Identification of New Candidate Genes Related to Semen Traits in Duroc Pigs through Weighted Single-Step GWAS
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Population and Phenotype Data
2.2. Genotypic Data
2.3. Statistical analyses
2.4. Candidate Gene Detection and Functional Enrichment Analysis
3. Results
3.1. Descriptive Statistics and Genetic Parameters for the Semen Traits
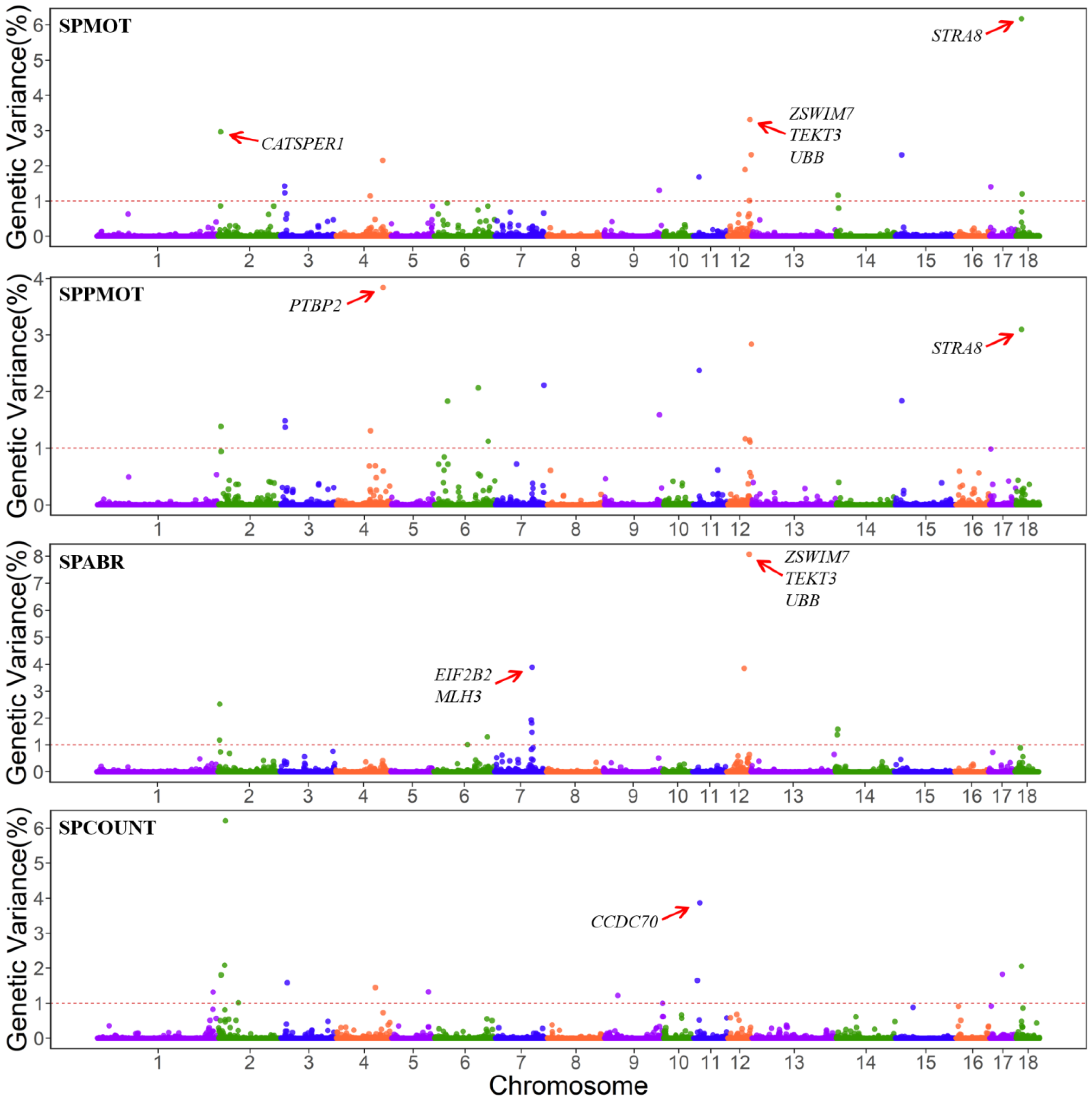
3.2. WssGWAS Results of Semen Traits
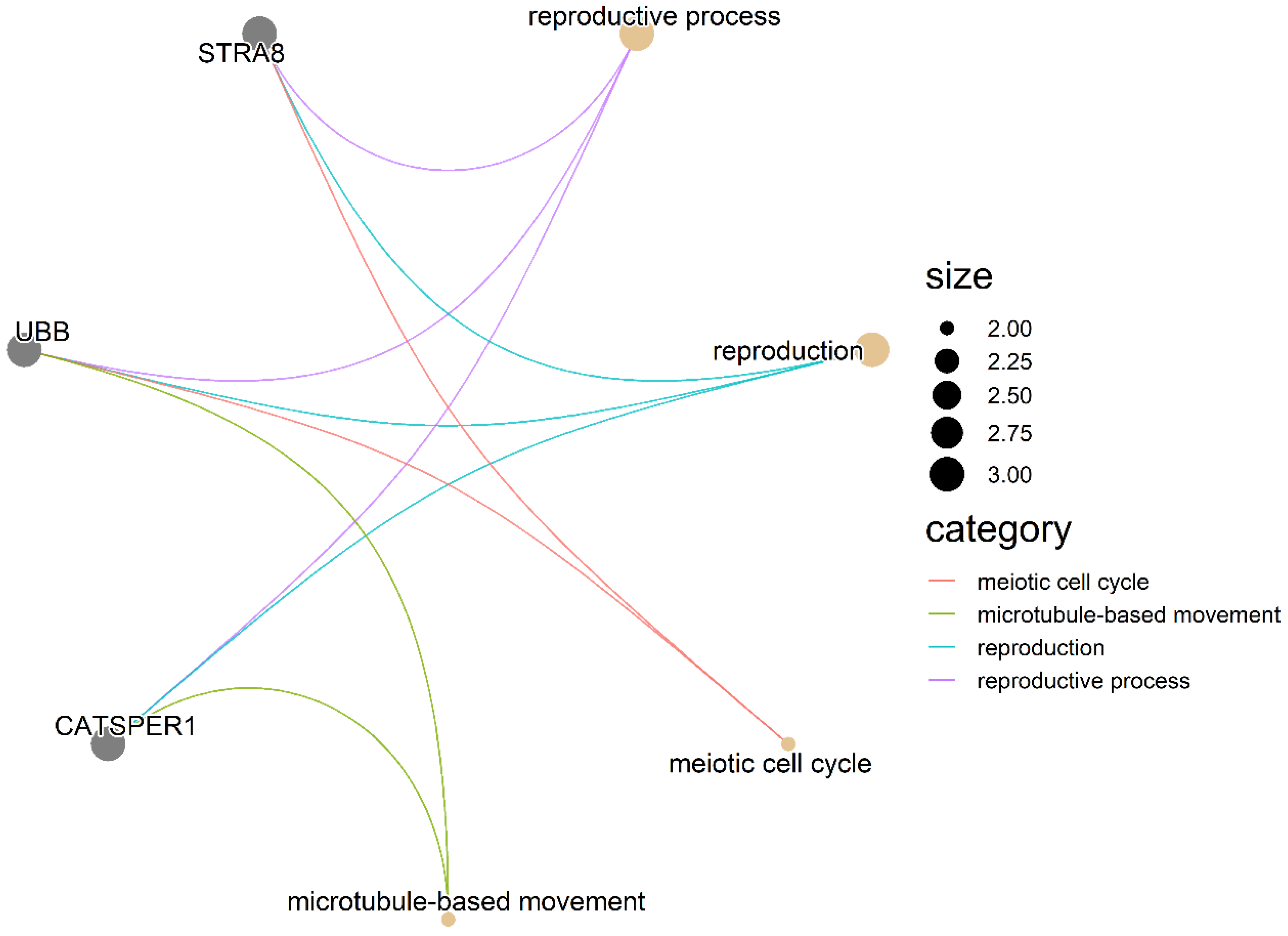
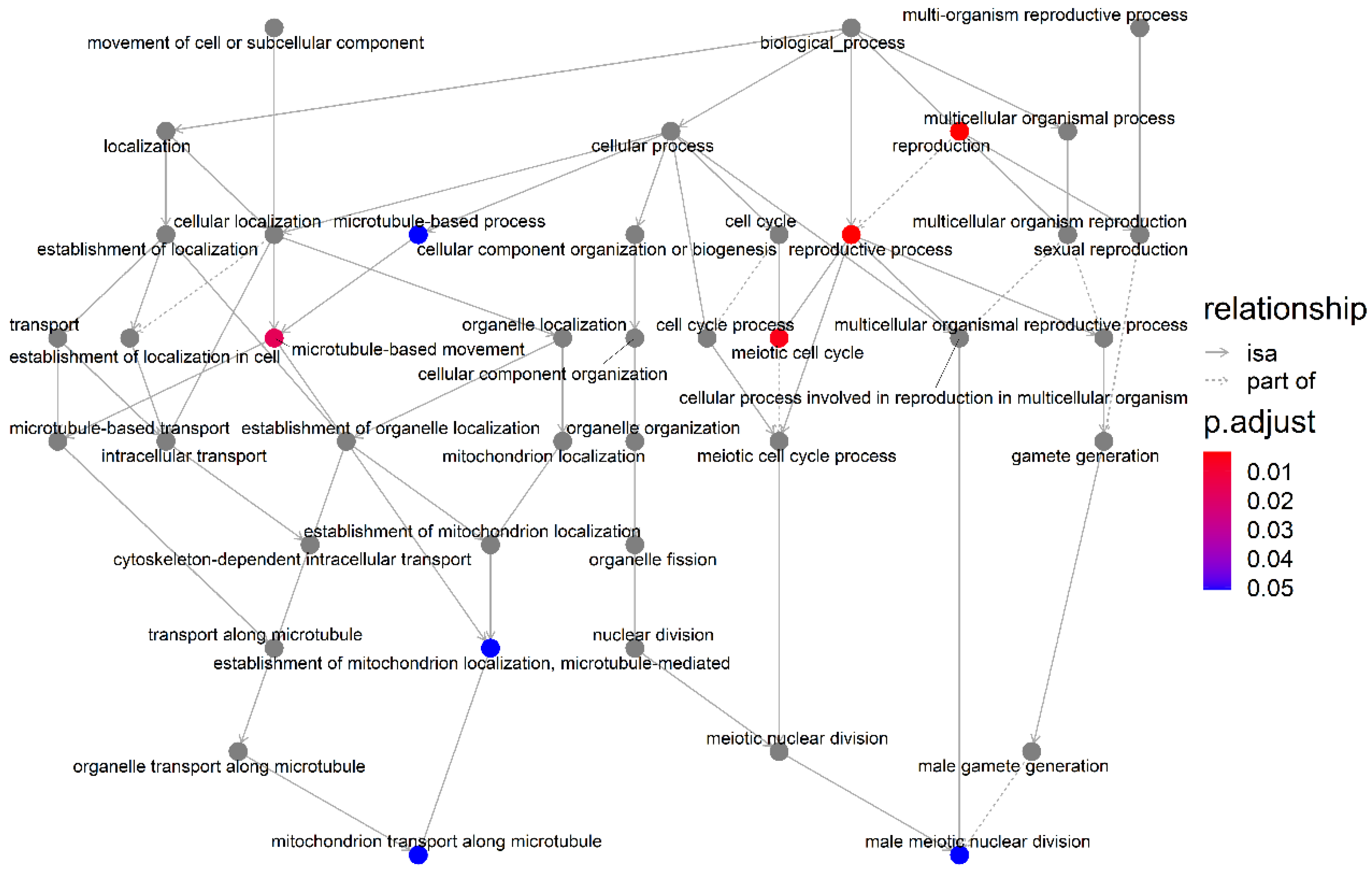
3.3. GO Terms and KEGG Pathway Enrichment Analysis
3.4. Association Network Diagram between GO Terms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Robinson, J.A.; Buhr, M.M. Impact of genetic selection on management of boar replacement. Theriogenology 2005, 63, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Koketsu, Y.; Sasaki, Y. Boar culling and mortality in commercial swine breeding herds. Theriogenology 2009, 71, 1186–1191. [Google Scholar] [CrossRef] [PubMed]
- Hirschhorn, J.N.; Daly, M.J. Genome-wide association studies for common diseases and complex traits. Nat. Rev. Genet. 2005, 6, 95–108. [Google Scholar] [CrossRef]
- De, R.; Bush, W.S.; Moore, J.H. Bioinformatics challenges in genome-wide association studies (GWAS). Methods Mol. Biol. 2014, 1168, 63–81. [Google Scholar] [CrossRef]
- Marques, D.B.D.; Bastiaansen, J.W.M.; Broekhuijse, M.; Lopes, M.S.; Knol, E.F.; Harlizius, B.; Guimaraes, S.E.F.; Silva, F.F.; Lopes, P.S. Weighted single-step GWAS and gene network analysis reveal new candidate genes for semen traits in pigs. Genet. Sel. Evol. 2018, 50, 40. [Google Scholar] [CrossRef]
- Gao, N.; Chen, Y.; Liu, X.; Zhao, Y.; Zhu, L.; Liu, A.; Jiang, W.; Peng, X.; Zhang, C.; Tang, Z.; et al. Weighted single-step GWAS identified candidate genes associated with semen traits in a Duroc boar population. BMC Genomics 2019, 20, 797. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gao, N.; Li, X.; El-Ashram, S.; Wang, Z.; Zhu, L.; Jiang, W.; Peng, X.; Zhang, C.; Chen, Y.; et al. Identifying candidate genes associated with sperm morphology abnormalities using weighted single-step GWAS in a Duroc boar population. Theriogenology 2020, 141, 9–15. [Google Scholar] [CrossRef]
