Characterization of Type I and Type III Collagen in the Intramuscular Connective Tissue of Wuzhumuqin Sheep
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Expression Analysis of Genes Related to Collagen Type I and III
2.3. Extraction of Type I and III Collagen
2.4. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) of Type I and III Collagen
2.5. Distribution of Type I and III Collagen in Intramuscular Connective Tissue (IMCT)
2.6. Fourier Transform Infrared Spectroscopy (FTIR) Analysis of Type I and III Collagen
2.7. Amino Acid Composition of Collagen Types I and III
2.8. Differential Scanning Calorimetry of Type I and III Collagen
2.9. Solubility Analysis of Type I and III Collagen
2.10. Determination of Pyridinoline (Pyr) in IMCT
2.11. Statistical Analysis
3. Results and Discussion
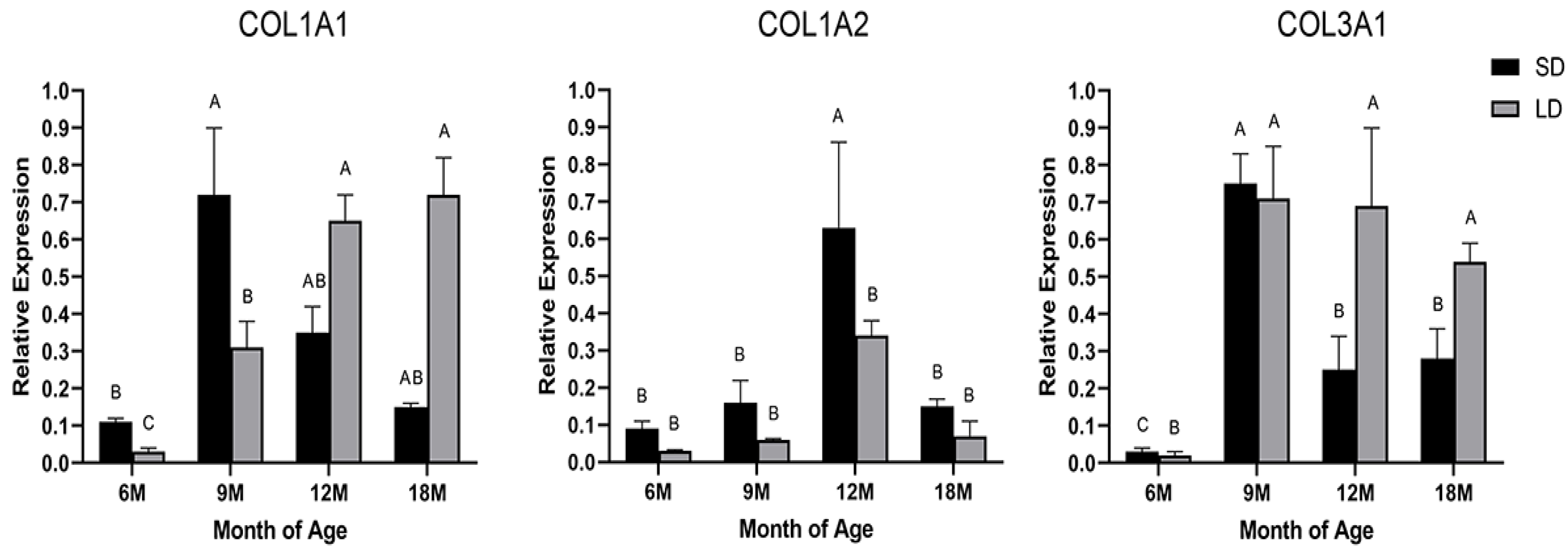
3.1. Expression Analysis of the COL1A1, COL1A2, and COL3A1 Genes
3.2. Distribution of Type I and III Collagen in Intramuscular Connective Tissue
3.3. Isolation and Identification of Type I and III Collagen
3.4. Amino Acid Composition of Type I and III Collagen
3.5. Collagen Solubility Analysis
3.6. Analysis of the Denaturation Temperature of Collagen Types I and III
3.7. Collagen Cross-Linking Content Analysis
3.8. Correlation Analysis of Collagen Characteristics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wojtysiak, D. Effect of age on structural properties of intramuscular connective tissue, muscle fibre, collagen content and meat tenderness in pig longissimus lumborum muscle. Folia Biol. 2013, 61, 221–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purslow, P.P. The structure and role of intramuscular connective tissue in muscle function. Front. Physiol. 2020, 11, 495. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.; Ertbjerg, P.; Failla, S.; Sañudo, C.; Richardson, R.I.; Nute, G.R.; Williams, J.L. Relationship between collagen characteristics, lipid content and raw and cooked texture of meat from young bulls of fifteen European breeds. Meat Sci. 2011, 87, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Fang, S.; Wakamatsu, J.I.; Takahashi, K. Relationships between physical and structural properties of intramuscular connective tissue and toughness of raw pork. Anim. Sci. J. 2009, 80, 85–90. [Google Scholar] [CrossRef]
- Frylinck, L.; Strydom, P.E.; O’Neil, A.; Du Toit, E.; Webb, E.C. The beef tenderness model. S. Afr. J. Anim. Sci. 2015, 45, 234–248. [Google Scholar] [CrossRef]
- Abdullah, A.Y.; Qudsieh, R.I. Effect of slaughter weight and aging time on the quality of meat from Awassi ram lambs. Meat Sci. 2009, 82, 309–316. [Google Scholar] [CrossRef]
