Total Selenium Level and Its Distribution between Organs in Beef Cattle in Different Selenium Status
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
- (a)
- Cows: 25 kg of haylage, 6–8 kg of pomace, 0.1 kg of mineral feed containing 12 mg/kg of inorganic Se and 8 mg/kg of organic Se, bulk straw and mineral lick containing 10 mg of sodium selenium/kg.
- (b)
- Heifers: 14–16 kg of haylage, 2 kg of concentrated feed, 6–8 kg of bagasse, as much straw as needed and a mineral lick containing 10 mg of sodium selenium/kg.
- (c)
- Bulls: 8 kg of maize, 6 kg of haylage, 6–8 kg of wet feed based on stillage (20% protein, 7.5 MJ), 4–6 kg of beet pulp, straw as desired and a mineral lick containing 10 mg of sodium selenium/kg.
2.2. Sample Collection
2.3. Ethical Consent
2.4. Chemical Analyses
2.4.1. Reagents
2.4.2. Se Determination
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klecha, B.; Bukowska, B. Selenium in the human body–characteristics of the element and potential therapeutic use. Bromat. Chem. Toksykol. 2016, 4, 818–829. (In Polish) [Google Scholar]
- Niwińska, B.; Andrzejewski, M. Selenium—An essential element in cattle feeding. Przegląd Hod. 2014, 2, 9–11. (In Polish) [Google Scholar]
- Galbraith, M.L.; Vorachek, W.R.; Estill, C.T.; Whanger, P.D.; Bobe, G.; Davis, T.Z.; Hall, J.A. Rumen Microorganisms Decrease Bioavailability of Inorganic Selenium Supplements. Biol. Trace Elem. Res. 2016, 171, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Duntas, L.H.; Benvega, S. Selenium: An element for life. Endocrine 2015, 48, 756–775. [Google Scholar] [CrossRef] [PubMed]
- Meschy, F. Mineral Nutrition of Ruminants; Editions Quae: Versaille, France, 2010; p. 208. (In French) [Google Scholar]
- Combs, G.F. Selenium in global food systems. Br. J. Nutr. 2001, 85, 517–547. [Google Scholar] [CrossRef] [PubMed]
- Reilly, C. Selenium in Food and Health, 2nd ed.; Queensland University of Technology: Brisbane, Australia, 2006; pp. 1–206. [Google Scholar]
- Mehdi, Y.; Dufrasne, I. Selenium in Cattle: A Review. Molecules 2016, 21, 545. [Google Scholar] [CrossRef]
- Dumont, E.; Vanhaecke, F.; Cornelis, R. Selenium speciation from food source to metabolites: A critical review. Anal. Bioanal. Chem. 2006, 385, 1304–1323. [Google Scholar] [CrossRef]
- Chen, J.; Berry, M.J. Selenium and selenoproteins in the brain and brain diseases. J. Neurochem. 2003, 86, 1–12. [Google Scholar] [CrossRef]
- Błażejak, J.; Milewski, S. The biological role of selenium in ruminants and diseases caused by its deficiency. Przegląd Hod. 2016, 84, 23–25. [Google Scholar]
- Akahoshi, N.; Anan, Y.; Hashimoto, Y.; Tokoro, N.; Mizuno, R.; Hayashi, S.; Yamamoto, S.; Shimada, K.; Kamata, S.; Ishii, I. Dietary selenium deficiency or selenomethionine excess drastically alters organ selenium contents without altering the expression of most selenoproteins in mice. J. Nutr. Biochem. 2019, 69, 120–129. [Google Scholar] [CrossRef]
- Surai, P.F.; Kochish, I.I.; Fisini, V.I.; Juniper, D.T. Revisiting oxidative stress and the use of organic selenium in dairy cow nutrition. Animals 2019, 9, 462. [Google Scholar] [CrossRef]
- Rossi, C.; Compiani, R.; Baldi, G.; Muraro, M.; Marden, J.; Rossi, R.; Pastorelli, G.; Corino, C.; Dell’Orto, V. Organic selenium supplementation improves growth parameters, immune and antioxidant status of newly received beef cattle. J. Anim. Feed Sci. 2017, 26, 100–108. [Google Scholar] [CrossRef]
- Juniper, D.T.; Phipps, R.H.; Ramos-Moralesa, E.; Bertin, G. Effect of high dose selenium enriched yeast diets on the distribution of total selenium and selenium species within lamb tissues. Lives. Sci. 2009, 122, 63–67. [Google Scholar] [CrossRef]
