ♂ | 2004 | 7161 | 5-4 | |
| 443 | ♀ | 2004 | 7147 | 5-3 | |
| 334 | ♀ | 2003 | 2661 | 5-3 | |
| 603 | ♀ | 2007 | 2640 | 5-4 | |
| 527 | ♀ | 2005 | 5283 | 5-4 | |
| For Black Substance | 307 | ♀ | 2002 | NA | 5-4 |
| 501 | ♂ | 2005 | 7072 | 5-4 |
| Month | January | February | March | April | May | June | July |
|---|---|---|---|---|---|---|---|
| Daubing Frequency | 6 | 23 | 34 | 11 | 0 | 0 | 0 |
| 4 | 25 | 34 | 33 | 2 | 0 | 0 | |
| 6 | 25 | 38 | 28 | 0 | 0 | 0 | |
| 6 | 20 | 36 | 32 | 0 | 0 | 0 | |
| 5 | 18 | 40 | 33 | 0 | 0 | 0 | |
| 3 | 20 | 38 | 35 | 0 | 0 | 0 | |
| 3 | 22 | 43 | 38 | 0 | 0 | 0 | |
| SD | 1.38 | 2.67 | 3.26 | 8.91 | 0.76 | 0.00 | 0.00 |
| Water Bathing Frequency | 7 | 25 | 37 | 30 | 34 | 25 | 5 |
| 5 | 28 | 36 | 35 | 38 | 28 | 8 | |
| 6 | 26 | 40 | 33 | 34 | 28 | 9 | |
| 7 | 24 | 38 | 35 | 32 | 24 | 8 | |
| 5 | 22 | 42 | 35 | 28 | 25 | 9 | |
| 7 | 23 | 40 | 38 | 26 | 22 | 6 | |
| 4 | 26 | 46 | 40 | 24 | 24 | 10 | |
| SD | 1.21 | 2.04 | 3.39 | 3.24 | 5.01 | 2.19 | 1.77 |
| The Total Duration of Daubing (Min) | 42 | 188 | 313 | 78 | 0 | 0 | 0 |
| 45 | 190 | 345 | 80 | 12 | 0 | 0 | |
| 46 | 205 | 330 | 76 | 0 | 0 | 0 | |
| 48 | 186 | 356 | 90 | 0 | 0 | 0 | |
| 43 | 198 | 346 | 85 | 0 | 0 | 0 | |
| 46 | 178 | 380 | 87 | 0 | 0 | 0 | |
| 36 | 179 | 400 | 96 | 0 | 0 | 0 | |
| SD | 3.95 | 9.74 | 29.41 | 7.11 | 4.54 | 0.00 | 0.00 |
| Categories | Compound Name and Molecular Formula | Compound Name and Molecular Formula |
|---|---|---|
| Ketones and aldehydes | Di-n-decylsulfone, | 1-Oxaspiro[2.5]octan-4-one,2,2,6-trimethyl-, trans-, |
| 4-Octanone, | 10-Nonadecanone, | |
| 5,10-Tetradecanedione, | Cyclohexanone,3-(3,3-dimethylbutyl)-, | |
| 2-Nonenal, (E)-, | 2-Hexenal, 2-ethyl-, | |
| (Z)-7-Hexadecenal, | 2(3H)-furanone,5-butyl-5-ethyldihydro- | |
| Esters | 2-Aminopent-4-enoic acid, N-vinyloxycarbonyl-, nonyl ester, | Acetic acid, chloro-, isobutyl ester, |
| Decanoic acid, 2,3-dihydroxypropyl ester, | 3-Chlropropionic acid, nonyl ester, | |
| Nonanoic acid, 6-phenyl-, methyl ester, | Butanoic acid, 2-methyl-, octyl ester, | |
| Succinic acid, 2-chloro-6-fluorophenyl non-3-en-1-yl ester, | 1,3,5-Triazine-2,4(1H,3H)-dione,6-(ethylamino)-, | |
| Docosanoic acid, docosyl ester, | 9,12,15-Octadecatrienoic acid,2-phenyl-1,3-dioxan-5-yl ester, | |
| Sulfurous acid, isohexyl 2-pentyl ester | Nonanoic acid, heptyl ester, | |
| vinyl laurate, | 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester, | |
| Esters | cyclohexylmethyl hexadecyl ester, | Formic acid, 2,4,4-trimethylpentyl ester, |
| Eicosyl trifluoroacetate, | Heptacosyl acetate, | |
| Sulfurous acid, octadecyl 2-propyl ester, | Diglycolic acid, 2-methylphenyl tridecyl ester, | |
| Bis(2-ethylhexyl) methylphosphonate, | Carbonic acid, decyl 2-ethylhexyl ester, | |
| Eicosanoic acid, 15-oxo-, methyl ester, | ||
| Alcohols | 2-Hexen-1-ol, (Z)-, | trans-1,4-Cyclohexanediol, |
| 1-Decanol, 2-hexyl-, | E,E,Z-1,3,12-Nonadecatriene-5,14-diol, | |
| Octacosanol, | 1,2-Cyclohexanediol,1-methyl-4-(1-methylethenyl)-, | |
| 1,2-Dihydrolinalool, | ||
| Hydrocarbons | Heptane, 3,3-dimethyl-, | Octane, 2-methyl-, |
| Octane, 2,6-dimethyl-, | Tridecane, | |
| Undecane, 2, 6-dimethyl-, | Hexadecane, | |
| Nonane, 5-(2-methylpropyl)-, | 2,6,10-Trimethyltridecane, | |