- Torrescano, G.; Sanchez-Escalante, A.; Gimenez, B.; Roncalés, P.; Beltrán, J.A. Shear values of raw samples of 14 bovine muscles and their relation to muscle collagen characteristics. Meat Sci. 2003, 64, 85–91. [Google Scholar] [CrossRef]
- Voutila, L.; Mullen, A.M.; Ruusunen, M.; Troy, D.; Puolanne, E. Thermal stability of connective tissue from porcine muscles. Meat Sci. 2007, 76, 474–480. [Google Scholar] [CrossRef]
- Li, X.; Ha, M.; Warner, R.D.; Dunshea, F.R. Collagen characteristics affect the texture of pork Longissimus and Biceps femoris. Transl. Anim. Sci. 2022, 6, txac129. [Google Scholar] [CrossRef]
- Slatter, D.A.; Avery, N.C.; Bailey, A.J. Collagen in its fibrillar state is protected from glycation. Int. J. Biochem. Cell Biol. 2008, 40, 2253–2263. [Google Scholar] [CrossRef]
- Bosselmann, A.; Möller, C.; Steinhart, H.; Kirchgessner, M.; Schwarz, F.J. Pyridinoline cross-links in bovine muscle collagen. J. Food Sci. 1995, 60, 953–958. [Google Scholar] [CrossRef]
- Rodríguez, M.I.A.; Barroso, L.G.R.; Sánchez, M.L. Collagen: A review on its sources and potential cosmetic applications. J. Cosmet. Dermatol. 2018, 17, 20–26. [Google Scholar] [CrossRef]
- Burson, D.E.; Hunt, M.C. Proportion of collagen types I and III in four bovine muscles differing in tenderness. J. Food Sci. 1986, 51, 51–53. [Google Scholar] [CrossRef]
- Siqin, Q.; Nishiumi, T.; Yamada, T.; Wang, S.; Liu, W.; Wu, R.; Borjigin, G. Relationships among muscle fiber type composition, fiber diameter and MRF gene expression in different skeletal muscles of naturally grazing Wuzhumuqin sheep during postnatal development. Anim. Sci. J. 2017, 88, 2033–2043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rihan, W.; Yueying, Y.; Qinghui, F.; Xia, Q.; Xige, H.; Lu, C.; Gerelt, B. Expression of myogenic regulatory factor genes in skeletal muscle satellite cells from Wuzhumuqin sheep in vitro. Small Rumin. Res. 2020, 193, 106251. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Chandrarajan, J. Separation of type Ⅲ collagen from typeⅠcollagen and pepsin by differential denaturation and renaturation. Biochem. Biophys. Res. Commun. 1978, 83, 180–186. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Listrat, A.; Lethias, C.; Hocquette, J.F.; Renand, G.; Ménissier, F.; Geay, Y.; Picard, B. Age-related changes and location of types I, III, XII and XIV collagen during development of skeletal muscles from genetically different animals. Histochem. J. 2000, 32, 349–356. [Google Scholar] [CrossRef]
- Nakamura, Y.N.; Iwamoto, H.; Etoh, T.; Shiotsuka, Y.; Yamaguchi, T.; Ono, Y.; Gotoh, T. Three-dimensional observation of connective tissue of bovine masseter muscle under concentrate-and roughage-fed conditions by using immunohistochemical/confocal laser-scanning microscopic methods. J. Food Sci. 2007, 72, 375–381. [Google Scholar] [CrossRef]
