Zoonotic Bacteria in Anolis sp., an Invasive Species Introduced to the Canary Islands (Spain)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Isolation of Pathogenic Bacteria
2.3. Molecular Identification of Isolates and Pathogenicity Genes
2.3.1. DNA Isolation
2.3.2. PCR Assays
2.4. Index Co-Infection (Ic)
2.5. Statistical Analysis
3. Results
3.1. Campylobacter spp.
3.2. Vibrio spp.
3.3. Enteropathogenic Shiga Toxin Producing Escherichia coli (STEC)
3.4. Salmonella spp.
3.5. Staphylococcus sp.
3.6. Pseudomonas spp.
3.7. Listeria monocytogenes, Mycobacterium sp., and Yersinia enterocolitica
3.8. Co-Infection and Index of Co-Infection (Ic)
4. Discussion
4.1. Salmonella spp.
4.2. Campylobacter spp.
4.3. Vibrio spp.
4.4. E. coli STEC
4.5. Staphylococcus sp.
4.6. Pseudomonas sp.
4.7. Listeria monocytogenes
4.8. Mycobacterium spp.
4.9. Yersinia enterocolitica
4.10. General
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skillings, D. Holobionts and the ecology of organisms: Multi-species communities or integrated individuals? Biol. Philos. 2016, 31, 875–892. [Google Scholar] [CrossRef]
- Capdevila-Argüelles, L.; Zilletti, B.; Suárez Álvarez, V.A. Causas de la pérdida de biodiversidad: Especies invasoras. Memorias R. Soc. Esp. Hist. Nat. 2013, 2, 10. [Google Scholar]
- Daszak, P.; Cunningham, A.A.; Hyatt, A.D. Emerging infectious diseases of wildlife—Threats to biodiversity and human health. Science 2000, 287, 443–449. [Google Scholar] [PubMed]
- Crump, J.A.; Murdoch, D.R.; Baker, M.G. Emerging infectious diseases in an island ecosystem: The New Zealand perspective. Emerg. Infect. Dis. 2001, 7, 767–772. [Google Scholar] [CrossRef]
- Mazza, G.; Tricarico, E.; Genovesi, P.; Gherardi, F. Biological invaders are threats to human health: An overview. Ethol. Ecol. Evol. 2014, 26, 112–129. [Google Scholar] [CrossRef]
- Young, H.S.; Parker, I.M.; Gilbert, G.S.; Guerra, A.S.; Nunn, C.L. Introduced species, disease ecology, and biodiversity–Disease relationships. Trends Ecol. Evol. 2017, 32, 41–54. [Google Scholar] [PubMed]
- Beever, R.; Waipara, N.; Ramsfield, T.D.; Dick, M.; Horner, I. Kauri (Agathis australis) under threat from Phytophthora. In Phytophthoras in Forests and Natural Ecosystems; Goheen, E., Frankel, S., Eds.; USDA/Forest Service, Pacific Southwest Research Station: Albany, CA, USA, 2009; pp. 74–85. [Google Scholar]
- Gobierno de Canarias. Available online: https://www.cienciacanaria.es/secciones/a-fondo/1174-invasoras-las-especies-que-acechan-en-las-islas (accessed on 19 September 2022).
- Medio Ambiente de Tenerife—Especies Exóticas Invasoras—Cabildo de Tenerife. Available online: https://www.tenerife.es/portalcabtfe/es/temas/medio-ambiente-de-tenerife/biodiversidad/especies-exoticas-invasoras (accessed on 19 September 2022).
- Gobierno de Canarias. Available online: https://www.biodiversidadcanarias.es/exos/especie/E09272 (accessed on 19 September 2022).
