Effects of Dietary Microbial Muramidase on the Growth, Liver Histoarchitecture, Antioxidant Status, and Immunoexpression of Pro-Inflammatory Cytokines in Broiler Chickens
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Feed Additive Used
2.2. Birds
2.3. Experimental Design and Diets
2.4. Growth Performance
2.5. Sample Collection
2.6. Clinico-Biochemical Analysis
2.7. Immune and Antioxidant Indices
2.8. Histological and Immunohistochemical Examination
2.9. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Clinic-Biochemical Indices
3.3. Antioxidant and Inflammatory Responses
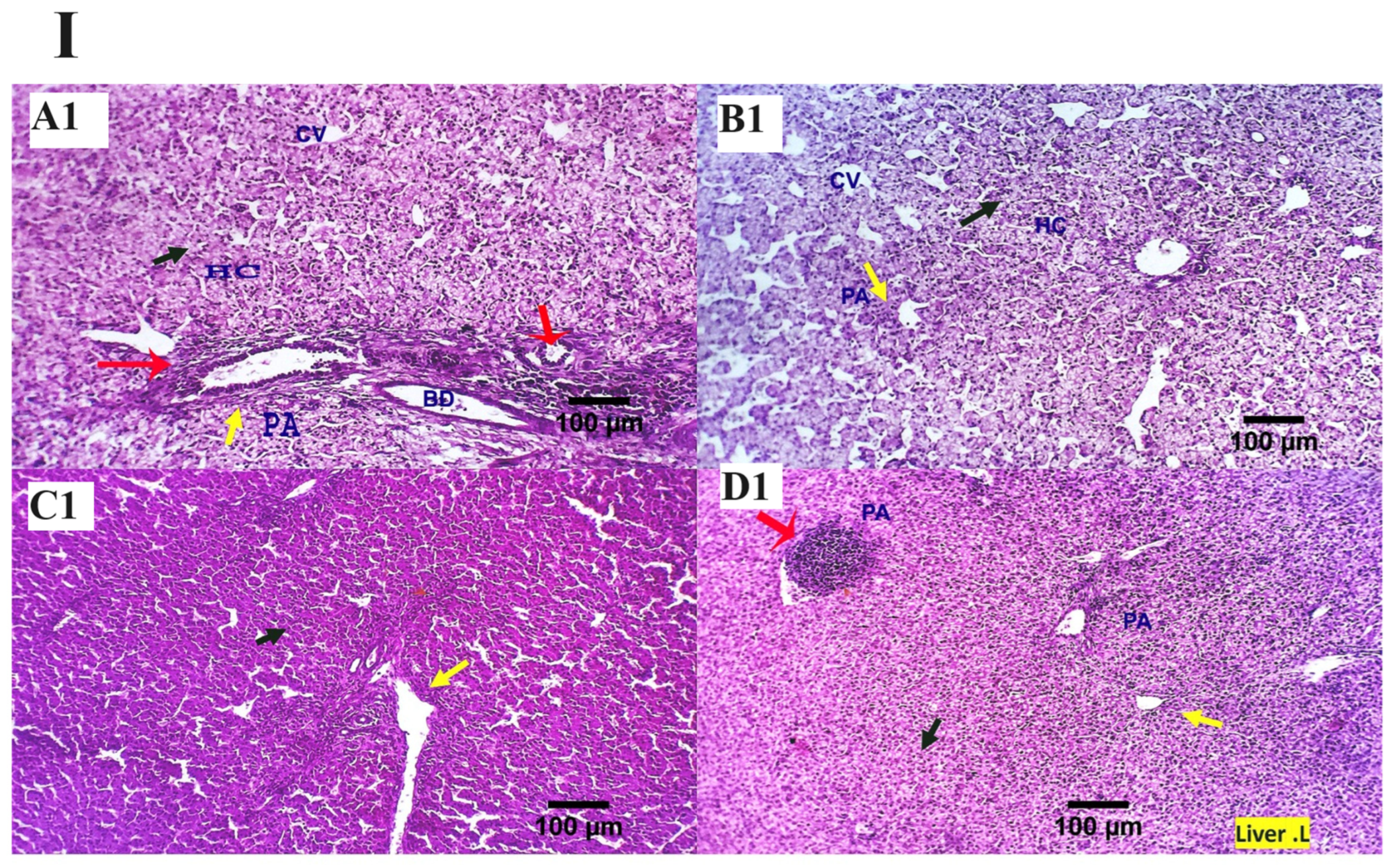
3.4. Histopathological Findings
3.5. TGF-β Immunostaining
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bedford, M.; Cowieson, A. Exogenous enzymes and their effects on intestinal microbiology. Anim. Feed Sci. Technol. 2012, 173, 76–85. [Google Scholar] [CrossRef]
- Adeola, O.; Cowieson, A. Board-invited review: Opportunities and challenges in using exogenous enzymes to improve nonruminant animal production. J. Anim. Sci. 2011, 89, 3189–3218. [Google Scholar] [CrossRef]
- Meale, S.J.; Beauchemin, K.A.; Hristov, A.N.; Chaves, A.; McAllister, T. Board-invited review: Opportunities and challenges in using exogenous enzymes to improve ruminant production. J. Anim. Sci. 2014, 92, 427–442. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Lin, S.; Zhu, J.; Pang, X.; Fang, Z.; Lin, Y.; Che, L.; Xu, S.; Li, J.; Huang, Y. Effects of dietary lysozyme levels on growth performance, intestinal morphology, non-specific immunity and mRNA expression in weanling piglets. Anim. Sci. J. 2016, 87, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Sais, M.; Barroeta, A.C.; López-Colom, P.; Nofrarías, M.; Majó, N.; Lopez-Ulibarri, R.; Calvo, E.P.; Martín-Orúe, S.M. Evaluation of dietary supplementation of a novel microbial muramidase on gastrointestinal functionality and growth performance in broiler chickens. Poult. Sci. 2020, 99, 235–245. [Google Scholar] [CrossRef]
- Sahoo, N.; Kumar, P.; Bhusan, B.; Bhattacharya, T.; Dayal, S.; Sahoo, M. Lysozyme in livestock: A guide to selection for disease resistance: A review. J. Anim. Sci. Adv 2012, 2, 347–360. [Google Scholar]
- Boroojeni, F.G.; Männer, K.; Rieger, J.; Calvo, E.P.; Zentek, J. Evaluation of a microbial muramidase supplementation on growth performance, apparent ileal digestibility, and intestinal histology of broiler chickens. Poult. Sci. 2019, 98, 2080–2086. [Google Scholar] [CrossRef]
- Lee, W.-J.; Hase, K. Gut microbiota–generated metabolites in animal health and disease. Nat. Chem. Biol. 2014, 10, 416–424. [Google Scholar] [CrossRef]
- Broom, L.J.; Kogut, M.H. Inflammation: Friend or foe for animal production? Poult. Sci. 2018, 97, 510–514. [Google Scholar] [CrossRef]