- Costa, N.D.; Glled, P.T.; Sansom, B.F.; Symonds, H.; Allen, W.M. Monensin and narasin increase selenium and zinc absorption in steers. Trace Elem. Man Anim. 1985, 6, 635–636. [Google Scholar]
- Cousins, F.B.; Cairney, I.M. Some aspects of selenium metabolism in sheep. Aust. J. Agric. Res. 1961, 12, 927–943. [Google Scholar] [CrossRef]
- Cristaldi, L.A.; McDowell, L.R.; Buergelt, C.D.; Davis, P.A.; Wilkinson, N.S.; Martin, F.G. Tolerance of inorganic selenium in wether sheep. Small Rumin. Res. 2005, 56, 205–213. [Google Scholar] [CrossRef]
- Wright, P.L.; Bell, M.C. Selenium and Vitamin E Influence upon the in vitro Uptake of Se75 by Ovine Blood Cells. Proc. Soc. Exp. Biol. Med. 1963, 114, 379–382. [Google Scholar] [CrossRef]
- Mainville, A.M.; Odongo, N.E.; Bettger, W.J.; McBride, B.W.; Osborne, V.R. Selenium uptake by ruminal microorganisms from organic and inorganic sources in dairy cows. Can. J. Anim. Sci. 2009, 89, 105–110. [Google Scholar] [CrossRef]
- Peter, D.W.; Whanger, P.D.; Lindsay, J.P.; Buscall, D.J. Excretion of selenium, zinc and copper by sheep receiving continuous intraruminal infusions of selenite or selenomethionine. Proc. Nutr. Soc. Austr. 1982, 7, 178–181. [Google Scholar]
- Juniper, D.T.; Phipps, R.H.; Ramos-Morales, E.; Bertin, G. Effect of dietary supplementation with selenium-enriched yeast or sodium selenite on selenium tissue distribution and meat quality in beef cattle. J. Anim. Sci. 2008, 86, 3100–3109. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, C.; Liu, Q.; Guo, G.; Huo, W.; Zhang, Y.; Pei, C.; Zhang, S.; Zhang, J. Effects of sodium selenite addition on ruminal fermentation, microflora and urinary excretion of purine derivatives in Holstein dairy bulls. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1719–1726. [Google Scholar] [CrossRef]
- Ortman, K.; Pehrson, B. Effect of selenate as a feed supplement to dairy cows in comparison to selenite and selenium yeast. J. Anim. Sci. 1999, 77, 3365–3370. [Google Scholar] [CrossRef]
- Netto, A.S.; Zanetti, M.A.; Claro, G.R.D.; de Melo, M.P.; Vilela, F.G.; Correa, L.B. Effects of copper and selenium supplementation on performance and lipid metabolism in confined brangus bulls. Asian-Australas. J. Anim. Sci. 2014, 27, 488–494. [Google Scholar] [CrossRef]
- Pilarczyk, R.; Pilarczyk, B. Selenium-importance and prevention of deficiency. Prz. Hod. 2001, 8, 11–12. (In Polish) [Google Scholar]
- Pilarczyk, B.; Tomza-Marciniak, A.; Mituniewicz-Małek, A.; Wieczorek, M.; Pilarczyk, R.; Wójcik, J.; Balicka-Ramisz, A.; Bąkowska, M.; Dmytrów, I. Selenium content in selected products of animal origin and estimation of the degree of cover daily Se requirement in Poland. Int. J. Food Sci. Technol. 2009, 45, 186–191. [Google Scholar] [CrossRef]
- Kubiński, T. Functional food. Życie Weter. 2010, 85, 932–935. [Google Scholar]
- Ratajczak, M.; Gietka-Czernel, M. The influence of selenium to human health. Postępy Nauk. Med. 2016, 29, 929–933. [Google Scholar]
- Reinoso-Maset, E.; Falk, M.; Bernhoft, A.; Ersdal, C.; Framstad, T.; Fuhrmann, H.; Salbu, B.; Oropeza-Moe, M. Selenium Speciation Analysis Reveals Improved Antioxidant Status in Finisher Pigs Fed l-Selenomethionine, Alone or Combined with Sodium Selenite, and Vitamin E. Biol. Trace Elem. Res. 2022, 201, 4400–4418. [Google Scholar] [CrossRef]
- Amezcua, F.; Ruelas-Inzunza, J.; Coiraton, C.; Spanopoulos-Zarco, P.; Páez-Osuna, F. A Global Review of Cadmium, Mercury, and Selenium in Sharks: Geographical Patterns, Baseline Levels and Human Health Implications. Rev. Environ. Contam. Toxicol. 2022, 260, 4. [Google Scholar] [CrossRef]
- European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A21999A0824%2801%29 (accessed on 24 April 2023).