| Nonane, 3-methyl-5-propyl-, | Tridecane, 2-methyl-, | |
| Tetradecane, | ||
| 3,5-Dimethyldodecane, | 1H-Indene, octahydro-, cis-, | |
| Tetradecane, 4-methyl-, | Undecane, 3, 6-dimethyl-, | |
| Heneicosane, | Heptadecane, cis-, | |
| 2, 6, 10, 15-tetramethyl-, | Tetradecane, 5-methyl-, | |
| Heptadecane, 2,6,10,15-tetramethyl-, | Decane, 5-ethyl-5-methyl-, | |
| Heptadecane, 8-methyl-, | Undecane, 6-cyclohexyl-, | |
| Undecane, 3-methyl-, | Nonadecane, | |
| Pentadecane, 2,6,10-trimethyl-, | Eicosane, | |
| Decane, 3,8-dimethyl-, | 5-Butyl-5-ethylpentadecane, | |
| Pentadecane, 2,6,10,14-tetramethyl-, | 2-methyloctacosane, | |
| Octadecane, | Hexadecane, 2,6,10,14-tetramethyl-, | |
| Tetracosane, | 2-Methylhexacosane, | |
| Nonacosane, | Squalane, | |
| Hexatriacontane, | 1-Cyclopentyleicosane, | |
| 5-Butyl-5-ethylheptadecane, | Dodecylcyclohexane, | |
| 2-Pentadecane, 2,6,10,14-tetramethyl-, | 2-Methyltetracosane, | |
| Pentacosane, | 15-Isobutyl-(13.alpha.H)-isocopalane, | |
| 2,2,4,4,6,6,8,8-Heptamethyl-1-nonene, | 7-Tetradecene, | |
| 9-Octadecene, (E)-, | 2,4-Dimethyl-1-hexene, | |
| Octadecane, 3-methyl-, | ||
| Others | Butanoyl Chloride, 3-methyl-, | Cyclotetrasiloxane, octamethyl-, |
| Anabasine, | 2-Propen-1-amine, N-2-propenyl-, | |
| (1S, 2S)-(+)-1, 2-Diaminocyclohexane, | Cycloheptasiloxane, tetradecamethyl-, | |
| Azocine, octahydro-, | (R)-2,4-Dihydroxy-N-(3-hydroxypropyl)-3,3-dimethylbutyramide, | |
| Others | Disulfide, di-tert-dodecyl, | Cyclohexasiloxane, dodecamethyl-, |
| Piperazine, 1,4-bis(5-methyl-1,2,3-2H-triazol-4-yl)-, | 1-Bromoeicosane, | |
| 4-O-.beta.-D-galactopyranosyl-, | Cycloheptasiloxane, tetradecamethyl-, | |
| Phenol, 3,5-bis(1,1-dimethylethyl)-, | Cyclooctasiloxane, hexadecamethyl, | |
| Chlorguanide, | Octadecane, 1-chloro-, | |
| 2-Bromotetradecane, | Hexacosyl nonyl ether, | |
| Cyclodecasiloxane, eicosamethyl-, | Octacosane, 1-iodo-, | |
| Dotriacontane, 1-iodo-, | Cyclononasiloxane, octadecamethyl-, | |
| Triacontane, 1-bromo-, | Heptasiloxane, hexadecamethyl-, | |
| 2(3H)-Furanone, 4,5-dihydro-5-methoxy-4-(2,3-dimethyl-2-buten-4-yl)-, |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Tong, Y.; Dong, R.; Ye, X.; Yu, X. A Breeding Plumage in the Making: The Unique Process of Plumage Coloration in the Crested Ibis in Terms of Chemical Composition and Sex Hormones. Animals 2023, 13, 3820. https://doi.org/10.3390/ani13243820
Liu D, Tong Y, Dong R, Ye X, Yu X. A Breeding Plumage in the Making: The Unique Process of Plumage Coloration in the Crested Ibis in Terms of Chemical Composition and Sex Hormones. Animals. 2023; 13(24):3820. https://doi.org/10.3390/ani13243820
Chicago/Turabian StyleLiu, Danni, Yiwei Tong, Rong Dong, Xinping Ye, and Xiaoping Yu. 2023. "A Breeding Plumage in the Making: The Unique Process of Plumage Coloration in the Crested Ibis in Terms of Chemical Composition and Sex Hormones" Animals 13, no. 24: 3820. https://doi.org/10.3390/ani13243820
APA StyleLiu, D., Tong, Y., Dong, R., Ye, X., & Yu, X. (2023). A Breeding Plumage in the Making: The Unique Process of Plumage Coloration in the Crested Ibis in Terms of Chemical Composition and Sex Hormones. Animals, 13(24), 3820. https://doi.org/10.3390/ani13243820





