Predicting the Potential Distribution of the Szechwan Rat Snake (Euprepiophis perlacea) and Its Response to Climate Change in the Yingjing Area of the Giant Panda National Park
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
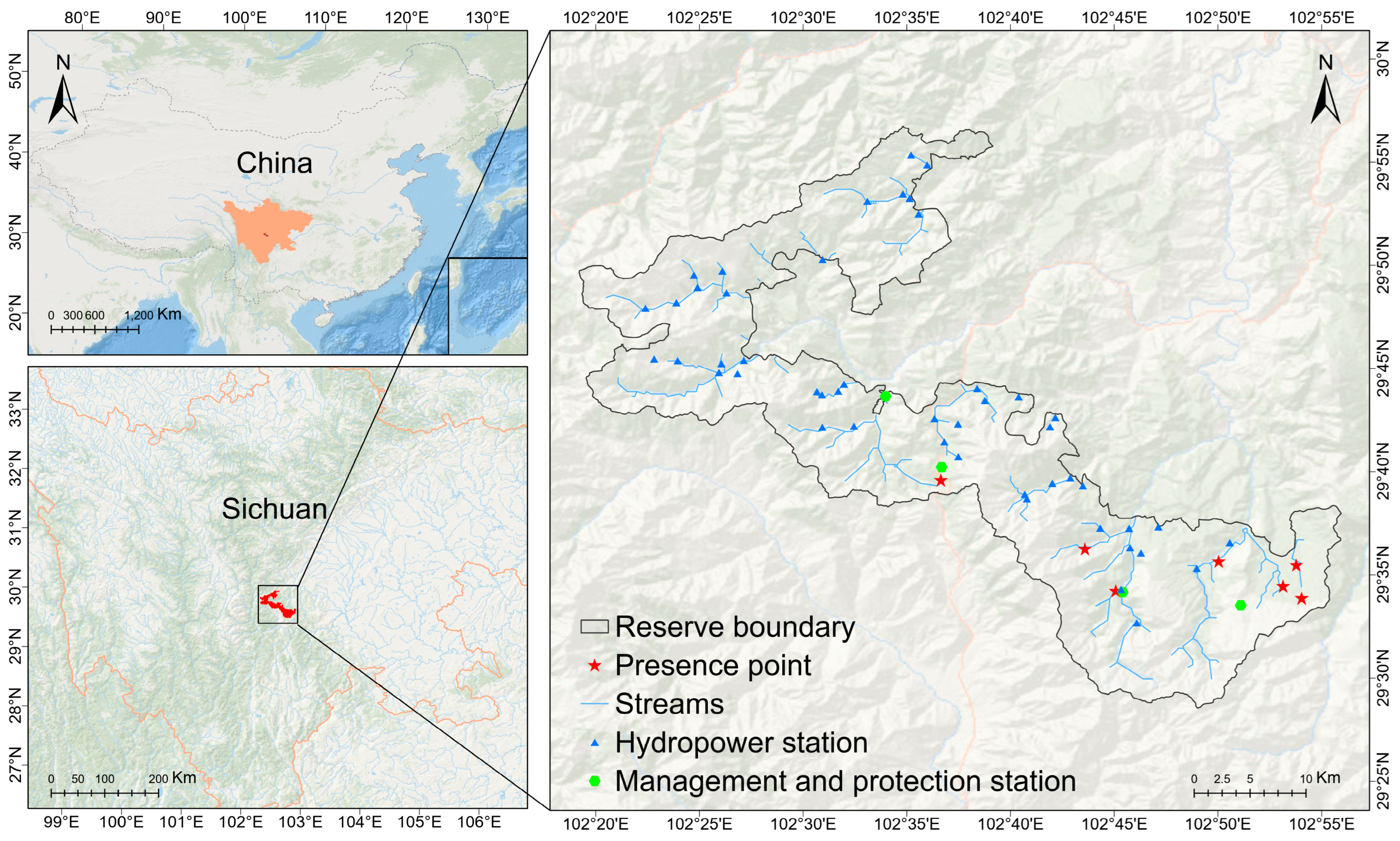
2.1. Study Site
2.2. Input Data
2.3. Ecological Niche Modeling (ENM)
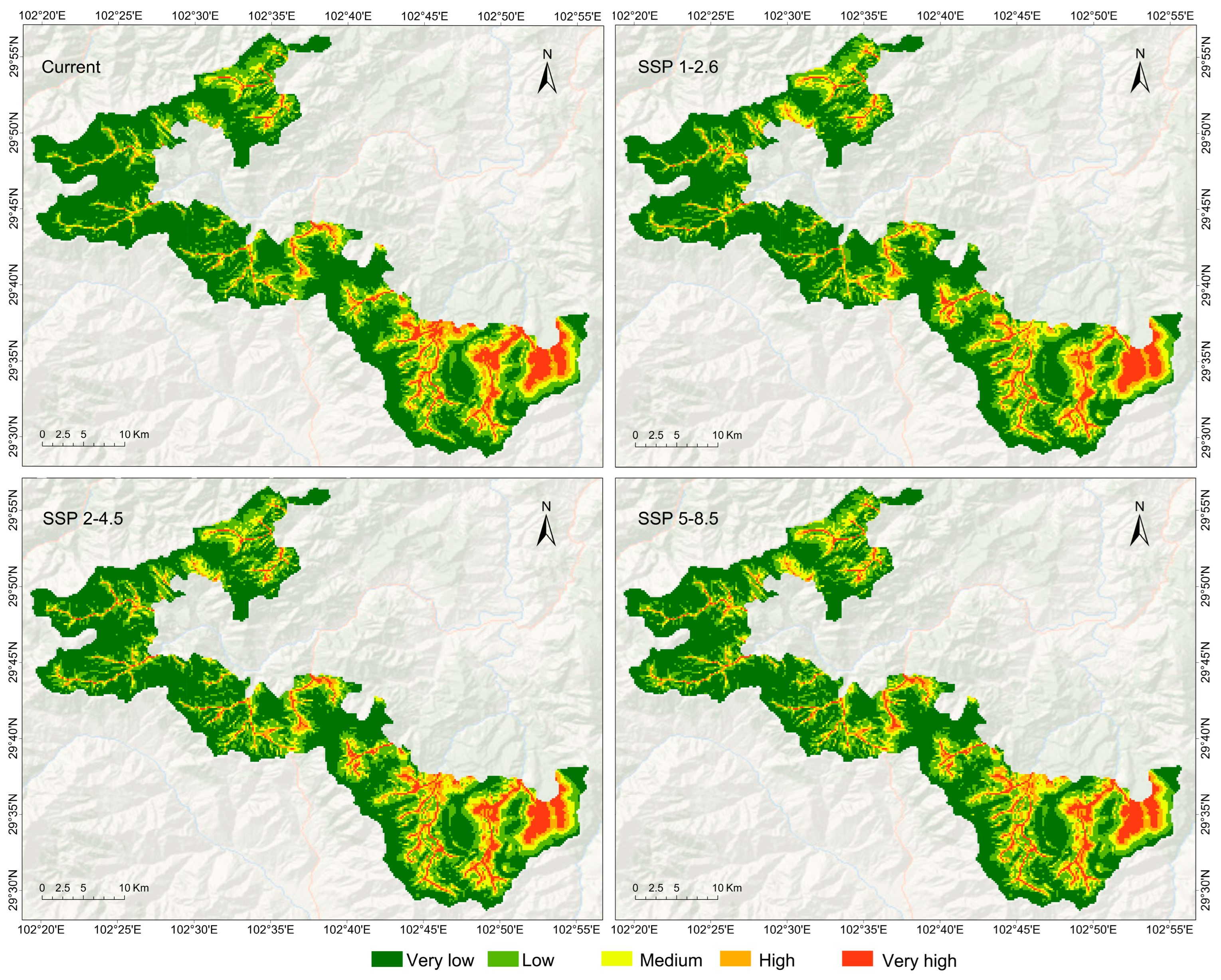
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ceballos, G.; Ehrlich, P.R.; Dirzo, R. Biological annihilation via the ongoing sixth mass extinction signaled by vertebrate population losses and declines. Proc. Natl. Acad. Sci. USA 2017, 114, E6089–E6096. [Google Scholar] [CrossRef] [PubMed]
- Barnosky, A.D.; Matzke, N.; Tomiya, S.; Wogan, G.O.U.; Swartz, B.; Quental, T.B.; Marshall, C.; McGuire, J.L.; Lindsey, E.L.; Maguire, K.C.; et al. Has the Earth’s sixth mass extinction already arrived? Nature 2011, 471, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, M.; Foden, W.B.; Visconti, P.; Watson, J.E.; Butchart, S.H.; Kovacs, K.M.; Scheffers, B.R.; Hole, D.G.; Martin, T.G.; Resit Akçakaya, H.; et al. Assessing species vulnerability to climate change. Nat. Clim. Chang. 2015, 5, 215–224. [Google Scholar] [CrossRef]
- Pimm, S.L.; Jenkins, C.N.; Abell, R.; Brooks, T.M.; Gittleman, J.L.; Joppa, L.N.; Raven, P.H.; Roberts, C.M.; Sexton, J.O. The biodiversity of species and their rates of extinction, distribution, and protection. Science 2014, 344, 1246752. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.D.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, B.F.N.; de Siqueira, M.F.; Grainger, A.; Hannah, L.; et al. Extinction risk from climate change. Nature 2004, 427, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Obregon, R.L.; Scolaro, J.A.; IbargÜengoytÍa, N.R.; Medina, M. Thermal biology and locomotor performance in Phymaturus calcogaster: Are Patagonian lizards vulnerable to climate change? Integr. Zool. 2021, 16, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Gardner, J.L.; Peters, A.; Kearney, M.R.; Joseph, L.; Heinsohn, R. Declining body size: A third universal response to warming? Trends Ecol. Evol. 2011, 26, 285–291. [Google Scholar] [CrossRef]
- Dillon, M.E.; Wang, G.; Huey, R.B. Global metabolic impacts of recent climate warming. Nature 2010, 467, 704–706. [Google Scholar] [CrossRef]
- Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Perry, A.L.; Low, P.J.; Ellis, J.R.; Reynolds, J.D. Climate change and distribution shifts in marine fishes. Science 2005, 308, 1912–1915. [Google Scholar] [CrossRef]
- Visser, M.E.; Both, C. Shifts in phenology due to global climate change: The need for a yardstick. Proc. R. Soc. B Biol. Sci. 2005, 272, 2561–2569. [Google Scholar] [CrossRef] [PubMed]
- Butikofer, L.; Ji, W.; Sacchi, R.; Mangiacotti, M. Climate migrants’ survival threatened by “C” shaped anthropic barriers. Integr. Zool. 2020, 15, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Rugiero, L.; Milana, G.; Petrozzi, F.; Capula, M.; Luiselli, L. Climate-change-related shifts in annual phenology of a temperate snake during the last 20 years. Acta Oecologica 2013, 51, 42–48. [Google Scholar] [CrossRef]
- VanDerWal, J.; Murphy, H.T.; Kutt, A.S.; Perkins, G.C.; Bateman, B.L.; Perry, J.J.; Reside, A.E. Focus on poleward shifts in species’ distribution underestimates the fingerprint of climate change. Nat. Clim. Chang. 2013, 3, 239–243. [Google Scholar] [CrossRef]
- Lenoir, J.; Gégout, J.C.; Marquet, P.A.; de Ruffray, P.; Brisse, H. A significant upward shift in plant species optimum elevation during the 20th century. Science 2008, 320, 1768–1771. [Google Scholar] [CrossRef]
- Parmesan, C.; Ryrholm, N.; Stefanescu, C.; Hill, J.K.; Thomas, C.D.; Descimon, H.; Huntley, B.; Kaila, L.; Kullberg, J.; Tammaru, T.; et al. Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature 1999, 399, 579–583. [Google Scholar] [CrossRef]
- Lourenço-de-Moraes, R.; Lansac-Toha, F.M.; Schwind, L.T.F.; Arrieira, R.L.; Rosa, R.R.; Terribile, L.C.; Lemes, P.; Rangel, T.F.; Diniz-Filho, J.A.F.; Bastos, R.P.; et al. Climate change will decrease the range size of snake species under negligible protection in the Brazilian Atlantic Forest hotspot. Sci. Rep. 2019, 9, 8523. [Google Scholar] [CrossRef]
- Zacarias, D.; Loyola, R. Climate change impacts on the distribution of venomous snakes and snakebite risk in Mozambique. Clim. Chang. 2019, 152, 195–207. [Google Scholar] [CrossRef]
- Currie, D.J. Projected effects of climate change on patterns of vertebrate and tree species richness in the conterminous United States. Ecosystems 2001, 4, 216–225. [Google Scholar] [CrossRef]