- Mei, Q.; Fu, C.; Sahana, G.; Chen, Y.; Yin, L.; Miao, Y.; Zhao, S.; Xiang, T. Identification of new semen trait-related candidate genes in Duroc boars through genome-wide association and weighted gene co-expression network analyses. J. Anim. Sci. 2021, 99, skab188. [Google Scholar] [CrossRef]
- Wang, H.; Misztal, I.; Aguilar, I.; Legarra, A.; Muir, W.M. Genome-wide association mapping including phenotypes from relatives without genotypes. Genet. Res. 2012, 94, 73–83. [Google Scholar] [CrossRef]
- Aguilar, I.; Misztal, I.; Johnson, D.L.; Legarra, A.; Tsuruta, S.; Lawlor, T.J. Hot topic: A unified approach to utilize phenotypic, full pedigree, and genomic information for genetic evaluation of Holstein final score. J. Dairy Sci. 2010, 93, 743–752. [Google Scholar] [CrossRef]
- Wang, H.; Misztal, I.; Aguilar, I.; Legarra, A.; Fernando, R.L.; Vitezica, Z.; Okimoto, R.; Wing, T.; Hawken, R.; Muir, W.M. Genome-wide association mapping including phenotypes from relatives without genotypes in a single-step (ssGWAS) for 6-week body weight in broiler chickens. Front. Genet. 2014, 5, 134. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.T.; Jiao, S.; Tiezzi, F.; Huang, Y.; Gray, K.A.; Maltecca, C. Genome-wide association study on legendre random regression coefficients for the growth and feed intake trajectory on Duroc Boars. BMC Genet. 2015, 16, 59. [Google Scholar] [CrossRef] [PubMed]
- Tiezzi, F.; Parker-Gaddis, K.L.; Cole, J.B.; Clay, J.S.; Maltecca, C. A genome-wide association study for clinical mastitis in first parity US Holstein cows using single-step approach and genomic matrix re-weighting procedure. PLoS ONE 2015, 10, e0114919. [Google Scholar] [CrossRef]
- Lemos, M.V.; Chiaia, H.L.; Berton, M.P.; Feitosa, F.L.; Aboujaoud, C.; Camargo, G.M.; Pereira, A.S.; Albuquerque, L.G.; Ferrinho, A.M.; Mueller, L.F.; et al. Genome-wide association between single nucleotide polymorphisms with beef fatty acid profile in Nellore cattle using the single step procedure. BMC Genomics 2016, 17, 213. [Google Scholar] [CrossRef] [PubMed]
- Valente, T.S.; Baldi, F.; Sant’Anna, A.C.; Albuquerque, L.G.; Paranhos da Costa, M.J. Genome-Wide Association Study between Single Nucleotide Polymorphisms and Flight Speed in Nellore Cattle. PLoS ONE 2016, 11. [Google Scholar] [CrossRef]