- Fritts, T.; Rodda, G. The role of introduced species in the degradation island ecosystems: A case history of Guam. Annu. Rev. Ecol. Syst. 1998, 29, 113–140. [Google Scholar] [CrossRef] [Green Version]
- Haddock, R.L.; Toda Nocon, F.A.; Santos, E.A.; Taylor, T.G. Reservoirs and vehicles of Salmonella infection on Guam. Environ. Int. 1990, 16, 11–16. [Google Scholar] [CrossRef]
- Beletsky, L. Hawaii: The Ecotravellers Wildlife Guide; Academic Press: London, UK, 2000; p. 416. [Google Scholar]
- Toda, M.; Takahashi, H.; Nakagawa, N.; Sukigara, N. Ecology and control of the green anole (Anolis carolinensis), an invasive alien species on the Ogasawara Islands. In Restoring the Oceanic Island Ecosystem; Kawakami, K., Okochi, I., Eds.; Springer: Tokyo, Japan, 2010; pp. 145–152. [Google Scholar]
- López, C.; Clemente, S.; Almeida, C.; Brito, A.; Hernández, M. A genetic approach to the origin of Millepora sp. in the eastern Atlantic. Coral. Reefs 2015, 34, 631–638. [Google Scholar]
- Wang, G.; Clark, C.G.; Taylor, T.M.; Pucknell, C.; Barton, C.; Price, L.; Woodward, D.L.; Rodgers, F.G. Colony Multiplex PCR Assay for Identification and Differentiation of Campylobacter jejuni, C. coli, C. lari, C. upsaliensis, and C. fetus subsp. Fetus. J. Clin. Microbiol. 2002, 40, 4744–4747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco, M.; Blanco, J.E.; Mora, A.; Dahbi, G.; Alonso, M.P.; González, E.A.; Bernárdez, M.I.; Blanco, J. Serotypes, virulence genes, and intimin types of shiga toxin (verotoxin)-producing Escherichia coli isolates from cattle in Spain and identification of a new intimin variant gene (eae-ξ). J. Clin. Microbiol. 2004, 42, 645–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Choi, Y.; Jeon, B.Y.; Jin, H.; Cho, S.N.; Lee, H. A simple and efficient Multiplex PCR assay for the identification of Mycobacterium genus and Mycobacterium tuberculosis complex to the species level. Yonsei Med. J. 2013, 54, 1220–1226. [Google Scholar] [CrossRef] [PubMed]
- Wannet, W.J.B.; Reessink, M.; Brunings, H.A.; Maas, H.M.E. Detection of pathogenic Yersinia enterocolitica by rapid and sensitive duplex PCR assay. J. Clin. Microbiol. 2001, 39, 4483–4486. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Yang, G.; Wang, H.; Chen, J.; Shi, X.; Zou, G.; Wei, Q.; Sun, X. Design of Vibrio 16S rRNA gene specific primers and their application in the analysis of seawater Vibrio community. J. Ocean Univ. China 2006, 5, 157–164. [Google Scholar]
- Neogi, S.B.; Chowdhury, N.; Asakura, M.; Hinenoya., A.; Haldar., S.; Saidi, S.M.; Kogure, K.; Lara, R.J.; Yamasaki, S. A highly sensitive and specific multiplex PCR assay for simultaneous detection of Vibrio cholerae, Vibrio parahaemolyticus and Vibrio vulnificus. Lett. Appl. Microbiol. 2010, 51, 293–300. [Google Scholar] [CrossRef]
- Guimarães de Freitas, C.; Patrícia Santana, A.; Helena Caldeira da Silva, P. Salvador Picão Gonçalves, V.; Ferreira Barros, M.A.; Gonçalves Torres, F.A.; Sayori Murata, L.; Perecmanis, S. PCR multiplex for detection of Salmonella Enteritidis, Typhi and Typhimurium and occurrence in poultry meat. Int. J. Food Microbiol. 2010, 139, 15–22. [Google Scholar] [CrossRef]
- Pérez-Roth, E.; Claverie-Martín, F.; Villar, J.; Méndez-Álvarez, S. Multiplex PCR for simultaneous identification of Staphylococcus aureus and detection of methicillin and mupirocin resistance. J. Clin. Microbiol. 2001, 39, 4037–4041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Vos, D.; Lim, A., Jr.; Pirnay, J.P.; Struelens, M.; Vandenvelde, C.; Duinslaeger, L.; Vanderkelen, A.; Cornelis, P. Direct detection and identification of Pseudomonas aeruginosa in clinical samples such as skin biopsy specimens and expectorations by Multiplex PCR Based on two outer membrane lipoprotein genes, oprI and oprL. J. Clin. Microbiol. 1997, 35, 1295–1299. [Google Scholar]
- Jaton, K.; Sahli, R.; Bille, J. Development of Polymerase Chain Reaction assays for detection of Listeria monocytogenes in clinical cerebrospinal fluid samples. J. Clin. Microbiol. 1992, 30, 1931–1936. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, H.S. Potential Effects of Mixed Infections in Ticks on Transmission Dynamics of Pathogens: Comparative Analysis of Published Records. Exp. Appl. Acarol. 2008, 46, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Home | Typhoid Fever | CDC. Available online: https://www.cdc.gov/typhoid-fever/ (accessed on 2 September 2021).