- Hansen, A.J.; Neilson, R.P.; Dale, V.H.; Flather, C.H.; Iverson, L.R.; Currie, D.J.; Shafer, S.; Cook, R.; Bartlein, P.J. Global change in forests: Responses of species, communities and biomes. BioScience 2001, 51, 765–779. [Google Scholar] [CrossRef]
- Araújo, M.B.; Thuiller, W.; Pearson, R.G. Climate warming and the decline of amphibians and reptiles in Europe. J. Biogeogr. 2006, 33, 1712–1728. [Google Scholar] [CrossRef]
- Archis, J.N.; Akcali, C.; Stuart, B.L.; Kikuchi, D.; Chunco, A.J. Is the future already here? The impact of climate change on the distribution of the eastern coral snake (Micrurus fulvius). PeerJ 2018, 6, e4647. [Google Scholar] [CrossRef]
- Kamdem, M.M.; Ngakou, A.; Yanou, N.N.; Otomo, V.P. Habitat components and population density drive plant litter consumption by Eudrilus eugeniae (Oligochaeta) under tropical conditions. Integr. Zool. 2021, 16, 255–269. [Google Scholar] [CrossRef]
- Kubisch, E.L.; Fernández, J.B.; Ibargüengoytía, N.R. Thermophysiological plasticity could buffer the effects of global warming on a Patagonian lizard. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2023, 339, 590–601. [Google Scholar] [CrossRef]
- McNeely, J.A. Today’s protected areas: Supporting a more sustainable future for humanity. Integr. Zool. 2020, 15, 603–616. [Google Scholar] [CrossRef]
- Sharnuud, R.; Ameca, E.I. Taxonomy, distribution, and contemporary exposure of terrestrial mammals to floods and human pressure across different areas for biodiversity conservation in China. Integr. Zool. 2023, 00, 1–10. [Google Scholar] [CrossRef]
- Carvalho, S.B.; Brito, J.C.; Crespo, E.J.; Possingham, H.P. Incorporating evolutionary processes into conservation planning using species distribution data: A case study with the western Mediterranean herpetofauna. Divers. Distrib. 2011, 17, 408–421. [Google Scholar] [CrossRef]
- Martínez-Freiría, F.; Argaz, H.; Fahd, S.; Brito, J.C. Climate change is predicted to negatively influence Moroccan endemic reptile richness. Implications for conservation in protected areas. Naturwissenschaften 2013, 100, 877–889. [Google Scholar] [CrossRef]
- Li, X.; Tian, H.; Wang, Y.; Li, R.; Song, Z.; Zhang, F.; Xu, M.; Li, D. Vulnerability of 208 endemic or endangered species in China to the effects of climate change. Reg. Environ. Chang. 2013, 13, 843–852. [Google Scholar] [CrossRef]