- Melo, T.P.; de Camargo, G.M.F.; de Albuquerque, L.G.; Carvalheiro, R. Genome-wide association study provides strong evidence of genes affecting the reproductive performance of Nellore beef cows. PLoS ONE 2017, 12, e0178551. [Google Scholar] [CrossRef] [PubMed]
- Verardo, L.L.; Silva, F.F.; Varona, L.; Resende, M.D.; Bastiaansen, J.W.; Lopes, P.S.; Guimaraes, S.E. Bayesian GWAS and network analysis revealed new candidate genes for number of teats in pigs. J. Appl. Genet. 2015, 56, 123–132. [Google Scholar] [CrossRef]
- Marques, D.B.D.; Lopes, M.S.; Broekhuijse, M.; Guimaraes, S.E.F.; Knol, E.F.; Bastiaansen, J.W.M.; Silva, F.F.; Lopes, P.S. Genetic parameters for semen quality and quantity traits in five pig lines. J. Anim. Sci. 2017, 95, 4251–4259. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Wei, H.; Zhou, Y.; Tan, J.; Sun, H.; Jiang, S.; Peng, J. Effects of feeding regimen on weight gain, semen characteristics, libido, and lameness in 170- to 250-kilogram Duroc boars. J. Anim. Sci. 2016, 94, 4666–4676. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Browning, B.L.; Browning, S.R. A unified approach to genotype imputation and haplotype-phase inference for large data sets of trios and unrelated individuals. Am. J. Hum. Genet. 2009, 84, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.; Mantysaari, E.A.; Madsen, P.; Thompson, R. Residual maximum likelihood estimation of (Co)variance components in multivariate mixed linear models using average information. J. Indian Soc. Agric. Stat. 1997, 49, 215–236. [Google Scholar]
- Misztal, I.; Tsuruta, S.; Strabel, T.; Auvray, B.; Druet, T.; Lee, D.H. BLUPF90 and related programs (BGF90). In Proceedings of the 7th World Congress on Genetics Applied to Livestock Production, Montpellier, France, 19–23 August 2002; pp. 743–744. [Google Scholar]
- Lund, O.F.C.M.S. Genomic prediction when some animals are not genotyped. Genet. Sel. Evol. 2010, 42, 2. [Google Scholar] [CrossRef]