- Veeruraj, A.; Arumugam, M.; Balasubramanian, T. Isolation and characterization of thermostable collagen from the marine eel-fish (Evenchelys macrura). Process. Biochem. 2013, 48, 1592–1602. [Google Scholar] [CrossRef]
- Chang, H.J.; Wang, Q.; Zhou, G.H.; Xu, X.L.; Li, C.B. Influence of weak organic acids and sodium chloride marination on characteristics of connective tissue collagen and textural properties of beef semitendinosus muscle. J. Texture Stud. 2010, 41, 279–301. [Google Scholar] [CrossRef]
- Hill, F. The solubility of intramuscular collagen in meat animals of various ages. J. Food Sci. 1966, 31, 161–166. [Google Scholar] [CrossRef]
- Bergman, I.; Loxley, R. Two improved and simplified methods for the spectrophotometric determination of hydroxyproline. Anal. Chem. 1963, 35, 1961–1965. [Google Scholar] [CrossRef]
- Grant, R.A. Estimation of hydroxyproline by the autoanalyser. J. Clin. Pathol. 1964, 17, 685–686. [Google Scholar] [CrossRef] [Green Version]
- Light, N.; Champion, A.E.; Voyle, C.; Bailey, A. The role of epimysial, perimysial and endomysial collagen in determining texture in six bovine muscles. Meat Sci. 1985, 13, 137–149. [Google Scholar] [CrossRef]
- Ahtikoski, A.M.; Koskinen, S.O.A.; Virtanen, P.; Kovanen, V.; Takala, T.E.S. Regulation of synthesis of fibrillar collagens in rat skeletal muscle during immobilization in shortened and lengthened positions. Acta Physiol. 2001, 172, 131–140. [Google Scholar] [CrossRef]
- Bjarnadottir, S.G.; Hollung, K.; Høy, M.; Bendixen, E.; Codrea, M.C.; Veiseth-Kent, E. Changes in protein abundance between tender and tough meat from bovine Longissimus thoracis muscle assessed by isobaric Tag for Relative and Absolute Quantitation (iTRAQ) and 2-dimensional gel electrophoresis analysis. J. Anim. Sci. 2012, 90, 2035–2043. [Google Scholar] [CrossRef] [Green Version]
- Sheng, H.; Guo, Y.; Zhang, L.; Zhang, J.; Miao, M.; Tan, H.; Hu, D.; Li, X.; Ding, X.; Li, G.; et al. Proteomic Studies on the Mechanism of Myostatin Regulating Cattle Skeletal Muscle Development. Front. Genet. 2021, 12, 752129. [Google Scholar] [CrossRef]
- Dos Santos Fontes, M.M.; Costa, T.C.; Lopes, M.M.; Souza, R.O.; Carneiro, L.S.; Paulino, P.V.R.; De Souza Duarte, M. Intramuscular collagen characteristics and expression of related genes in skeletal muscle of cull cows receiving a high-energy diet. Meat Sci. 2021, 177, 108495. [Google Scholar] [CrossRef]
- Wang, Y.H.; Bower, N.I.; Reverter, A.; Tan, S.H.; De Jager, N.; Wang, R.; Lehnert, S.A. Gene expression patterns during intramuscular fat development in cattle. J. Anim. Sci. 2009, 87, 119–130. [Google Scholar] [CrossRef]