- Bond, N.; Thomson, J.; Reich, P.; Stein, J. Using species distribution models to infer potential climate change-induced range shifts of freshwater fish in south-eastern Australia. Mar. Freshw. Res. 2011, 62, 1043–1061. [Google Scholar] [CrossRef]
- Wang, B.; Xu, Y.; Ran, J.H. Predicting suitable habitat of the Chinese monal (Lophophorus lhuysii) using ecological niche modeling in the Qionglai Mountains, China. PeerJ 2017, 5, e3477. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.S.; Graham, B.; Bocksberger, G.; Stewart, F.A.; Sunderland-Groves, J.; Tagg, N.; Todd, A.; Vosper, A.; Wenceslau, J.F.C.; Wessling, E.G.; et al. Predicting range shifts of African apes under global change scenarios. Divers. Distrib. 2021, 27, 1663–1679. [Google Scholar] [CrossRef]
- Tang, J.; Swaisgood, R.R.; Owen, M.A.; Zhao, X.; Wei, W.; Hong, M.; Zhou, H.; Zhang, Z. Assessing the effectiveness of protected areas for panda conservation under future climate and land use change scenarios. J. Environ. Manag. 2023, 342, 118319. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, J.R.; Huettmann, F.; Leathwick, R.J.; Lehmann, A.; et al. Novel methods improve prediction of species’distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Song, K.; Mi, C.R.; Yang, N.; Sun, L.; Sun, Y.H.; Xu, J.L. Improve the roles of nature reserves in conservation of endangered pheasant in a highly urbanized region. Sci. Rep. 2020, 10, 17673. [Google Scholar] [CrossRef]
- Mays, H.L.; Hung, C.M.; Shaner, P.J.; Denvir, J.; Justice, M.; Yang, S.F.; Roth, T.L.; Oehler, D.A.; Fan, J.; Rekulapally, S.; et al. Genomic analysis of demographic history and ecological niche modeling in the endangered Sumatran rhinoceros Dicerorhinus sumatrensis. Curr. Biol. 2018, 28, 70–76. [Google Scholar] [CrossRef]
- Qin, A.; Liu, B.; Guo, Q.; Bussmann, R.W.; Ma, F.; Jian, Z.; Xu, G.; Pei, S. Maxent modeling for predicting impacts of climate change on the potential distribution of Thuja sutchuenensis Franch., an extremely endangered conifer from southwestern China. Glob. Ecol. Conserv. 2017, 10, 139–146. [Google Scholar] [CrossRef]
- Zhao, E. The validity of Elaphe perlacea-a rare endemic snake from Sichuan Province, China. Asiat. Herpetol. Res. 1990, 3, 101–103. [Google Scholar]
- IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org/en (accessed on 17 August 2023).