- VanRaden, P.M. Efficient methods to compute genomic predictions. J. Dairy Sci. 2008, 91, 4414–4423. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, I.; Misztal, I.; Legarra, A.; Tsuruta, S. Efficient computation of the genomic relationship matrix and other matrices used in single-step evaluation. J. Anim. Breed. Genet. 2011, 128, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Legarra, A.; Robert-Granie, C.; Manfredi, E.; Elsen, J.M. Performance of genomic selection in mice. Genetics 2008, 180, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lourenco, D.; Aguilar, I.; Legarra, A.; Misztal, I. Weighting strategies for single-step genomic BLUP: An iterative approach for accurate calculation of GEBV and GWAS. Front. Genet. 2016, 7, 151. [Google Scholar] [CrossRef]
- Veroneze R, L.P.; Guimarães, S.E.; Silva, F.F.; Lopes, M.S.; Harlizius, B.; Knol, E.F. Linkage disequilibrium and haplotype block structure in six commercial pig lines. J. Anim. Sci. 2013, 91, 3493–3501. [Google Scholar] [CrossRef]
- Amaral, A.J.; Megens, H.J.; Crooijmans, R.P.; Heuven, H.C.; Groenen, M.A. Linkage disequilibrium decay and haplotype block structure in the pig. Genetics 2008, 179, 569–579. [Google Scholar] [CrossRef]
- Gonzalez-Pena, D.; Gao, G.; Baranski, M.; Moen, T.; Cleveland, B.M.; Kenney, P.B.; Vallejo, R.L.; Palti, Y.; Leeds, T.D. Genome-wide association study for identifying loci that affect fillet yield, carcass, and body weight traits in rainbow trout (Oncorhynchus mykiss). Front. Genet. 2016, 7, 203. [Google Scholar] [CrossRef]
- Irano, N.; de Camargo, G.M.; Costa, R.B.; Terakado, A.P.; Magalhaes, A.F.; Silva, R.M.; Dias, M.M.; Bignardi, A.B.; Baldi, F.; Carvalheiro, R.; et al. Genome-wide association study for indicator traits of sexual precocity in Nellore cattle. PLoS ONE 2016, 11, e0159502. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic. Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Odegard, J.; Meuwissen, T.H. Estimation of heritability from limited family data using genome-wide identity-by-descent sharing. Genet. Sel. Evol. 2012, 44, 16. [Google Scholar] [CrossRef]
- Sun, S.; Jiang, Y.; Zhang, Q.; Pan, H.; Li, X.; Yang, L.; Huang, M.; Wei, W.; Wang, X.; Qiu, M.; et al. Znhit1 controls meiotic initiation in male germ cells by coordinating with Stra8 to activate meiotic gene expression. Dev. Cell. 2022, 57, 901–913. [Google Scholar] [CrossRef]