- Bao, X.; Zeng, Y.; Wei, S.; Wang, G.; Liu, C.; Sun, Y.; Li, H. Developmental changes of Col3a1 mRNA expression in muscle and their association with intramuscular collagen in pigs. J. Genet. Genom. 2007, 34, 223–228. [Google Scholar] [CrossRef]
- Chuan-Hao, L.; Wei, C.; Jia-Qing, H.; Yan-Dong, W.; Shou-Dong, W.; Yong-Qing, Z.; Hui, W. miRNA-29a targets COL3A1 to regulate the level of type III collagen in pig. Gene 2016, 592, 140–147. [Google Scholar] [CrossRef]
- Nishimura, T.; Ojima, K.; Hattori, A.; Takahashi, K. Developmental expression of extracellular matrix components in intramuscular connective tissue of bovine semitendinosus muscle. Histochem. Cell Biol. 1997, 107, 215–221. [Google Scholar] [CrossRef]
- Stoilov, I.; Starcher, B.C.; Mecham, R.P.; Broekelmann, T.J. Measurement of elastin, collagen, and total protein levels in tissues. Method Cell Biol. 2018, 143, 133–146. [Google Scholar]
- Liu, D.; Wei, G.; Li, T.; Hu, J.; Lu, N.; Regenstein, J.M.; Zhou, P. Effects of alkaline pretreatments and acid extraction conditions on the acid-soluble collagen from grass carp (Ctenopharyngodon idella) skin. Food Chem. 2015, 172, 836–843. [Google Scholar] [CrossRef]
- Liu, D.; Liang, L.; Regenstein, J.M.; Zhou, P. Extraction and characterisation of pepsin-solubilised collagen from fins, scales, skins, bones and swim bladders of bighead carp (Hypophthalmichthys nobilis). Food Chem. 2012, 133, 1441–1448. [Google Scholar] [CrossRef]
- Gao, L.L.; Wang, Z.Y.; Zheng, L.I.; Zhang, C.X.; Zhang, D.Q. The characterization of acid and pepsin soluble collagen from ovine bones (Ujumuqin sheep). J. Integr. Agric. 2018, 17, 704–711. [Google Scholar] [CrossRef] [Green Version]
- Nagai, T.; Tanoue, Y.; Kai, N.; Suzuki, N. Characterization of collagen from emu (Dromaius novaehollandiae) skins. J. Food Sci. Technol. 2015, 52, 2344–2351. [Google Scholar] [CrossRef] [Green Version]
- Vidal, A.R.; Duarte, L.P.; Schmidt, M.M.; Cansian, R.L.; Fernandes, I.A.; de Oliveira Mello, R.; Dornelles, R.C.P. Extraction and characterization of collagen from sheep slaughter by-products. Waste Manag. 2020, 102, 838–846. [Google Scholar] [CrossRef]
- Okazaki, E.; Osako, K. Isolation and characterization of acid-soluble collagen from the scales of marine fishes from Japan and Vietnam. Food Chem. 2014, 149, 264–270. [Google Scholar]
- Huang, C.Y.; Kuo, J.M.; Wu, S.J.; Tsai, H.T. Isolation and characterization of fish scale collagen from tilapia (Oreochromis sp.) by a novel extrusion-hydro-extraction process. Food Chem. 2016, 190, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Truong, T.M.T.; Nguyen, V.M.; Tran, T.T.; Le, T.M.T. Characterization of acid-soluble collagen from food processing by-products of snakehead fish (Channa striata). Processes 2021, 9, 1188. [Google Scholar] [CrossRef]