- Stejneger, L. A new snake from China. Proc. Biol. Soc. Wash. 1929, 42, 129–130. [Google Scholar]
- Deng, Q.X.; Jiang, Y.M. The Szechwan rat snake in China. J. China West Norm. Univ. (Nat. Sci.) 1989, 10, 120–122. [Google Scholar]
- Chen, X.; Jiang, K.; Guo, P.; Huang, S.; Rao, D.; Ding, L.; Takeuchi, H.; Che, J.; Zhang, Y.P.; Myers, E.A.; et al. Assessing species boundaries and the phylogenetic position of the rare Szechwan rat snake, Euprepiophis perlaceus (Serpentes: Colubridae), using coalescent-based methods. Mol. Phylogenet. Evol. 2014, 70, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.N.; Gan, S.X.; Ding, L.; Zhou, C.Q.; Mao, X.T.; Zhao, K.L. The experiment of the captive breeding for the Euprepiophis perlacea. J. Sichuan For. Sci. Technol. 2017, 38, 1003–5508. [Google Scholar]
- Gan, S.; Ding, L.; Yang, H.; Zhou, C.; Zhao, K. A preliminary study of selecting key ecological factors of the suitable habitats for Euprepiophis perlacer. J. Sichuan For. Sci. Technol. 2017, 38, 40–44. [Google Scholar]
- Shao, W.; Song, X.; Chen, C.; Zhao, L.; Jin, L.; Liao, W. Diversity and altitudinal distribution pattern of amphibians and reptiles in Yingjing Area of Giant Panda National Park. Chin. J. Zool. 2022, 57, 707–721. [Google Scholar]
- Fu, J.; Sun, Z.; Liu, S.; Fu, Z.; Liu, Y.; Wang, X.; Zhao, J.; Cai, G. A survey of bird resources in Daxiangling Nature Reserve in Sichuan Province. J. Sichuan For. Sci. Technol. 2008, 29, 31–37. [Google Scholar]
- Jia, W. Giant Panda Microhabitat Study in the Daxiangling Niba Mountain Corridor, Sichuan Province. Master Thesis, Guizhou Normal University, Guizhou, China, 10 March 2023. [Google Scholar]
- Deb, C.R.; Jamir, N.S.; Kikon, Z.P. Distribution prediction model of a rare orchid species (Vanda bicolor Griff.) using small sample size. Am. J. Plant Sci. 2017, 8, 1388. [Google Scholar]
- Popp, A.; Calvin, K.; Fujimori, S.; Havlik, P.; Humpenöder, F.; Stehfest, E.; Bodirsky, B.L.; Dietrich, J.P.; Doelmann, J.C.; Gusti, M.; et al. Land-use futures in the shared socio-economic pathways. Glob. Environ. Chang. 2017, 42, 331–345. [Google Scholar] [CrossRef]
- Xin, X. Performance of BCC-CSM2-MR in simulating summer climate changes in East Asia. EGU Gen. Assem. Conf. Abstr. 2019, 21, 4711. [Google Scholar]
- Gong, J.; Li, Y.; Wang, R.; Yu, C.; Fan, J.; Shi, K. MaxEnt modeling for predicting suitable habitats of snow leopard (Panthera uncia) in the mid-eastern Tianshan Mountains. J. Resour. Ecol. 2023, 14, 1075–1085. [Google Scholar]
- Ye, W.J.; Yang, N.; Yang, B.; Li, Y.; Zhang, J.D.; Chen, D.M.; Zhou, C.Q.; Zhong, X.; Zhang, Z.J. Impacts of climate change on potential geographical distribution of golden pheasant (Chrysolophus pictus), an endemic species in China. Chin. J. Ecol. 2021, 40, 1783–1792. [Google Scholar]
- Fischer, G.; Nachtergaele, F.; Prieler, S.; van Velthuizen, H.T.; Verelst, L.; Wiberg, D. Global Agro-Ecological Zones Assessment for Agriculture (GAEZ 2008); IIASA: Laxenburg, Austria; FAO: Rome, Italy, 2008. [Google Scholar]
- Hernandez, P.A.; Graham, C.H.; Master, L.L.; Albert, D.L. The effect of sample size and species characteristics on performance of different species distribution modeling methods. Ecography 2006, 29, 773–785. [Google Scholar] [CrossRef]