- Sinha, N.; Whelan, E.C.; Tobias, J.W.; Avarbock, M.; Stefanovski, D.; Brinster, R.L. Roles of Stra8 and Tcerg1l in retinoic acid induced spermatogonial differentiation in mousedagger. Biol. Reprod. 2021, 105, 503–518. [Google Scholar] [CrossRef]
- Niu, C.; Guo, J.; Shen, X.; Ma, S.; Xia, M.; Xia, J.; Zheng, Y. Meiotic gatekeeper STRA8 regulates cell cycle by interacting with SETD8 during spermatogenesis. J. Cell. Mol. Med. 2020, 24, 4194–4211. [Google Scholar] [CrossRef]
- Shen, X.; Niu, C.; Guo, J.; Xia, M.; Xia, J.; Hu, Y.; Zheng, Y. Stra8 may inhibit apoptosis during mouse spermatogenesis via the AKT signaling pathway. Int. J. Mol. Med. 2018, 42, 2819–2830. [Google Scholar] [CrossRef]
- Feng, C.W.; Burnet, G.; Spiller, C.M.; Cheung, F.K.M.; Chawengsaksophak, K.; Koopman, P.; Bowles, J. Identification of regulatory elements required for Stra8 expression in fetal ovarian germ cells of the mouse. Development 2021, 148, dev194977. [Google Scholar] [CrossRef]
- Gewiss, R.L.; Shelden, E.A.; Griswold, M.D. STRA8 induces transcriptional changes in germ cells during spermatogonial development. Mol. Reprod. Dev. 2021, 88, 128–140. [Google Scholar] [CrossRef]
- Skaftnesmo, K.O.; Crespo, D.; Kleppe, L.; Andersson, E.; Edvardsen, R.B.; Norberg, B.; Fjelldal, P.G.; Hansen, T.J.; Schulz, R.W.; Wargelius, A. Loss of stra8 Increases Germ Cell Apoptosis but Is Still Compatible With Sperm Production in Atlantic Salmon (Salmo salar). Front. Cell. Dev. Biol. 2021, 9, 657192. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, Y.; Zhou, J.; Zhang, H.; Zhang, Y.; Ma, H.; Jiang, X.; Shi, Q. A recurrent ZSWIM7 mutation causes male infertility resulting from decreased meiotic recombination. Hum. Reprod. 2021, 36, 1436–1445. [Google Scholar] [CrossRef]
- Hussain, S.; Nawaz, S.; Khan, I.; Khan, N.; Hussain, S.; Ullah, I.; Fakhro, K.A.; Ahmad, W. A novel homozygous variant in homologous recombination repair gene ZSWIM7 causes azoospermia in males and primary ovarian insufficiency in females. Eur. J. Med. Genet. 2022, 65, 104629. [Google Scholar] [CrossRef]