- Journal of Laws 2017 Pos. 2132 Act of 29 June 2007 on the Organization of Breeding and Reproduction of Farm Animals. Available online: https://www.fao.org/faolex/results/details/en/c/LEX-FAOC182668/ (accessed on 27 April 2023).
- Journal of Laws 2015 Item 1703 Regulation of the Minister of Agriculture and Rural Development on Veterinary Requirements for the Production of Products of Animal Origin Intended for Direct Sale. Available online: https://www.fao.org/faolex/results/details/es/c/LEX-FAOC182497/ (accessed on 15 January 2023).
- Puls, R. Mineral levels in animal health. In Diagnostic Data, 2nd ed.; Sherpa International: Clearbrook, BC, Canada, 1994. [Google Scholar]
- Journal Laws of 2015 Item 266, ACT on the Protection of Animals Used for Scientific or Educational Purposes. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20150000266 (accessed on 15 January 2023).
- Council Regulation (WE) NR 1/2005. Available online: https://eur-lex.europa.eu/legal-content/PL/TXT/PDF/?uri=CELEX:32005R0001&from=PL (accessed on 2 May 2023).
- Council Regulation (EC) No 1099/2009 of 24 September 2009 on the Protection of Animals at the Time of Killing (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/eli/reg/2009/1099/oj (accessed on 25 May 2019).
- Darecki, A.; Saeid, A.; Górecki, H. Perspective of selenium fortifications of plants with economic importance to Poland. Wiadomości Chem. 2015, 69, 11–12. [Google Scholar]
- GUS, Statistical Analyses, Statistics Poland, Agriculture Department 2016. Available online: https://stat.gov.pl/en/topics/statistical-yearbooks/statistical-yearbooks/statistical-yearbook-of-agriculture-2016,6,11.html (accessed on 15 September 2022).
- GUS, Statistical Analyses, Statistics Poland, Agriculture Department 2020. Available online: https://stat.gov.pl/en/topics/agriculture-forestry/agricultural-census-2020/ (accessed on 21 July 2022).
- Food & Agricultural Policy Research Institute (FAPRI). Available online: www.fapri.missouri.edu (accessed on 5 January 2023).
- Ockerman, H.W.; Basu, L. By-Products. In Encyclopedia of Meat Sciences, 2nd ed.; Devine, C., Dikeman, M., Eds.; Elsevier Academic Press: Amsterdam, The Netherlands, 2004; pp. 104–112. [Google Scholar]
- Hintze, K.J.; Lardy, G.P.; Marchello, M.J.; Finley, J.W. Areas with high concentrations of selenium in the soil and forage produce beef with enhanced concentrations of selenium. J. Agric. Food Chem. 2001, 49, 1062–1067. [Google Scholar] [CrossRef]