- Nishimura, T.; Hattori, A.; Takahashi, K. Structural changes in intramuscular connective tissue during the fattening of Japanese black cattle: Effect of marbling on beef tenderization. J. Anim. Sci. 1999, 77, 93–104. [Google Scholar] [CrossRef] [Green Version]
- Purslow, P.P. Contribution of collagen and connective tissue to cooked meat toughness; some paradigms reviewed. Meat Sci. 2018, 144, 127–134. [Google Scholar] [CrossRef]
- Roy, B.C.; Sedgewick, G.; Aalhus, J.L.; Basarab, J.A.; Bruce, H.L. Modification of mature non-reducible collagen cross-link concentrations in bovine m. gluteus medius and semitendinosus with steer age at slaughter, breed cross and growth promotants. Meat Sci. 2015, 110, 109–117. [Google Scholar] [CrossRef]
- Eyre, D.R.; Wu, J.J. Collagen Cross-Links. In Collagen; Brinckmann, J., Notbohm, H., Müller, P.K., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 247, pp. 207–229. [Google Scholar]
- Roy, B.C.; Das, C.; Aalhus, J.L.; Bruce, H.L. Relationship between meat quality and intramuscular collagen characteristics of muscles from calf-fed, yearling-fed and mature crossbred beef cattle. Meat Sci. 2021, 173, 108375. [Google Scholar] [CrossRef]
- Li, P.; Wu, G. Roles of dietary glycine, proline, and hydroxyproline in collagen synthesis and animal growth. Amino Acids 2018, 50, 29–38. [Google Scholar] [CrossRef]
- Starkey, C.P.; Geesink, G.H.; van de Ven, R.; Hopkins, D.L. The relationship between shear force, compression, collagen characteristics, desmin degradation and sarcomere length in lamb biceps femoris. Meat Sci. 2017, 126, 18–21. [Google Scholar] [CrossRef]
- Starkey, C.P.; Geesink, G.H.; Collins, D.; Oddy, V.H.; Hopkins, D.L. Do sarcomere length, collagen content, pH, intramuscular fat and desmin degradation explain variation in the tenderness of three ovine muscles? Meat Sci. 2016, 113, 51–58. [Google Scholar] [CrossRef]





| Amino Acids | Muscles | Type I Collagen | Type III Collagen | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 6M | 9M | 12M | 18M | 6M | 9M | 12M | 18M | ||
| Aspartic acid | SD | 0.24 ± 0.02 D | 1.61 ± 0.12 C | 2.72 ± 0.18 B | 4.14 ± 0.32 A | 4.46 ± 0.32 D | 3.48 ± 0.23 C | 3.27 ± 0.19 B | 1.86 ± 0.12 A |
| LD | 4.25 ± 0.21 D | 5.09 ± 0.26 C | 5.52 ± 0.31 B | 6.09 ± 0.32 A | 6.09 ± 0.22 D | 5.31 ± 0.21 C | 5.18 ± 0.18 B | 4.63 ± 0.15 A | |
| Threonine | SD | 0.57 ± 0.06 D | 0.61 ± 0.07 C | 1.00 ± 0.09 B | 3.79 ± 0.34 A | 2.60 ± 0.21 B | 2.66 ± 0.22 C | 2.57 ± 0.18 B | 1.08 ± 0.09 A |
| LD | 3.02 ± 0.11 D | 3.97 ± 0.13 C | 3.47 ± 0.16 B | 3.67 ± 0.21 A | 3.09 ± 0.14 D | 2.21 ± 0.11 C | 2.09 ± 0.13 B | 1.53 ± 0.08 A | |