- Roy, A.; Lin, Y.N.; Agno, J.E.; DeMayo, F.J.; Matzuk, M.M. Tektin 3 is required for progressive sperm motility in mice. Mol. Reprod. Dev. 2009, 76, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, M.; Hiyama, E.; Hirotani, K.; Gotoh, T.; Inai, T.; Iida, H. Translocation of Tektin 3 to the equatorial segment of heads in bull spermatozoa exposed to dibutyryl cAMP and calyculin A. Mol. Reprod. Dev. 2017, 84, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Jung, B.K.; Park, S.H.; Song, K.J.; Anwar, M.A.; Ryu, K.Y.; Kim, K.P. Polyubiquitin gene Ubb is required for upregulation of Piwi protein level during mouse testis development. Cell. Death Discov. 2021, 7, 194. [Google Scholar] [CrossRef] [PubMed]
- Sinnar, S.A.; Small, C.L.; Evanoff, R.M.; Reinholdt, L.G.; Griswold, M.D.; Kopito, R.R.; Ryu, K.Y. Altered testicular gene expression patterns in mice lacking the polyubiquitin gene Ubb. Mol. Reprod. Dev. 2011, 78, 415–425. [Google Scholar] [CrossRef]
- Li, H.G.; Liao, A.H.; Ding, X.F.; Zhou, H.; Xiong, C.L. The expression and significance of CATSPER1 in human testis and ejaculated spermatozoa. Asian J. Androl. 2006, 8, 301–306. [Google Scholar] [CrossRef]
- Manfrevola, F.; Ferraro, B.; Sellitto, C.; Rocco, D.; Fasano, S.; Pierantoni, R.; Chianese, R. CRISP2, CATSPER1 and PATE1 Expression in Human Asthenozoospermic Semen. Cells. 2021, 10, 1956. [Google Scholar] [CrossRef]
- Wang, Y.N.; Wang, B.; Liang, M.; Han, C.Y.; Zhang, B.; Cai, J.; Sun, W.; Xing, G.G. Down-regulation of CatSper1 channel in epididymal spermatozoa contributes to the pathogenesis of asthenozoospermia, whereas up-regulation of the channel by Sheng-Jing-San treatment improves the sperm motility of asthenozoospermia in rats. Fertil. Steril. 2013, 99, 579–587. [Google Scholar] [CrossRef]
- Yu, Q.; Mei, X.Q.; Ding, X.F.; Dong, T.T.; Dong, W.W.; Li, H.G. Construction of a catsper1 DNA vaccine and its antifertility effect on male mice. PLoS ONE 2015, 10, e0127508. [Google Scholar] [CrossRef] [PubMed]
- Forero-Forero, A.; Lopez-Ramirez, S.; Felix, R.; Hernandez-Sanchez, J.; Tesoro-Cruz, E.; Orozco-Suarez, S.; Murbartian, J.; Soria-Castro, E.; Olivares, A.; Bekker-Mendez, C.; et al. Down Regulation of Catsper1 Expression by Calmodulin Inhibitor (Calmidazolium): Possible Implications for Fertility. Int. J. Mol. Sci. 2022, 23, 8070. [Google Scholar] [CrossRef] [PubMed]