- Pedersen, K.; Lassen-Nielsen, A.M.; Nordentoft, S.; Hammer, A.S. Serovars of Salmonella from captive reptiles. Zoonoses Public Health 2009, 56, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Collard, P.; Montefiore, C. Agama as a reservoir of Salmonella infection in Ibadan. West Afr. Med. J. 1954, 5, 154–156. [Google Scholar]
- Kourany, M.; Telford, S.R. Lizards in the ecology of salmonelosis in Panama. Appl. Environ. Microbiol. 1981, 41, 1248–1253. [Google Scholar] [CrossRef]
- Gugnani, H.C.; Oguike, J.U.; Sakazaki, R. Salmonellae and other enteropathogenic bacteria in the intestines of wall geckos in Nigeria. Anton. Leeuw. 1986, 52, 117–120. [Google Scholar] [CrossRef]
- Cambre, R.C.; Green, D.E.; Smith, E.E.; Montali, R.J.; Bush, M. Salmonellosis and arizonosis in the reptile collection at the National Zoological Park. J. Am. Vet. Med. Assoc. 1980, 177, 800–803. [Google Scholar]
- Onderka, D.K.; Finlayson, M.C. Salmonella and salmonelosis in captive reptiles. Can. J. Comp. Med. 1985, 49, 268–270. [Google Scholar]
- Kalvig, B.A.; Maggio-Price, L.; Tsuji, J.; Giddens, W.E. Salmonellosis in laboratory-housed iguanid lizards (Sceloporus spp.). J. Wil. Dis. 1991, 2, 551–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baylet, R.; Diop, S.; Baylet, J.F. Simultaneous study of Salmonella infection in the population of eleven villages in Senegal and among lizards (species Agama). Pathol. Biol. 1981, 29, 617–619. [Google Scholar]
- Tauxe, R.V.; Rigau-Pérez, J.G.; Wells, J.G.; Blake, P.A. Turtle-associated salmonellosis in Puerto Rico: Hazards of the global turtle trade. J. Am. Med. Assoc. 1985, 254, 237–239. [Google Scholar] [CrossRef]
- Hoff, G.L.; White, F.H. Salmonella in reptiles: Isolation from free-ranging lizards (Reptilia, Lacertilia) in Florida. J. Herpetol. 1977, 11, 123–129. [Google Scholar] [CrossRef]
- Sumiyama, D.; Shimizu, A.; Kanazawa, T.; Anzai, H.; Murta, K. Prevalence of Salmonella in green anoles (Anolis carolinensis), an invasive alien species in Naha and Tomigusuku Cities, Okinawa Main Island, Japan. J. Vet. Med. Sci. 2020, 82, 678–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sumiyama, D.; Izumiya, H.; Kanazawa, T.; Murata, K. Salmonella infection in green anoles (Anolis carolinensis), an invasive alien species on Chichi Island of the Ogasawara archipelago in Japan. J. Vet. Med. Sci. 2014, 76, 461–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monzón Moreno, C.; Ojeda-Vargas, M.M.; Echeita, A.; Usera, M.A. Ocurrence of Salmonella in cold-blooded animals in Gran Canaria, Canary Islands. Ant. Leeuw. 1995, 68, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Ebani, V.V. Domestic reptiles as source of zoonotic bacteria: A mini review. Asian Pac. J. Trop. Med. 2017, 10, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Sumiyama, D.; Hayashida, I.; Kanazawa, T.; Anzai, H.; Murata, K. Prevalence and antimicrobial-resistance profiles of Salmonella spp. isolated from green anoles (Anolis carolinensis) collected on the Haha-jima of the Ogasawara archipelago, Japan. J. Vet. Med. Sci. 2020, 82, 1558–1561. [Google Scholar] [CrossRef]