| Serine | SD | 0.51 ± 0.05 D | 0.85 ± 0.08 C | 1.32 ± 0.12 B | 2.40 ± 0.21 A | 2.36 ± 0.22 C | 2.44 ± 0.23 B | 2.38 ± 0.21 B | 1.68 ± 0.15 A |
| LD | 2.85 ± 0.09 C | 3.22 ± 0.13 B | 3.74 ± 0.12 A | 2.81 ± 0.11 C | 2.95 ± 0.17 D | 2.37 ± 0.15 C | 2.25 ± 0.12 B | 1.95 ± 0.10 A | |
| Glutamic acid | SD | 1.30 ± 0.12 D | 1.84 ± 0.16 C | 2.99 ± 0.26 B | 6.97 ± 0.54 A | 6.8 ± 0.51 D | 5.17 ± 0.43 C | 4.58 ± 0.36 B | 2.45 ± 0.16 A |
| LD | 4.62 ± 0.22 D | 5.25 ± 0.26 C | 6.05 ± 0.18 B | 6.72 ± 0.27 A | 6.44 ± 0.31 D | 5.12 ± 0.26 C | 4.66 ± 0.15 B | 3.34 ± 0.12 A | |
| Glycine | SD | 4.55 ± 0.43 D | 8.44 ± 0.61 C | 13.16 ± 0.98 B | 26.61 ± 1.14 A | 23.79 ± 1.61 D | 23.37 ± 1.54 C | 23.03 ± 1.22 B | 16.89 ± 0.86 A |
| LD | 18.21 ± 0.82 D | 23.96 ± 0.63 C | 26.25 ± 0.71 B | 29.31 ± 0.46 A | 27.33 ± 0.81 D | 23.66 ± 0.62 C | 23.18 ± 0.46 B | 19.86 ± 0.37 A | |
| Alanine | SD | 1.29 ± 0.11 D | 2.51 ± 0.22 C | 3.63 ± 0.31 B | 7.21 ± 0.64 A | 6.5 ± 0.32 C | 6.78 ± 0.36 B | 6.79 ± 0.34 B | 4.44 ± 0.19 A |
| LD | 5.41 ± 0.21 D | 7.05 ± 0.24 C | 7.73 ± 0.13 B | 8.62 ± 0.18 A | 7.93 ± 0.41 D | 6.70 ± 0.36 C | 6.58 ± 0.34 B | 5.58 ± 0.31 A | |
| Cystine | SD | 0.01 ± 0 B | 0.01 ± 0 B | 0.02 ± 0 B | 0.02 ± 0 A | 0.01 ± 0 C | 0.07 ± 0 B | 0.04 ± 0 A | 0.05 ± 0 A |
| LD | 0.09 ± 0 B | 0.07 ± 0 B | 0.08 ± 0 B | 0.03 ± 0 A | 0.04 ± 0 C | 0.05 ± 0 B | 0.03 ± 0 A | 0.03 ± 0 A | |
| Valine | SD | 0.52 ± 0.05 D | 0.71 ± 0.07 C | 0.98 ± 0.08 B | 1.49 ± 0.13 A | 1.80 ± 0.14 C | 1.77 ± 0.13 C | 1.73 ± 0.11 B | 0.93 ± 0.06 A |
| LD | 2.26 ± 0.08 C | 3.26 ± 0.12 A | 2.27 ± 0.13 BC | 2.36 ± 0.11 B | 1.91 ± 0.12 D | 1.74 ± 0.11 C | 1.65 ± 0.02 B | 1.28 ± 0.07 A | |
| Methionine | SD | 0.14 ± 0.01 C | 0.17 ± 0.02 BC | 0.18 ± 0.02 B | 0.76 ± 0.06 A | 0.14 ± 0.01 D | 0.43 ± 0.03 C | 0.47 ± 0.04 B | 0.70 ± 0.06 A |
| LD | 0.32 ± 0.01 D | 0.52 ± 0.02 C | 0.63 ± 0.02 B | 0.99 ± 0.04 A | 0.63 ± 0.03 D | 0.53 ± 0.03 C | 0.49 ± 0.02 B | 0.28 ± 0.02 A | |
| Isoleucine | SD | 0.30 ± 0.03 D | 0.41 ± 0.04 C | 0.53 ± 0.04 B | 0.79 ± 0.06 A | 0.23 ± 0.02 D | 0.19 ± 0.02 C | 0.09 ± 0.01 B | 0.42 ± 0.03 A |
| LD | 0.29 ± 0.01 D | 0.23 ± 0.01 C | 0.34 ± 0.02 B | 0.55 ± 0.01 A | 0.69 ± 0.04 D | 0.47 ± 0.03 C | 0.37 ± 0.03 B | 0.22 ± 0.01 A | |
| Leucine | SD | 0.13 ± 0.01 D | 0.21 ± 0.02 C | 0.42 ± 0.04 B | 0.92 ± 0.08 A | 0.52 ± 0.05 C | 0.56 ± 0.05 C | 0.29 ± 0.24 B | 0.74 ± 0.053 A |
| LD | 0.28 ± 0.01 D | 0.36 ± 0.02 C | 0.41 ± 0.02 B | 0.45 ± 0.03 A | 0.49 ± 0.03 C | 0.41 ± 0.02 B | 0.32 ± 0.02 A | 0.30 ± 0.02 A | |
| Tyrosine | SD | 0.03 ± 0 A | 0.02 ± 0 A | 0.04 ± 0 A | 0.02 ± 0 A | 0.10 ± 0 A | 0.09 ± 0 A | 0.04 ± 0 C | 0.09 ± 0 A |
| LD | 0.04 ± 0 D | 0.03 ± 0 C | 0.08 ± 0 B | 0.06 ± 0 A | 0.06 ± 0 C | 0.04 ± 0 B | 0.04 ± 0 B | 0.03 ± 0 AB | |