- Lee, M.S.; Kim, Y.-J. Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annu. Rev. Biochem. 2007, 76, 447–480. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Lichtenberg, J.; Calvo, E.P.; Madsen, K.; Lund, T.Ø.; Birkved, F.K.; van Cauwenberghe, S.; Mourier, M.; Wulf-Andersen, L.; Jansman, A.; Lopez-Ulibarri, R. Safety evaluation of a novel muramidase for feed application. Regul. Toxicol. Pharmacol. 2017, 89, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Oliver, W.; Wells, J. Lysozyme as an alternative to antibiotics improves growth performance and small intestinal morphology in nursery pigs. J. Anim. Sci. 2013, 91, 3129–3136. [Google Scholar] [CrossRef] [PubMed]
- Oliver, W.; Wells, J. Lysozyme as an alternative to growth promoting antibiotics in swine production. J. Anim. Sci. Biotechnol. 2015, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Amer, S.A.; Farahat, M.; Gouda, A.; Abdel-Wareth, A.A.; Abdel-Warith, A.-W.A.; Younis, E.M.; Elshopakey, G.E.; Baher, W.M.; Saleh, G.K.; Davies, S.J. New Insights into the Effects of Microbial Muramidase Addition in the Diets of Broiler Chickens. Animals 2023, 13, 1356. [Google Scholar] [CrossRef]
- Aviagen, R. Ross Broiler Management Manual, 2009; ROSS: Richmond, VA, USA, 2014; Volume 9, pp. 350–364. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International; AOAC: Rockville, MD, USA, 2000. [Google Scholar]
- Simpson, R.J.; Neuberger, M.R.; Liu, T.-Y. Complete amino acid analysis of proteins from a single hydrolysate. J. Biol. Chem. 1976, 251, 1936–1940. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.; Furrow, R.; Bradley, B. Subchronic toxicity of monensin in broiler chickens. Vet. Pathol. 1983, 20, 353–359. [Google Scholar] [CrossRef]
- Trinder, P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann. Clin. Biochem. 1969, 6, 24–27. [Google Scholar] [CrossRef]
- Mcdonald, R.E.; Hultin, H.O. Some characteristics of the enzymic lipid peroxidation system in the microsomal fraction of flounder skeletal muscle. J. Food Sci. 1987, 52, 15–21. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.J. 241 Total antioxidant status in plasma and body fluids. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1994; Volume 234, pp. 279–293. [Google Scholar]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Nishikimi, M.; Rao, N.A.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Bancroft, J.; Layton, C.; Suvarna, S. Bancroft’s Theory and Practice of Histological Techniques; Churchill Livingstone: London, UK, 2013. [Google Scholar]
- Saber, S.; Khalil, R.M.; Abdo, W.S.; Nassif, D.; El-Ahwany, E. Olmesartan ameliorates chemically-induced ulcerative colitis in rats via modulating NFκB and Nrf-2/HO-1 signaling crosstalk. Toxicol. Appl. Pharmacol. 2019, 364, 120–132. [Google Scholar] [CrossRef]