- Pearson, R.G.; Raxworthy, C.J.; Nakamura, M.; Peterson, A.T. Predicting species distributions from small numbers of occurrence records: A test case using cryptic geckos in Madagascar. J. Biogeogr. 2007, 34, 102–117. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting pseudo-absences for species distribution models: How, where and how many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Qu, M.; Zhang, Y.; Gao, Z.; Zhang, Z.; Liu, Y.; Wan, S.; Lin, Q. The genetic basis of the leafy seadragon’s unique camouflage morphology and avenues for its efficient conservation derived from habitat modeling. Sci. China Life Sci. 2023, 66, 1213–1230. [Google Scholar] [CrossRef] [PubMed]
- Terribile, L.C.; Diniz-Filho, J.A.F. How many studies are necessary to compare niche-based models for geographic distributions? Inductive reasoning may fail at the end. Braz. J. Biol. 2010, 70, 263–269. [Google Scholar] [CrossRef]
- Hosseinzadeh, M.S.; Ghezellou, P.; Kazemi, S.M. Predicting the potential distribution of the endemic snake Spalerosophis microlepis (Serpentes: Colubridae), in the Zagros Mountains, western Iran. Salamandra 2010, 53, 294–298. [Google Scholar]
- Chefaoui, R.M.; Hosseinzadeh, M.S.; Mashayekhi, M.; Safaei-Mahroo, B.; Kazemi, S.M. Identifying suitable habitats and current conservation status of a rare and elusive reptile in Iran. Amphib. Reptil. 2018, 39, 355–362. [Google Scholar] [CrossRef]
- Andrade-Díaz, M.S.; Sarquis, J.A.; Loiselle, B.A.; Giraudo, A.R.; Díaz-Gómez, J.M. Expansion of the agricultural frontier in the largest South American Dry Forest: Identifying priority conservation areas for snakes before everything is lost. PLoS ONE 2019, 14, e0221901. [Google Scholar] [CrossRef]
- Kirk, D.A.; Karimi, S.; Maida, J.R.; Harvey, J.A.; Larsen, K.W.; Bishop, C.A. Using ecological niche models for population and range estimates of a threatened snake species (Crotalus oreganus) in Canada. Diversity 2021, 13, 467. [Google Scholar] [CrossRef]
- Mizsei, E.; Szabolcs, M.; Szabó, L.O.R.Á.N.D.; Boros, Z.; Mersini, K.; Roussos, S.A.; Lengyel, S. Determining priority areas for an endangered cold-adapted snake on warming mountaintops. Oryx 2021, 55, 334–343. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Pearman, P.B.; Guisan, A.; Broennimann, O.; Randin, C.F. Niche dynamics in space and time. Trends Ecol. Evol. 2008, 23, 149–158. [Google Scholar] [CrossRef]
- Lin, L.H.; Zhu, X.M.; Du, Y.; Fang, M.C.; Ji, X. Global, regional, and cladistic patterns of variation in climatic niche breadths in terrestrial elapid snakes. Curr. Zool. 2019, 65, 1–9. [Google Scholar] [CrossRef]
- Kalboussi, M.; Achour, H. Modelling the spatial distribution of snake species in northwestern Tunisia using maximum entropy (Maxent) and Geographic Information System (GIS). J. For. Res. 2018, 29, 233–245. [Google Scholar] [CrossRef]
- Snyder, S.D.; Sutton, W.B.; Steen, D.A. Species distribution modeling reveals insights into the occurrence of a locally rare snake at the periphery of its geographic range. Herpetologica 2023, 79, 98–107. [Google Scholar]
- Richardson, D.M.; Holmes, P.M.; Esler, K.J.; Galatowitsch, S.M.; Stromberg, J.C.; Kirkman, S.P.; Pysek, P.; Hobbs, R.J. Riparian vegetation: Degradation, alien plant invasions, and restoration prospects. Divers. Distrib. 2007, 13, 126–139. [Google Scholar] [CrossRef]
- Bale, J.S.; Masters, G.J.; Hodkinson, I.D.; Awmack, C.; Bezemer, T.M.; Brown, V.K.; Butterfield, J.; Buse, A.; Coulson, J.C.; Farrar, J.; et al. Herbivory in global climate change research: Direct effects of rising temperature on insect herbivores. Glob. Chang. Biol. 2022, 8, 1–16. [Google Scholar] [CrossRef]