| Phenylalanine | SD | 0.37 ± 0.04 D | 0.56 ± 0.05 C | 0.88 ± 0.07 B | 1.28 ± 0.11 A | 1.48 ± 0.12 C | 1.45 ± 0.11 C | 1.41 ± 0.09 B | 1.04 ± 0.08 A |
| LD | 1.73 ± 0.08 C | 1.47 ± 0.09 B | 1.46 ± 0.10 B | 1.28 ± 0.08 A | 1.94 ± 0.14 D | 1.55 ± 0.12 C | 1.50 ± 0.11 B | 1.08 ± 0.08 A | |
| Lysine | SD | 0.43 ± 0.04 D | 0.73 ± 0.06 C | 1.02 ± 0.10 B | 2.35 ± 0.21 A | 2.11 ± 0.16 D | 5.09 ± 0.37 C | 5.03 ± 0.29 B | 1.49 ± 0.12 A |
| LD | 1.43 ± 0.08 D | 3.26 ± 0.11 C | 3.55 ± 0.16 B | 3.76 ± 0.12 A | 4.40 ± 0.36 D | 3.30 ± 0.27 C | 3.14 ± 0.22 B | 2.64 ± 0.14 A | |
| Histidine | SD | 0.12 ± 0.01 D | 0.21 ± 0.02 C | 0.32 ± 0.03 B | 0.98 ± 0.07 A | 0.68 ± 0.07 C | 0.39 ± 0.03 B | 0.54 ± 0.04 A | 0.55 ± 0.05 A |
| LD | 0.16 ± 0.01 D | 0.26 ± 0.01 C | 0.34 ± 0.02 B | 0.47 ± 0.03 A | 0.35 ± 0.02 C | 0.34 ± 0.02 C | 0.32 ± 0.01 B | 0.20 ± 0.01 A | |
| Arginine | SD | 0.66 ± 0.06 D | 1.00 ± 0.09 C | 1.61 ± 0.12 B | 2.12 ± 0.16 A | 6.14 ± 0.51 D | 5.03 ± 0.46 C | 4.91 ± 0.41 B | 3.99 ± 0.21 A |
| LD | 2.31 ± 0.09 D | 4.52 ± 0.21 C | 5.13 ± 0.14 B | 6.02 ± 0.24 A | 5.95 ± 0.32 D | 5.47 ± 0.31 C | 5.38 ± 0.27 B | 4.45 ± 0.19 A | |
| Hydroxyproline | SD | 1.75 ± 0.16 D | 3.3 ± 0.22 C | 5.22 ± 0.43 B | 11.72 ± 1.03 A | 5.51 ± 0.34 D | 5.69 ± 0.36 C | 6.14 ± 0.42 B | 7.78 ± 0.51 A |
| LD | 1.48 ± 0.07 D | 2.58 ± 0.14 C | 3.98 ± 0.17 B | 8.01 ± 0.43 A | 5.02 ± 0.21 D | 5.91 ± 0.31 C | 6.56 ± 0.27 B | 7.53 ± 0.23 A | |
| Proline | SD | 1.4 ± 0.12 D | 2.65 ± 0.21 C | 4.08 ± 0.28 B | 7.66 ± 0.44 A | 7.28 ± 0.67 D | 7.16 ± 0.55 C | 6.99 ± 0.48 B | 5.09 ± 0.29 A |
| LD | 6.25 ± 0.31 D | 7.38 ± 0.28 C | 8.13 ± 0.34 B | 9.3 ± 0.42 A | 8.03 ± 0.46 D | 7.19 ± 0.39 C | 6.99 ± 0.37 B | 5.81 ± 0.28 A | |
| Total imino acids | SD | 3.15 ± 0.26 D | 5.95 ± 0.41 C | 9.30 ± 0.53 B | 19.38 ± 0.32 A | 12.79 ± 0.41 C | 12.85 ± 0.39 A | 13.13 ± 0.31 B | 12.87 ± 0.33 A |
| LD | 7.73 ± 0.41 D | 9.96 ± 0.37 C | 12.11 ± 0.64 B | 17.31 ± 0.71 A | 13.05 ± 0.39 D | 13.1 ± 0.51 C | 13.55 ± 0.48 B | 13.34 ± 0.25 A | |
| Total amino acids | SD | 14.32 ± 0.36 D | 25.84 ± 0.46 C | 40.12 ± 0.63 B | 81.23 ± 0.96 A | 72.51 ± 0.63 D | 71.82 ± 0.78 C | 70.3 ± 0.46 B | 51.27 ± 0.28 A |
| LD | 55.00 ± 0.72 D | 72.48 ± 0.91 C | 79.16 ± 0.67 B | 90.5 ± 0.74 A | 83.34 ± 0.44 D | 72.37 ± 0.38 C | 70.73 ± 0.31 B | 60.74 ± 0.29 A | |
| 6M | 9M | 12M | 18M | ||
|---|---|---|---|---|---|
SD | Type Ⅰ Collagen | 15.24 ± 0.43 aA | 10.63 ± 0.47 bA | 9.96 ± 0.42 cA | 8.72 ± 0.31 dA |
| Type Ⅲ Collagen | 13.89 ± 0.62 aB | 10.02 ± 0.43 bB | 9.41 ± 0.34 bcB | 8.14 ± 0.32 cB | |
LD | Type Ⅰ Collagen | 16.01 ± 0.54 aA | 11.82 ± 0.51 bA | 10.46 ± 0.39 cA | 9.12 ± 0.29 dA |