- Chauhan, R.; Awasthi, S.; Srivastava, S.; Dwivedi, S.; Pilon-Smits, E.; Dhankher, O.; Tripathi, R. Understanding selenium metabolism in plants and its role as a beneficial element. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1937–1958. [Google Scholar] [CrossRef]
- Pereira, V.; Carbajales, P.; López-Alonso, M.; Miranda, M. Trace Element Concentrations in Beef Cattle Related to the Breed Aptitude. Biol. Trace Elem. Res. 2019, 186, 135–142. [Google Scholar] [CrossRef]
- Biel, W.; Czerniawska-Piątkowska, E.; Kowalczyk, A. Offal Chemical Composition from Veal, Beef, and Lamb Maintained in Organic Production Systems. Animals 2019, 9, 489. [Google Scholar] [CrossRef]
- Ullah, H.; Ullah Khan, R.; Tufarelli, V.; Laudadio, V. Selenium: An Essential Micronutrient for Sustainable Dairy Cows Production. Sustainability 2020, 12, 10693. [Google Scholar] [CrossRef]
- Clerens, S.; Thomas, A.; Gathercole, J.; Plowman, J.E.; Yu, T.; Grosvenor, A.J.; Haines, S.R.; Dobbie, P.; Taukiri, K.; Rosenvold, K.; et al. Proteomic and peptidomic differences and similarities between four muscle types from New Zealand raised Angus steers. Meat Sci. 2016, 212, 53–63. [Google Scholar] [CrossRef]
- Rederstorff, M.; Krol, A.; Lescure, A. Understanding the importance of selenium and selenoproteins in muscle function. Cell Mol. Life Sci. 2006, 63, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Hai-Bing, Y.; Wei, Y.; Wei-Dong, L.; Shang, M. Selenium Supplementation Protects against Arsenic-Trioxide-Induced Cardiotoxicity via Reducing Oxidative Stress and Inflammation through Increasing NAD+ Pool. Biol. Trace Elem. Res. 2022, 201, 3941–3950. [Google Scholar]
- Wang, X.; Lu, W.; Xia, X.; Zhu, Y.; Ge, C.; Guo, X.; Zhang, N.; Chen, H.; Xu, S. Selenomethionine mitigate PM2.5-induced cellular senescence in the lung via attenuating inflammatory response mediated by cGAS/STING/NF-κB pathway. Ecotoxicol. Environ. Saf. 2022, 247, 114266. [Google Scholar] [CrossRef] [PubMed]
- Lawler, T.; Taylor, J.; Finley, J.; Caton, J. Effect of supranutritional and organically bound selenium on performance, carcass characteristics, and selenium distribution in finishing beef steers. J. Anim. Sci. 2004, 82, 1488–1493. [Google Scholar] [CrossRef] [PubMed]
- Salwa, A.; Kopczewski, A.; Borkowska-Opacka, B.; Przewoski, W.; Strzałkowski, L.; Sroka, A.; Arent, Z.; Malinowski, E.; Lachowski, A. Epizootic of listeriosis in cows in the Kaszuby region. Med. Wet. 2007, 63, 1579–1582. (In Polish) [Google Scholar]