- Rizzardi, A.E.; Johnson, A.T.; Vogel, R.I.; Pambuccian, S.E.; Henriksen, J.; Skubitz, A.P.; Metzger, G.J.; Schmechel, S.C. Quantitative comparison of immunohistochemical staining measured by digital image analysis versus pathologist visual scoring. Diagn. Pathol. 2012, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Deplancke, B.; Gaskins, H.R. Microbial modulation of innate defense: Goblet cells and the intestinal mucus layer. Am. J. Clin. Nutr. 2001, 73, 1131S–1141S. [Google Scholar] [CrossRef] [PubMed]
- Goes, E.C.; Dal Pont, G.C.; Maiorka, A.; Bittencourt, L.C.; Bortoluzzi, C.; Fascina, V.B.; Lopez-Ulibarri, R.; Calvo, E.P.; Beirão, B.C.; Caron, L.F. Effects of a microbial muramidase on the growth performance, intestinal permeability, nutrient digestibility, and welfare of broiler chickens. Poult. Sci. 2022, 101, 102232. [Google Scholar] [CrossRef] [PubMed]
- Kogut, M.H.; Genovese, K.J.; Swaggerty, C.L.; He, H.; Broom, L. Inflammatory phenotypes in the intestine of poultry: Not all inflammation is created equal. Poult. Sci. 2018, 97, 2339–2346. [Google Scholar] [CrossRef]
- Lee, M.; Kovacs-Nolan, J.; Yang, C.; Archbold, T.; Fan, M.Z.; Mine, Y. Hen egg lysozyme attenuates inflammation and modulates local gene expression in a porcine model of dextran sodium sulfate (DSS)-induced colitis. J. Agric. Food Chem. 2009, 57, 2233–2240. [Google Scholar] [CrossRef] [PubMed]
- May, K.; Wells, J.; Maxwell, C.; Oliver, W. Granulated lysozyme as an alternative to antibiotics improves growth performance and small intestinal morphology of 10-day-old pigs. J. Anim. Sci. 2012, 90, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Latif, M.A.; El-Far, A.H.; Elbestawy, A.R.; Ghanem, R.; Mousa, S.A.; Abd El-Hamid, H.S. Exogenous dietary lysozyme improves the growth performance and gut microbiota in broiler chickens targeting the antioxidant and non-specific immunity mRNA expression. PLoS ONE 2017, 12, e0185153. [Google Scholar] [CrossRef]
- Brugaletta, G.; De Cesare, A.; Laghi, L.; Manfreda, G.; Zampiga, M.; Oliveri, C.; Pérez-Calvo, E.; Litta, G.; Lolli, S.; Sirri, F. A multi-omics approach to elucidate the mechanisms of action of a dietary muramidase administered to broiler chickens. Sci. Rep. 2022, 12, 5559. [Google Scholar] [CrossRef]
- Humphrey, B.D.; Huang, N.; Klasing, K.C. Rice expressing lactoferrin and lysozyme has antibiotic-like properties when fed to chicks. J. Nutr. 2002, 132, 1214–1218. [Google Scholar]