| Type Ⅲ Collagen | 14.62 ± 0.52 aB | 10.21 ± 0.43 bB | 9.85 ± 0.41 cB | 8.63 ± 0.36 dB | |
| Denaturation Temperature | 6M | 9M | 12M | 18M | ||
|---|---|---|---|---|---|---|
| SD | Type Ⅰ Collagen | To (°C) | 30.5 ± 0.45 A | 32.7 ± 0.37 B | 36.3 ± 0.21 C | 41.7 ± 0.26 D |
| Tp (°C) | 40.7 ± 0.70 A | 41.3 ± 0.62 B | 44.8 ± 0.42 C | 48.7 ± 0.48 D | ||
| Tf (°C) | 49.7 ± 0.38 A | 50.5 ± 0.71 B | 51.4 ± 0.56 C | 60.5 ± 0.82 D | ||
| Type Ⅲ Collagen | To (°C) | 33.1 ± 0.51 A | 35.1 ± 0.45 B | 36.9 ± 0.54 C | 38.4 ± 0.48 D | |
| Tp (°C) | 41.9 ± 0.46 A | 42.6 ± 0.73 B | 43.6 ± 0.62 C | 45.0 ± 0.57 D | ||
| Tf (°C) | 49.7 ± 0.62 A | 50.7 ± 0.61 B | 51.2 ± 0.73 C | 52.4 ± 0.69 D | ||
| LD | Type Ⅰ Collagen | To (°C) | 31.6 ± 0.37 A | 37.9 ± 0.27 B | 32.1 ± 0.31 C | 39.3 ± 0.31 D |
| Tp (°C) | 42.1 ± 0.43 A | 43.1 ± 0.31 B | 45.8 ± 0.36 C | 50.7 ± 0.38 D | ||
| Tf (°C) | 50.7 ± 0.58 A | 52.4 ± 0.29 B | 51.8 ± 0.35 C | 59.1 ± 0.61 D | ||
| Type Ⅲ Collagen | To (°C) | 30.4 ± 0.36 A | 33.3 ± 0.49 B | 35.9 ± 0.27 C | 40.1 ± 0.42 D | |
| Tp (°C) | 41.2 ± 0.39 A | 44.3 ± 0.51 B | 48.1 ± 0.39 C | 50.6 ± 0.47 D | ||
| Tf (°C) | 48.7 ± 0.41 A | 52.1 ± 0.68 B | 54.4 ± 0.48 C | 61.1 ± 0.64 D | ||
| 6M | 9M | 12M | 18M | ||
|---|---|---|---|---|---|
Endomysium | SD | 1.023 ± 0.063 A | 1.098 ± 0.103 B | 1.206 ± 0.072 C | 2.506 ± 0.025 D |
| LD | 0.962 ± 0.063 A | 1.055 ± 0.074 B | 1.196 ± 0.104 C | 2.33 ± 0.083 D | |
Perimysium | SD | 0.305 ± 0.030 A | 0.314 ± 0.025 AB | 0.320 ± 0.021 B | 0.704 ± 0.052 C |
| LD | 0.291 ± 0.018 A | 0.305 ± 0.022 A | 0.398 ± 0.028 B | 0.66 ± 0.048 C | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Wu, Q.; Xue, W.; Wu, R.; Huang, Y.; Chen, L.; Han, Y.; Wu, J.; Borjigin, G.; Sha, R. Characterization of Type I and Type III Collagen in the Intramuscular Connective Tissue of Wuzhumuqin Sheep. Animals 2023, 13, 395. https://doi.org/10.3390/ani13030395
He X, Wu Q, Xue W, Wu R, Huang Y, Chen L, Han Y, Wu J, Borjigin G, Sha R. Characterization of Type I and Type III Collagen in the Intramuscular Connective Tissue of Wuzhumuqin Sheep. Animals. 2023; 13(3):395. https://doi.org/10.3390/ani13030395
Chicago/Turabian StyleHe, Xige, Qiong Wu, Wenjun Xue, Rihan Wu, Yajuan Huang, Lu Chen, Yunfei Han, Jindi Wu, Gerelt Borjigin, and Rina Sha. 2023. "Characterization of Type I and Type III Collagen in the Intramuscular Connective Tissue of Wuzhumuqin Sheep" Animals 13, no. 3: 395. https://doi.org/10.3390/ani13030395
APA StyleHe, X., Wu, Q., Xue, W., Wu, R., Huang, Y., Chen, L., Han, Y., Wu, J., Borjigin, G., & Sha, R. (2023). Characterization of Type I and Type III Collagen in the Intramuscular Connective Tissue of Wuzhumuqin Sheep. Animals, 13(3), 395. https://doi.org/10.3390/ani13030395