- Balicka-Ramisz, A.; Jastrzębski, G. Selenium concentration in dairy cows and its influence on production traits. Acta Sci. Pol. Zoot. 2012, 11, 49–58. [Google Scholar]
- Andrzejewski, M. The influence of the form of selenium in the diet on its status in cows and calves. Rozpr. Dokt. IZ PIB 2012. (In Polish) [Google Scholar]
- Pavlata, L.; Illek, J.; Pechowa, A.; Matujlaek, M. Selenium status of cattle in the Czech Republic. Acta Vet. Brno. 2002, 71, 3–8. [Google Scholar] [CrossRef]
- Davy, J.; Forero, L.; Tucker, T.; Mayo, C.; Drake, D.; Maas, J.; Oltjen, J. Efficacy of selenium supplementation methods in California yearling beef cattle and resulting effect on weight gain. Calif. Agric. 2016, 70, 187–193. [Google Scholar] [CrossRef]
- Franke, B.M.; Gremaud, G.; Hadorn, R.; Kreuzer, M. Geographic origin of meat-elements of an analytical approach to its authentication. Eur. Food Res. Technol. 2005, 221, 493–503. [Google Scholar] [CrossRef]
- Hintze, K.J.; Lardy, G.P.; Marchello, M.J.; Finley, J.W. Selenium accumulation in beef: Effect of dietary selenium and geographical area of animal origin. J. Agric. Food Chem. 2002, 50, 3938–3942. [Google Scholar] [CrossRef]

| Chemical Composition | Cows | Heifers | Bulls |
|---|---|---|---|
| Dry matter (g) | 10,200 ± 158.1 | 8370 ± 103.4 | 11,900 ± 115.8 |
| Crude protein (g) | 802.1 ± 68.7 | 761.3 ± 54.5 | 1331.2 ± 98.3 |
| Raw fibre (g) | 1628.2 ± 111.2 | 1524.7 ± 95.8 | 2387.2 ± 167.7 |
| Metabolic energy (MJ) | 93.28 ± 14.6 | 79.24 ± 7.4 | 115.74 ± 19.2 |
| Raw ash (%) | 6.34 ± 0.35 | 5.95 ± 0.51 | 8.29 ±0.07 |
| Selenium (mg) | 3.06 ± 0.02 | 3.08 ± 0.05 | 2.52 ± 0.1 |
| Selenium (mg/kg) | 0.09 ± 0.03 | 0.12 ± 0.06 | 0.01 ± 0.01 |
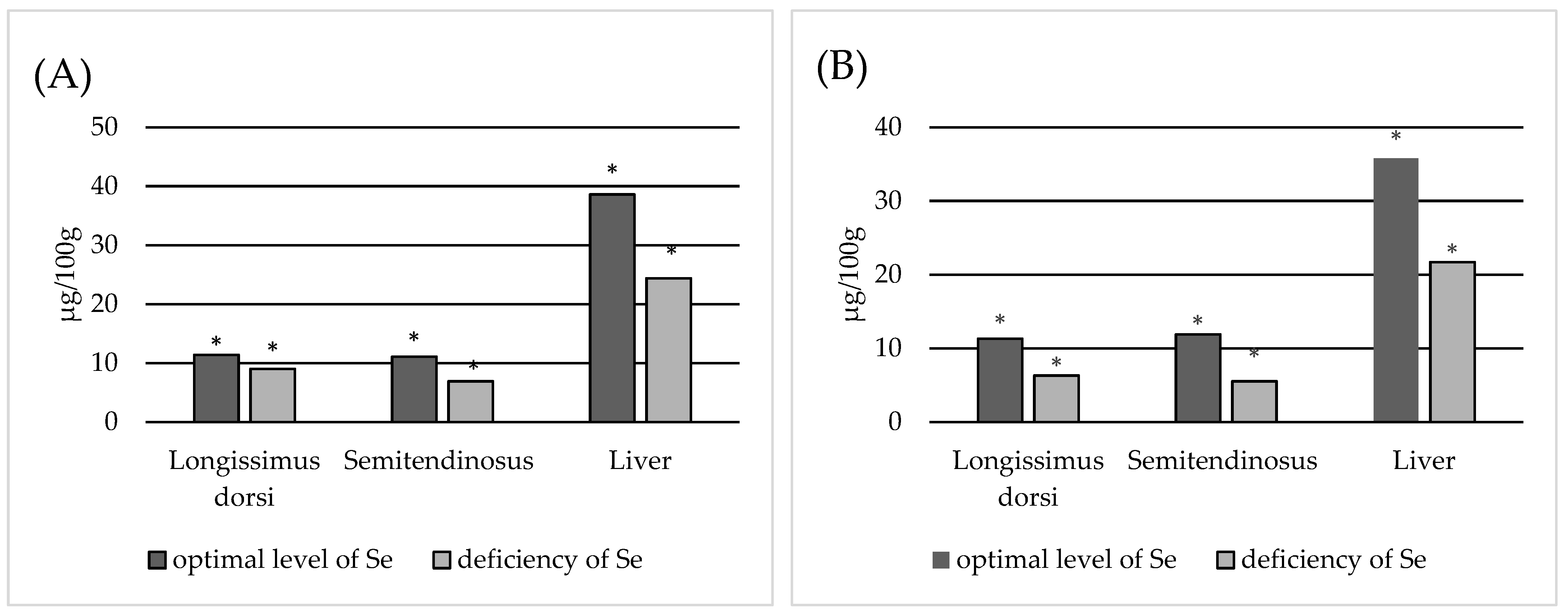
| Tissue | Se Concentration (µg/g w.w.) | Anova p Value | Normal Range [33] | ||
|---|---|---|---|---|---|
| Optimal Se Level | Deficient Se Status | ||||
| Serum μg/mL | Mean | 0.121 abcde | 0.053 abcde | ||
| Sd | 0.024 | 0.020 | <0.01 | 0.08–0.3 | |
| Range | 0.081–0.167 | 0.019–0.079 | |||
| Longissimus dorsi muscle | Mean | 0.114 fghij | 0.09 fgh | 0.07–0.15 | |
| Sd | 0.049 | 0.044 | 0.05 | (muscle) | |
| Range | 0.028–0.232 | 0.027–0.182 | |||
| Semitendinosus muscle | Mean | 0.111 klmno | 0.069 ijkl | 0.07–0.15 | |
| Sd | 0.031 | 0.036 | <0.01 | (muscle) | |
| Range | 0.042–0.171 | 0.021–0.158 | |||
| Kidney | Mean | 1.526 afkprst | 1.313 afimnop | ||
| Sd | 0.463 | 0.413 | 0.10 | 1–1.5 | |
| Range | 0.862–2.47 | 0.452–1.966 | |||
| Lungs | Mean | 0.215 bglpu | 0.165 bjm | ||
| Sd | 0.090 | 0.072 | 0.01 | ||