- Liu, D.; Guo, Y.; Wang, Z.; Yuan, J. Exogenous lysozyme influences Clostridium perfringens colonization and intestinal barrier function in broiler chickens. Avian Pathol. 2010, 39, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Anderson, D.; Rathgeber, B.; MacIsaac, J. The effect of dietary lysozyme with EDTA on growth performance and intestinal microbiota of broiler chickens in each period of the growth cycle. J. Appl. Poult. Res. 2017, 26, 1–8. [Google Scholar] [CrossRef]
- Schliffka, W.; Zhai, H.-X.; Calvo, E.P.; van Cauwenberghe, S.; Walsh, M.C.; Lopez-Ulibarri, R. Safety and efficacy evaluation of a novel dietary muramidase for swine. Heliyon 2019, 5, e02600. [Google Scholar] [CrossRef] [PubMed]
- Robertson, F.P.; Bessell, P.R.; Diaz-Nieto, R.; Thomas, N.; Rolando, N.; Fuller, B.; Davidson, B.R. High serum Aspartate transaminase levels on day 3 postliver transplantation correlates with graft and patient survival and would be a valid surrogate for outcome in liver transplantation clinical trials. Transpl. Int. 2016, 29, 323–330. [Google Scholar] [CrossRef]
- Abdelazeem, A.S.; Fayed, A.M.; Basyony, M.M.; Abu Hafsa, S.H.; Mahmoud, A.E. Hematology profile, digestive enzymes, thyroid hormones, productivity, and nitrogen balance of growing male rabbits supplemented with exogenous dietary lysozyme. Anim. Biotechnol. 2023, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Scanes, C.G.; Harvey, S.; Marsh, J.A.; King, D.B. Hormones and growth in poultry. Poult. Sci. 1984, 63, 2062–2074. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, S.; Xia, Z.; Yuan, J. Effects of different types of polyunsaturated fatty acids on immune function and PGE2 synthesis by peripheral blood leukocytes of laying hens. Anim. Feed Sci. Technol. 2004, 116, 249–258. [Google Scholar] [CrossRef]
- Johnson, R.W. Inhibition of growth by pro-inflammatory cytokines: An integrated view. J. Anim. Sci. 1997, 75, 1244–1255. [Google Scholar] [CrossRef]
- Spurlock, M. Regulation of metabolism and growth during immune challenge: An overview of cytokine function. J. Anim. Sci. 1997, 75, 1773–1783. [Google Scholar] [CrossRef]
- Cooper, C.A.; Brundige, D.R.; Reh, W.A.; Maga, E.A.; Murray, J.D. Lysozyme transgenic goats’ milk positively impacts intestinal cytokine expression and morphology. Transgenic Res. 2011, 20, 1235–1243. [Google Scholar] [CrossRef][Green Version]
- Masschalck, B.; Michiels, C.W. Antimicrobial properties of lysozyme in relation to foodborne vegetative bacteria. Crit. Rev. Microbiol. 2003, 29, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Brundige, D.R.; Maga, E.A.; Klasing, K.C.; Murray, J.D. Lysozyme transgenic goats' milk influences gastrointestinal morphology in young pigs. J. Nutr. 2008, 138, 921–926. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ma, X.; Zhang, S.; Pan, L.; Piao, X. Effects of lysozyme on the growth performance, nutrient digestibility, intestinal barrier, and microbiota of weaned pigs fed diets containing spray-dried whole egg or albumen powder. Can. J. Anim. Sci. 2017, 97, 466–475. [Google Scholar] [CrossRef]