- Kocmánková, E.; Trnka, M.; Juroch, J.; Dubrovský, M.; Semerádová, D.; Možný, M.; Žalud, Z. Impact of climate change on the occurrence and activity of harmful organisms. Plant Prot. Sci. 2009, 45, S48–S52. [Google Scholar] [CrossRef]
- Dos Santos, L.A.; Mendes, M.F.; Krüger, A.P.; Blauth, M.L.; Gottschalk, M.S.; Garcia, F.R. Global potential distribution of Drosophila suzukii (Diptera, Drosophilidae). PLoS ONE 2017, 12, e0174318. [Google Scholar] [CrossRef]
- Wei, J.; Peng, L.; He, Z.; Lu, Y.; Wang, F. Potential distribution of two invasive pineapple pests under climate change. Pest Manag. Sci. 2020, 76, 1652–1663. [Google Scholar] [CrossRef] [PubMed]
- Staley, J.T.; Hodgson, C.J.; Mortimer, S.R.; Morecroft, M.D.; Masters, G.J.; Brown, V.K.; Taylor, M.E. Effects of summer rainfall manipulations on the abundance and vertical distribution of herbivorous soil macro-invertebrates. Eur. J. Soil Biol. 2007, 43, 189–198. [Google Scholar] [CrossRef]
- Kumar, R.; Nagrare, V.S.; Nitharwal, M.; Swami, D.; Prasad, Y.G. Within-plant distribution of an invasive mealybug, Phenacoccus solenopsis, and associated losses in cotton. Phytoparasitica 2014, 42, 311–316. [Google Scholar] [CrossRef]
- Wen, H.; Luo, T.; Wang, Y.; Wang, S.; Liu, T.; Xiao, N.; Zhou, J. Molecular phylogeny and historical biogeography of the cave fish genus Sinocyclocheilus (Cypriniformes: Cyprinidae) in southwest China. Integr. Zool. 2022, 17, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Sun, Y.; Wang, T.; Skidmore, A.K.; Ding, C.; Ye, X. Linking the past and present to predict the distribution of Asian crested ibis (Nipponia nippon) under global changes. Integr. Zool. 2022, 17, 1095–1105. [Google Scholar] [CrossRef]
- Tourinho, L.; Vale, M.M. Choosing among correlative, mechanistic, and hybrid models of species’ niche and distribution. Integr. Zool. 2023, 18, 93–109. [Google Scholar] [CrossRef] [PubMed]
- Muthoni, F.K. Modelling the Spatial Distribution of Snake Species under Changing Climate Scenario in Spain. Master’s Thesis, University of Twente, Enschede, The Netherlands, 2010. [Google Scholar]
- Muñoz, I.; Rigarlsford, G.; Canals, L.M.; King, H. Accounting for greenhouse gas emissions from the degradation of chemicals in the environment. Int. J. Life Cycle Assess. 2013, 18, 252–262. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, J.; Ren, G.; Zhao, K.; Wang, X. Global potential distribution prediction of Xanthium italicum based on Maxent model. Sci. Rep. 2021, 11, 16545. [Google Scholar] [CrossRef]
- Della, R.F.; Milanesi, P. Combining climate, land use change and dispersal to predict the distribution of endangered species with limited vagility. J. Biogeogr. 2020, 47, 1427–1438. [Google Scholar] [CrossRef]
- Liu, S.; Xia, S.; Wu, D.; Behm, J.E.; Meng, Y.; Yuan, H.; Wen, P.; Hughes, A.C.; Yang, X. Understanding global and regional patterns of termite diversity and regional functional traits. iScience 2022, 25, 105538. [Google Scholar] [CrossRef] [PubMed]
- Sentís, M.; Pacioni, C.; De Cuyper, A.; Janssens, G.P.J.; Lens, L.; Strubbe, D. Biophysical models accurately characterize the thermal energetics of a small invasive passerine bird. iScience 2023, 26, 107743. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Tang, L.; He, H.; Yang, F.; Tao, J.; Wang, W. Assessing the impact of climate change on the distribution of Osmanthus fragrans using Maxent. Environ. Sci. Pollut. Res. 2021, 28, 34655–34663. [Google Scholar] [CrossRef] [PubMed]