| Range | 0.118–504 | 0.045–0.273 | |||
| Heart | Mean | 0.223 chmrw | 0.158 cn | ||
| Sd | 0.061 | 0.076 | <0.01 | ||
| Range | 0.107–0.337 | 0.039–0.293 | |||
| Spleen | Mean | 0.223 dinsz | 0.217 dgko | ||
| Sd | 0.110 | 0.104 | 0.19 | ||
| Range | 0.100–0.462 | 0.037–0.435 | |||
| Liver | Mean | 0.386 ejotuwz | 0.244 ehlp | ||
| Sd | 0.136 | 0.141 | <0.01 | 0.25–0.5 | |
| Range | 0.127–0.713 | 0.063–0.590 | |||
| Tissue | Se Concentration (µg/g w.w.) in Cows | Anova p Value | ||
|---|---|---|---|---|
| Optimal Se Level | Deficient Se Status | |||
| Serum μg/mL | Mean | 0.126 ab | 0.038 bc | |
| Sd | 0.027 | 0.014 | <0.01 | |
| Range | 0.081–0.167 | 0.023–0.075 | ||
| 25–95%CI | 0.128–0.142 | 0.039–0.047 | ||
| Longissimus dorsi muscle | Mean | 0.113 cd | 0.063 def | |
| Sd | 0.048 | 0.036 | 0.01 | |
| Range | 0.043–0.232 | 0.027–0.157 | ||
| 25–95%CI | 0.118–0.142 | 0.066–0.087 | ||
| Semitendinosus muscle | Mean | 0.119 ef | 0.055 ghi | |
| Sd | 0.039 | 0.030 | <0.01 | |
| Range | 0.042–0.171 | 0.021–0.117 | ||
| 25–95%CI | 0.123–0.143 | 0.058–0.075 | ||
| Kidney | Mean | 1.375 aceghij | 1.052 adgjklm | |
| Sd | 0.435 | 0.319 | 0.07 | |
| Range | 0.908–2.397 | 0.452–1.414 | ||
| 25–95%CI | 1.418–1.667 | 1.085–1.280 | ||
| Lungs | Mean | 0.184 gk | 0.122 j | |
| Sd | 0.061 | 0.067 | 0.03 | |
| Range | 0.119–0.285 | 0.045–0.258 | ||
| 25–95%CI | 0.190–0.221 | 0.129–0.167 | ||
| Heart | Mean | 0.214 hl | 0.137 k | |
| Sd | 0.067 | 0.086 | 0.03 | |
| Range | 0.128–0.337 | 0.039–0.291 | ||
| 25–95%CI | 0.220–0.257 | 0.145–0.195 | ||
| Spleen | Mean | 0.255 i | 0.199 behl | |
| Sd | 0.120 | 0.081 | 0.22 | |
| Range | 0.1–0.416 | 0.037–0.353 | ||
| 25–95%CI | 0.267–0.336 | 0.207–0.254 | ||
| Liver | Mean | 0.358 bdfjkl | 0.217 cfim | |
| Sd | 0.160 | 0.153 | 0.04 | |
| Range | 0.127–0.713 | 0.063–0.575 | ||
| 25–95%CI | 0.372–0.454 | 0.232–0.319 | ||
| Tissue | Se Concentration (µg/g w.w.) in Bulls | Anova p Value | ||
|---|---|---|---|---|
| Optimal Se Level | Deficient Se Status | |||
| Serum μg/mL | Mean | 0.123 ab | 0.066 ab | |
| Sd | 0.022 | 0.022 | <0.01 | |
| Range | 0.092–0.166 | 0.019–0.079 | ||
| 25–95%CI | 0.125–0.134 | 0.069–0.087 | ||
| Longissimus dorsi muscle | Mean | 0.121 cd | 0.118 cd | |
| Sd | 0.044 | 0.032 | 0.86 | |
| Range | 0.058–0.208 | 0.073–0.158 | ||
| 25–95%CI | 0.125–0.144 | 0.122–0.147 | ||
| Semitendinosus muscle | Mean | 0.108 ef | 0.085 ef | |
| Sd | 0.026 | 0.032 | 0.07 | |
| Range | 0.079–0.166 | 0.034–0.13 | ||