- Wang, Y.; Goossens, E.; Eeckhaut, V.; Calvo, E.P.; Lopez-Ulibarri, R.; Eising, I.; Klausen, M.; Debunne, N.; De Spiegeleer, B.; Ducatelle, R. Dietary muramidase degrades bacterial peptidoglycan to NOD-activating muramyl dipeptides and reduces duodenal inflammation in broiler chickens. Br. J. Nutr. 2021, 126, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Negroni, A.; Pierdomenico, M.; Cucchiara, S.; Stronati, L. NOD2 and inflammation: Current insights. J. Inflamm. Res. 2018, 49–60. [Google Scholar] [CrossRef]
- Strober, W.; Murray, P.J.; Kitani, A.; Watanabe, T. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat. Rev. Immunol. 2006, 6, 9–20. [Google Scholar] [CrossRef]
- Abu Hafsa, S.H.; Mahmoud, A.E.; Fayed, A.M.; Abdel-Azeem, A.-A.S. The Effect of Exogenous Lysozyme Supplementation on Growth Performance, Caecal Fermentation and Microbiota, and Blood Constituents in Growing Rabbits. Animals 2022, 12, 899. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, X.; Yang, Y.; Han, D.; Jin, J.; Xie, S. Effect of dietary lysozyme on growth, immune response, intestine microbiota, intestine morphology and resistance to A eromonas hydrophilia in gibel carp (C arassius auratus gibelio). Aquac. Nutr. 2014, 20, 229–241. [Google Scholar] [CrossRef]




| Ingredients | Starter Period (4–10 d) | Grower Period (11–23 d) | Finisher Period (24–35 d) |
|---|---|---|---|
| Yellow corn | 55.86 | 59.29 | 62.27 |
| Soybean meal, 48% | 33.67 | 28.085 | 23.625 |
| Corn gluten, 60% | 3.725 | 5.3 | 6 |
| Soybean oil | 2.2 | 3 | 4 |
| Calcium carbonate | 1.2 | 1.2 | 1.1 |
| Calcium dibasic phosphate | 1.5 | 1.4 | 1.3 |
| Common salt | 0.15 | 0.15 | 0.15 |
| Premix * | 0.3 | 0.3 | 0.3 |
| DL-methionine, 98% | 0.4 | 0.3 | 0.33 |
| Lysine HCl, 78% | 0.47 | 0.45 | 0.40 |
| Choline 60% | 0.07 | 0.07 | 0.07 |
| Threonine | 0.1 | 0.1 | 0.1 |
| Phytase | 0.005 | 0.005 | 0.005 |
| Na2Co3 | 0.25 | 0.25 | 0.25 |
| Anti-mycotoxin | 0.1 | 0.1 | 0.1 |
| Chemical composition | |||
| ME (Kcal/kg) | 3003 | 3101 | 3202 |
| Crude protein % | 23.04 | 21.5 | 20.04 |
| Calcium % | 0.941 | 0.903 | 0.832 |
| Available phosphorus % | 0.482 | 0.448 | 0.417 |
| Lysine % | 1.47 | 1.31 | 1.157 |
| Methionine % | 0.721 | 0.610 | 0.625 |
| Threonine % | 0.825 | 0.765 | 0.709 |
| Traits | MMUR Level (mg/kg) | p-Value | |||||
|---|---|---|---|---|---|---|---|
| 0 | 200 | 400 | 600 | ANOVA | Linear | Quadratic | |
| Initial BW (g) | 98.00 ± 0.001 | 97.1 ± 0.72 | 98.1 ± 0.63 | 97.5 ± 0.001 | 0.094 | 0.720 | 0.611 |
| Starter period (4–10th day) | |||||||
| BW (g) | 332 ± 7.5 b | 340 ± 5.9 ab | 336 ± 3.1 ab | 350 ± 12.3 a | 0.103 | 0.041 | 0.574 |