- Hannigan, M.M.; Zagore, L.L.; Licatalosi, D.D. Ptbp2 Controls an Alternative Splicing Network Required for Cell Communication during Spermatogenesis. Cell. Rep. 2017, 19, 2598–2612. [Google Scholar] [CrossRef] [PubMed]
- Zagore, L.L.; Grabinski, S.E.; Sweet, T.J.; Hannigan, M.M.; Sramkoski, R.M.; Li, Q.; Licatalosi, D.D. RNA Binding Protein Ptbp2 Is Essential for Male Germ Cell Development. Mol. Cell. Biol. 2015, 35, 4030–4042. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, Y.; Liu, X.; Zhang, X.; Ding, J.; Li, Y.; Tian, Y.; Wang, H.; Liu, W.; Lu, Z. A Novel Meiosis-Related lncRNA, Rbakdn, Contributes to Spermatogenesis by Stabilizing Ptbp2. Front. Genet. 2021, 12, 752495. [Google Scholar] [CrossRef]
- Lin, Y.; Liang, A.; He, Y.; Li, Z.; Li, Z.; Wang, G.; Sun, F. Proteomic analysis of seminal extracellular vesicle proteins involved in asthenozoospermia by iTRAQ. Mol. Reprod. Dev. 2019, 86, 1094–1105. [Google Scholar] [CrossRef]
- Xu, K.; Lu, T.; Zhou, H.; Bai, L.; Xiang, Y. The role of MSH5 C85T and MLH3 C2531T polymorphisms in the risk of male infertility with azoospermia or severe oligozoospermia. Clin. Chim. Acta. 2010, 411, 49–52. [Google Scholar] [CrossRef]
- Nawaz, S.; Ullah, M.I.; Hamid, B.S.; Nargis, J.; Nawaz, M.; Hussain, S.; Ahmad, W. A loss-of-function variant in DNA mismatch repair gene MLH3 underlies severe oligozoospermia. J. Hum. Genet. 2021, 66, 725–730. [Google Scholar] [CrossRef]
| Trait | Number of Boars | Number of Phenotypes | Mean ± SD | CV(%) a | Minimum | Maximum |
|---|---|---|---|---|---|---|
| SPMOT/% | 583 | 24,833 | 82.35 ± 12.08 | 14.67 | 10.00 | 100.00 |
| SPPMOT/% | 583 | 24,730 | 28.84 ± 18.44 | 63.92 | 1.00 | 100.00 |
| SPABR/% | 583 | 24,917 | 28.81 ± 12.49 | 43.35 | 4.00 | 100.00 |
| SPCOUNT/108 | 583 | 24,492 | 364.67 ± 190.59 | 52.26 | 50.00 | 2529.09 |
| Trait | Models | (SE) | (SE) | (SE) | (SE) | (SE) |
|---|---|---|---|---|---|---|
| SPMOT | BLUP | 50.472 ± 11.526 | 15.672 ± 7.563 | 110.890 ± 1.026 | 0.285 ± 0.058 | 0.374 ± 0.020 |
| ssGBLUP | 29.428 ± 6.584 | 34.956 ± 4.471 | 110.860 ± 1.025 | 0.168 ± 0.034 | 0.367 ± 0.018 | |
| SPPMOT | BLUP | 67.082 ± 14.463 | 7.460 ± 9.143 | 258.340 ± 2.394 | 0.202 ± 0.040 | 0.224 ± 0.016 |
| ssGBLUP | 39.267 ± 8.315 | 31.831 ± 5.095 | 258.310 ± 2.394 | 0.119 ± 0.023 | 0.216 ± 0.014 | |