- Thapa, A.; Wu, R.; Hu, Y.; Nie, Y.; Singh, P.B.; Khatiwada, J.R.; Yan, L.; Gu, X.; Wei, F. Predicting the potential distribution of the endangered red panda across its entire range using MaxEnt modeling. Ecol. Evol. 2018, 8, 10542–10554. [Google Scholar] [CrossRef] [PubMed]
- De Kort, H.; Baguette, M.; Lenoir, J.; Stevens, V.M. Toward reliable habitat suitability and accessibility models in an era of multiple environmental stressors. Ecol. Evol. 2020, 10, 10937–10952. [Google Scholar] [CrossRef]
- Yang, S.; Wang, X.; Hu, J. Mountain frog species losing out to climate change around the Sichuan Basin. Sci. Total Environ. 2022, 806, 150605. [Google Scholar] [CrossRef]
- Bensch, S.; Caballero-López, V.; Cornwallis, C.K.; Sokolovskis, K. The evolutionary history of “suboptimal” migration routes. iScience 2023, 26, 108266. [Google Scholar] [CrossRef]
- Fricke, E.C.; Ordonez, A.; Rogers, H.S.; Svenning, J.C. The effects of defaunation on plants’ capacity to track climate change. Science 2022, 375, 210–214. [Google Scholar] [CrossRef]
| Different Scenarios | AUC Value * |
|---|---|
| Current | 0.83 ± 0.16 |
| 2050s SSP 1-2.6 | 0.81 ± 0.21 |
| 2050s SSP 2-4.5 | 0.80 ± 0.21 |
| 2050s SSP 5-8.5 | 0.79 ± 0.21 |
| Environmental Variable | Current | 2050s | ||
|---|---|---|---|---|
| SSP 1-2.6 | SSP 2-4.5 | SSP 5-8.5 | ||
| Distance from stream | 41.90 | 48.80 | 48.50 | 49.50 |
| Slope degree | 32.00 | 37.50 | 38.80 | 40.40 |
| NDVI | 0.40 | 0.30 | 0.30 | |
| Soil water regime | 0.10 | 0.40 | 0.40 | 0.50 |
| Bio2 (mean diurnal range) | 4.30 | 8.30 | 12.10 | 7.70 |
| Bio3 (isothermality) | 19.90 | 0.60 | 1.10 | |
| Bio7 (temperature annual range) | 0.10 | |||
| Bio14 (precipitation of driest month) | 1.40 | 0.10 | ||
| Bio15 (precipitation seasonality) | 0.10 | 3.90 | 0.20 | |
| Bio17 (precipitation of driest quarter) | 0.40 | 0.30 | ||
| Bio19 (precipitation of coldest quarter) | 0.20 | |||
| Distribution Potential | Current | 2050s | ||
|---|---|---|---|---|
| SSP 1-2.6 | SSP 2-4.5 | SSP 5-8.5 | ||
| very low | 53.59 | 51.88 | 50.45 | 49.56 |
| low | 23.30 | 24.06 | 24.95 | 25.40 |
| medium | 10.64 | 12.02 | 11.95 | 12.27 |
| high | 6.90 | 6.91 | 7.14 | 7.21 |
| very high | 5.58 | 5.14 | 5.50 | 5.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Jiang, Y.; Zhao, L.; Jin, L.; Yan, C.; Liao, W. Predicting the Potential Distribution of the Szechwan Rat Snake (Euprepiophis perlacea) and Its Response to Climate Change in the Yingjing Area of the Giant Panda National Park. Animals 2023, 13, 3828. https://doi.org/10.3390/ani13243828
Song X, Jiang Y, Zhao L, Jin L, Yan C, Liao W. Predicting the Potential Distribution of the Szechwan Rat Snake (Euprepiophis perlacea) and Its Response to Climate Change in the Yingjing Area of the Giant Panda National Park. Animals. 2023; 13(24):3828. https://doi.org/10.3390/ani13243828
Chicago/Turabian StyleSong, Xinqiang, Ying Jiang, Li Zhao, Long Jin, Chengzhi Yan, and Wenbo Liao. 2023. "Predicting the Potential Distribution of the Szechwan Rat Snake (Euprepiophis perlacea) and Its Response to Climate Change in the Yingjing Area of the Giant Panda National Park" Animals 13, no. 24: 3828. https://doi.org/10.3390/ani13243828