| BWG (g) | 234 ± 7.5 b | 243 ± 6.2 ab | 238 ± 3.6 ab | 252 ± 12.3 a | 0.094 | 0.041 | 0.600 |
| FI (g) | 268 ± 7.1 | 265 ± 6.1 | 264 ± 3.1 | 265 ± 8.4 | 0.854 | 0.520 | 0.592 |
| FCR | 1.15 ± 0.02 a | 1.10 ± 0.04 ab | 1.11 ± 0.01 ab | 1.05 ± 0.07 b | 0.094 | 0.033 | 0.989 |
| Grower period (11th–23rd day) | |||||||
| BW (g) | 1080 ± 72.5 b | 1170 ± 8.7 ab | 1182 ± 27.5 ab | 1212.62 ± 73 a | 0.074 | 0.018 | 0.359 |
| BWG (g) | 748 ± 65.1 b | 830 ± 14.1 ab | 846 ± 25.3 ab | 862.7 ± 80.6 a | 0.119 | 0.033 | 0.323 |
| FI (g) | 1139 ± 33.2 | 1147 ± 37.6 | 1202 ± 47.8 | 1107.08 ± 94 | 0.320 | 0.790 | 0.165 |
| FCR | 1.53 ± 0.09 a | 1.38 ± 0.07 bc | 1.42 ± 0.03 ab | 1.28 ± 0.01 c | 0.008 | 0.002 | 0.908 |
| 4th–23rd day | |||||||
| BW (g) | 1080 ± 72.5 b | 1170 ± 8.7 ab | 1182 ± 27.5 ab | 1212.62 ± 73 a | 0.074 | 0.018 | 0.359 |
| BWG (g) | 982.5 ±55.1 b | 1073.4 ± 13.1 ab | 1084.44 ± 15.3 ab | 1115.12 ± 80.9 a | 0.119 | 0.033 | 0.323 |
| FI (g) | 1405 ± 33.2 | 1412 ± 37.6 | 1466 ± 47.8 | 1371.9 ± 94 | 0.380 | 0.752 | 0.207 |
| FCR | 1.43 ± 0.05 a | 1.32 ± 0.06 bc | 1.35 ± 0.03 ab | 1.23 ± 0.01 c | 0.008 | 0.002 | 0.908 |
| Finisher period (24–35th day) | |||||||
| BW (g) | 1899 ± 78.9 b | 2040 ± 54.6 ab | 2062 ± 47.2 ab | 2131 ± 146.2 a | 0.071 | 0.015 | 0.511 |
| BWG (g) | 819 ± 49.2 | 869 ± 60.2 | 879 ± 38.3 | 918 ± 82.04 | 0.307 | 0.080 | 0.867 |
| FI (g) | 1511 ± 32.1 | 1508 ± 75.1 | 1560 ± 106.5 | 1422 ± 137.5 | 0.418 | 0.413 | 0.261 |
| FCR | 1.85 ± 0.14 | 1.74 ± 0.15 | 1.78 ± 0.19 | 1.55 ± 0.11 | 0.173 | 0.059 | 0.524 |
| Overall performance (4–35th day) | |||||||
| Final BW, g | 1899 ± 78.9 b | 2040 ± 54.6 ab | 2062 ± 47.2 ab | 2131 ± 146.2 a | 0.071 | 0.015 | 0.511 |
| Total BWG, g | 1801 ± 78.9 b | 1943 ± 55.3 ab | 1964 ± 46.7 ab | 2034 ± 146.2 a | 0.071 | 0.015 | 0.510 |
| Total FI, g | 2919± 68.9 | 2920 ± 104.9 | 3027 ± 152.4 | 2795 ± 239.1 | 0.396 | 0.524 | 0.229 |
| FCR | 1.62 ± 0.05 a | 1.50 ± 0.07 a | 1.54 ± 0.09 a | 1.37 ± 0.06 b | 0.014 | 0.004 | 0.546 |
| Traits | MMUR Level (mg/kg) | p-Value | |||||
|---|---|---|---|---|---|---|---|
| 0 | 200 | 400 | 600 | ANOVA | Linear | Quadratic | |
| ALT (U/L) | 5.00 ± 1.00 | 6.66 ± 1.15 | 7.33 ± 1.53 | 7.37 ± 1.53 | 0.181 | 0.055 | 0.307 |
| AST (U/L) | 52.0 ± 9.00 | 54.0 ± 6.25 | 49.7 ± 2.52 | 54.0 ± 6.25 | 0.819 | 0.922 | 0.761 |
| Creatinine (mg/dL) | 1.99 ± 0.04 c | 2.08 ± 0.05 b | 2.07 ± 0.04 b | 2.15 ± 0.02 a | 0.005 | 0.001 | 0.883 |
| Uric acid (mg/dL) | 2.003 ± 0.12 | 2.04 ± 0.02 | 2.05 ± 0.03 | 2.07 ± 0.04 | 0.674 | 0.251 | 0.898 |
| T3 (ng/mL) | 3.34 ± 0.73 c | 4.29 ± 0.11 b | 4.48 ± 0.15 ab | 5.21 ± 0.47 a | 0.006 | 0.001 | 0.671 |
| T4 (ng/mL) | 19.3 ± 0.47 c | 22.4 ± 0.80 b | 23.31 ± 1.45 b | 26.1 ± 1.20 a | <0.001 | <0.001 | 0.829 |