| SPABR | BLUP | 65.056 ± 17.395 | 47.887 ± 12.085 | 71.246 ± 0.658 | 0.353 ± 0.082 | 0.613 ± 0.019 |
| ssGBLUP | 44.741 ± 11.239 | 67.419 ± 7.871 | 71.246 ± 0.658 | 0.244 ± 0.054 | 0.612 ± 0.017 | |
| SPCOUNT | BLUP | 9436.900 ± 2092.000 | 2706.900 ± 1358.500 | 22884.000 ± 213.240 | 0.269 ± 0.053 | 0.347 ± 0.020 |
| ssGBLUP | 6116.500 ± 1247.900 | 5610.700 ± 782.780 | 22881.000 ± 213.200 | 0.177 ± 0.032 | 0.339 ± 0.018 |
| Traits a | Chr b | Position (Mb) | gVar (%) c | Nsnp | Candidate Genes |
|---|---|---|---|---|---|
| SOMOT | 18 | 14.23–14.60 | 6.18 | 13 | STRA8 |
| 12 | 58.83–59.21 | 3.31 | 10 | ZSWIM7, TEKT3, UBB | |
| 2 | 6.19–6.58 | 3.87 | 6 | CATSPER1 | |
| SPPMOT | 4 | 121.17–121.57 | 3.84 | 10 | PTBP2 |
| 18 | 14.23–14.60 | 3.10 | 13 | STRA8 | |
| 12 | 62.16–62.44 | 2.84 | 6 | - | |
| SPABR | 12 | 58.83–59.21 | 8.07 | 10 | ZSWIM7, TEKT3, UBB |
| 7 | 97.93–98.26 | 3.88 | 10 | EIF2B2, MLH3 | |
| 12 | 46.05–46.45 | 3.84 | 7 | - | |
| SPCOUNT | 2 | 17.69–18.09 | 6.21 | 8 | - |
| 11 | 15.98–16.37 | 3.86 | 10 | CCDC70 | |
| 2 | 15.98–16.09 | 2.08 | 3 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Lin, Q.; Liao, W.; Zhang, W.; Li, T.; Li, J.; Zhang, Z.; Huang, X.; Zhang, H. Identification of New Candidate Genes Related to Semen Traits in Duroc Pigs through Weighted Single-Step GWAS. Animals 2023, 13, 365. https://doi.org/10.3390/ani13030365
Zhang X, Lin Q, Liao W, Zhang W, Li T, Li J, Zhang Z, Huang X, Zhang H. Identification of New Candidate Genes Related to Semen Traits in Duroc Pigs through Weighted Single-Step GWAS. Animals. 2023; 13(3):365. https://doi.org/10.3390/ani13030365
Chicago/Turabian StyleZhang, Xiaoke, Qing Lin, Weili Liao, Wenjing Zhang, Tingting Li, Jiaqi Li, Zhe Zhang, Xiang Huang, and Hao Zhang. 2023. "Identification of New Candidate Genes Related to Semen Traits in Duroc Pigs through Weighted Single-Step GWAS" Animals 13, no. 3: 365. https://doi.org/10.3390/ani13030365
APA StyleZhang, X., Lin, Q., Liao, W., Zhang, W., Li, T., Li, J., Zhang, Z., Huang, X., & Zhang, H. (2023). Identification of New Candidate Genes Related to Semen Traits in Duroc Pigs through Weighted Single-Step GWAS. Animals, 13(3), 365. https://doi.org/10.3390/ani13030365