| 25–95%CI | 0.110–0.122 | 0.089–0.114 | ||
| Kidney | Mean | 1.557 aceghij | 1.564 aceghij | |
| Sd | 0.445 | 0.393 | 0.97 | |
| Range | 0.862–2.407 | 0.096–1.966 | ||
| 25–95%CI | 1.594–1.79 | 1.614–1.927 | ||
| Lungs | Mean | 0.233 gk | 0.204 g | |
| Sd | 0.099 | 0.075 | 0.48 | |
| Range | 0.133–0.504 | 0.078–0.273 | ||
| 25–95%CI | 0.241–0.286 | 0.213–0.273 | ||
| Heart | Mean | 0.234 hl | 0.183 h | |
| Sd | 0.044 | 0.070 | 0.05 | |
| Range | 0.163–0.316 | 0.042–0.256 | ||
| 25–95%CI | 0.237–0.257 | 0.192–0.247 | ||
| Spleen | Mean | 0.237 im | 0.203 i | |
| Sd | 0.100 | 0.124 | 0.49 | |
| Range | 0.136–0.462 | 0.067–0.435 | ||
| 25–95%CI | 0.245–0.29 | 0.219–0.317 | ||
| Liver | Mean | 0.388 bdfjklm | 0.309 bdfj | |
| Sd | 0.109 | 0.121 | 0.14 | |
| Range | 0.17–0.616 | 0.071–0.425 | ||
| 25–95%CI | 0.397–0.446 | 0.324–0.421 | ||
| Tissue | Se Concentration (µg/g w.w.) in Heifers | Anova p Value | ||
|---|---|---|---|---|
| Optimal Se Level | Deficient Se Status | |||
| Serum μg/mL | Mean | 0.107 ab | 0.063 ab | |
| Sd | 0.017 | 0.013 | <0.01 | |
| Range | 0.086–0.127 | 0.033–0.077 | ||
| 25–95%CI | 0.108–0.124 | 0.070–0.083 | ||
| Longissimus dorsi muscle | Mean | 0.095 cd | 0.101 cd | |
| Sd | 0.036 | 0.048 | 0.84 | |
| Range | 0.028–0.205 | 0.044–0.182 | ||
| 25–95%CI | 0.111–0.171 | 0.101–0.130 | ||
| Semitendinosus muscle | Mean | 0.100 ef | 0.076 ef | |
| Sd | 0.023 | 0.043 | 0.39 | |
| Range | 0.074–0.133 | 0.03–0.158 | ||
| 25–95%CI | 0.101–0.122 | 0.085–0.111 | ||
| Kidney | Mean | 1.473 aceghij | 1.383 aceghij | |
| Sd | 0.570 | 0.366 | 0.38 | |
| Range | 0.96–2.47 | 0.879–1.929 | ||
| 25–95%CI | 1.637–2.16 | 1.376–1.620 | ||
| Lungs | Mean | 0.212 g | 0.161 g | |
| Sd | 0.106 | 0.063 | 0.12 | |
| Range | 0.118–0.364 | 0.11–0.273 | ||
| 25–95%CI | 0.242–0.340 | 0.164–0.201 | ||
| Heart | Mean | 0.192 h | 0.176 h | |
| Sd | 0.087 | 0.071 | 0.36 | |
| Range | 0.107–0.31 | 0.1–0.293 | ||
| 25–95%CI | 0.219–0.297 | 0.176–0.219 | ||
| Spleen | Mean | 0.260 i | 0.270 i | |
| Sd | 0.134 | 0.116 | 0.83 | |
| Range | 0.125–0.456 | 0.127–0.433 | ||
| 25–95%CI | 0.294–0.422 | 0.272–0.341 | ||
| Liver | Mean | 0.420 bdfj | 0.230 bdfj | |
| Sd | 0.137 | 0.149 | 0.02 | |
| Range | 0.278–0.652 | 0.093–0.59 | ||
| 25–95%CI | 0.454–0.585 | 0.254–0.342 | ||
| Tissue | Se Concentration (µg/g w.w.) | ||
|---|---|---|---|
| Bulls | Heifers | Cows | |
| Serum μg/mL | |||
| Mean | 0.106 ± 0.034 abcd | 0.077 ± 0.028 a | 0.086 ± 0.050 a |
| Longissimus dorsi muscle | |||