| Growth hormone (ng/mL) | 2.63 ± 0.25 c | 3.60 ± 0.20 b | 4.96 ± 0.21 a | 5.20 ± 0.26 a | <0.001 | <0.001 | 0.026 |
| Leptin (ng/mL) | 2.01 ± 0.11 b | 2.18 ± 0.03 ab | 2.22 ± 0.16 ab | 2.31 ± 0.13 a | 0.081 | 0.017 | 0.579 |
| Glucose (mg/dL) | 334 ± 5.86 | 339± 4.58 | 335 ± 4.73 | 340 ± 4.04 | 0.506 | 0.341 | 0.908 |
| Traits | MMUR Level (mg/kg) | p-Value | |||||
|---|---|---|---|---|---|---|---|
| 0 | 200 | 400 | 600 | ANOVA | Linear | Quadratic | |
| TAC (U/mL) | 10.2 ± 0.11 c | 11.48 ± 0.99 b | 13.1 ± 0.39 a | 13.8 ± 0.25 a | <0.001 | <0.001 | 0.388 |
| CAT (U/mL) | 2.42 ± 0.52 b | 3.55 ± 1.32 b | 5.43 ± 0.60 a | 6.12 ± 0.93 a | 0.004 | 0.001 | 0.679 |
| SOD (U/mL) | 133± 0.90 c | 145 ± 2.91 b | 154± 8.69 ab | 162 ± 5.29 a | 0.001 | <0.001 | 0.540 |
| MDA nmol/mL | 5.51 ± 0.45 a | 2.89 ± 0.27 b | 2.23 ± 0.05 b | 2.52 ± 0.53 b | <0.001 | <0.001 | <0.001 |
| IL1β (ug/mL) | 140 ± 4.73 c | 153± 13.00 bc | 161 ± 3.79 ab | 171 ± 3.00 a | 0.005 | 0.001 | 0.790 |
| IFN-γ (pg/mL) | 7.5 ± 2.35 c | 11.33 ± 0.96 b | 13.6 ± 1.08 ab | 14.9 ± 1.13 a | 0.001 | <0.001 | 0.174 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omar, A.E.; El-Rahman, G.I.A.; Gouda, A.; Abdel-Warith, A.-W.A.; Younis, E.M.; Abdo, S.A.; Eltanahy, A.; Kamal, A.S.; Davies, S.J.; Amer, S.A. Effects of Dietary Microbial Muramidase on the Growth, Liver Histoarchitecture, Antioxidant Status, and Immunoexpression of Pro-Inflammatory Cytokines in Broiler Chickens. Animals 2023, 13, 3862. https://doi.org/10.3390/ani13243862
Omar AE, El-Rahman GIA, Gouda A, Abdel-Warith A-WA, Younis EM, Abdo SA, Eltanahy A, Kamal AS, Davies SJ, Amer SA. Effects of Dietary Microbial Muramidase on the Growth, Liver Histoarchitecture, Antioxidant Status, and Immunoexpression of Pro-Inflammatory Cytokines in Broiler Chickens. Animals. 2023; 13(24):3862. https://doi.org/10.3390/ani13243862
Chicago/Turabian StyleOmar, Anaam E., Ghada I. Abd El-Rahman, Ahmed Gouda, Abdel-Wahab A. Abdel-Warith, Elsayed M. Younis, Samar A. Abdo, Azhar Eltanahy, Ahmed Said Kamal, Simon J. Davies, and Shimaa A. Amer. 2023. "Effects of Dietary Microbial Muramidase on the Growth, Liver Histoarchitecture, Antioxidant Status, and Immunoexpression of Pro-Inflammatory Cytokines in Broiler Chickens" Animals 13, no. 24: 3862. https://doi.org/10.3390/ani13243862
APA StyleOmar, A. E., El-Rahman, G. I. A., Gouda, A., Abdel-Warith, A.-W. A., Younis, E. M., Abdo, S. A., Eltanahy, A., Kamal, A. S., Davies, S. J., & Amer, S. A. (2023). Effects of Dietary Microbial Muramidase on the Growth, Liver Histoarchitecture, Antioxidant Status, and Immunoexpression of Pro-Inflammatory Cytokines in Broiler Chickens. Animals, 13(24), 3862. https://doi.org/10.3390/ani13243862