| Mean | 0.120 ± 0.04 aefgh | 0.102 ± 0.051 | 0.09 ± 0.049 |
| Semitendinosus muscle | |||
| Mean | 0.101 ± 0.029 bei | 0.083 ± 0.037 | 0.09 ± 0.047 b |
| Kidney | |||
| Mean | 1.560 ± 0.421 fj | 1.490 ± 0.34 b | 1.221 ± 0.410 |
| Lungs | |||
| Mean | 0.224 ± 0.092 cgk | 0.189 ± 0.084 bcde | 0.156 ± 0.07 |
| Heart | |||
| Mean | 0.218 ± 0.057 dhijk | 0.188 ± 0.073 cfg | 0.177 ± 0.085 a |
| Spleen | |||
| Mean | 0.227 ± 0.106 l | 0.261 ± 0.124 dfh | 0.227 ± 0.104 |
| Liver | |||
| Mean | 0.364 ± 0.116 l | 0.306 ± 0.171 aegh | 0.293 ± 0.169 b |
| Longissimus Dorsi Muscle | Semitendinosus Muscle | Kidney | Lungs | Heart | Spleen | Liver | |
|---|---|---|---|---|---|---|---|
| All animals | |||||||
| Optimal Se level | −0.438 * | −0.218 | −0.139 | −0.113 | −0.048 | −0.402 * | −0.028 |
| Deficient Se status | 0.601 * | 0.615 * | 0.561 * | 0.597 * | 0.573 * | 0.451 * | 0.458 * |
| Cows | |||||||
| Optimal Se level | −0.500 | −0.022 | 0.238 | 0.563 | 0.594 | −0.233 | 0.055 |
| Deficient Se status | 0.270 | 0.706 * | 0.339 | 0.519 | 0.671 * | 0.385 | 0.377 |
| Bulls | |||||||
| Optimal Se level | −0.388 | −0.212 | −0.133 | −0.239 | −0.287 | −0.733 * | −0.040 |
| Deficient Se status | 0.723 | 0.756 * | 0.599 | 0.825 * | 0.932 * | 0.436 | 0.914 * |
| Heifers | |||||||
| Optimal Se level | 0.134 | 0.169 | −0.439 | −0.386 | −0.257 | −0.378 | 0.049 |
| Deficient Se status | −0.055 | 0.272 | 0.651 | 0.284 | 0.341 | 0.294 | 0.166 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juszczak-Czasnojć, M.; Tomza-Marciniak, A.; Pilarczyk, B.; Gączarzewicz, D. Total Selenium Level and Its Distribution between Organs in Beef Cattle in Different Selenium Status. Animals 2023, 13, 3885. https://doi.org/10.3390/ani13243885
Juszczak-Czasnojć M, Tomza-Marciniak A, Pilarczyk B, Gączarzewicz D. Total Selenium Level and Its Distribution between Organs in Beef Cattle in Different Selenium Status. Animals. 2023; 13(24):3885. https://doi.org/10.3390/ani13243885
Chicago/Turabian StyleJuszczak-Czasnojć, Marta, Agnieszka Tomza-Marciniak, Bogumiła Pilarczyk, and Dariusz Gączarzewicz. 2023. "Total Selenium Level and Its Distribution between Organs in Beef Cattle in Different Selenium Status" Animals 13, no. 24: 3885. https://doi.org/10.3390/ani13243885
APA StyleJuszczak-Czasnojć, M., Tomza-Marciniak, A., Pilarczyk, B., & Gączarzewicz, D. (2023). Total Selenium Level and Its Distribution between Organs in Beef Cattle in Different Selenium Status. Animals, 13(24), 3885. https://doi.org/10.3390/ani13243885