- Kaakoush, N.O.; Castaño-Rodríguez, N.; Mitchell, H.M.; Man, S.M. Global epidemiology of Campylobacter infection. Clin. Microbiol. Rev. 2015, 28, 687–720. [Google Scholar] [CrossRef]
- Hsieh, Y.H.; Sulaiman, I.M. Campylobacteriosis: An emerging infectious foodborne disease. In Foodborne Diseases; Academic Press: Cambridge, MA, USA, 2018; pp. 119–155. [Google Scholar]
- Epps, S.V.; Harvey, R.B.; Hume, M.E.; Phillips, T.D.; Anderson, R.C.; Nisbet, D.J. Foodborne Campylobacter: Infections, metabolism, pathogenesis, and reservoirs. Int. J. Environ. Res. Public Health 2013, 10, 6292–6304. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, M.; Duim, B.; Zomer, A.L.; Wagenaar, A. Living in cold blood: Arcobacter, Campylobacter, and Helicobacter in reptiles. Front. Microbiol. 2019, 10, 1086. [Google Scholar] [CrossRef]
- Masila, N.M.; Ross, K.E.; Gardner, M.G.; Whiley, H. Zoonotic and public health implications of Campylobacter species and squamates (lizards, snakes and amphisbaenians). Pathogens 2020, 9, 799. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.J.; Miller, W.G.; Yee, E.; Kik, M.; Wagenaar, J.A.; Duim, B. Complete genome sequence of Campylobacter iguaniorum strain 1485ET, isolated from a bearded dragon (Pogona vitticeps). Genome Announc. 2014, 2, e00844-14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mihaljevic, R.R.; Sikic, M.; Klancnik, A.; Brumini, G.; Mozina, S.S.; Abram, M. Environmental stress factors affecting survival and virulence of Campylobacter jejuni. Microb. Pathog. 2007, 43, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Vitt, L.J.; Zug, G.R.; Caldwell, J.P. Herpetology: An Introductory Biology of Amphibians and Reptiles, 2nd ed.; Academic press: Waltham, MA, USA, 2001. [Google Scholar]
- Stintzi, A. Gene expression profile of Campylobacter jejuni in response to growth temperature variation. J. Bacteriol. 2003, 185, 2009–2016. [Google Scholar] [CrossRef] [Green Version]
- Tu, Z.C.; Zeitlin, G.; Gagner, J.P.; Keo, T.; Hanna, B.A.; Blaser, M.J. Campylobacter fetus of reptile origin as a human pathogen. J. Clin. Microbiol. 2004, 42, 4405–4407. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, M.J.; Miller, W.G.; Yee, E.; Kik, M.; Zomer, A.L.; Wagenaar, J.A.; Duim, B. Comparative genomics of Campylobacter iguaniorum to unravel genetic regions associated with reptilian hosts. Genome Biol. Evol. 2016, 8, 3022–3029. [Google Scholar] [CrossRef] [Green Version]
- Whiley, H.; McLean, R.; Ross, K. Detection of Campylobacter jejuni in lizard faeces from central Australia using quantitative PCR. Pathogens 2017, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- Garrity, G.; Bell, J.; Lilburn, T. Taxonomic Outline of the Prokaryotes. Bergey’s Manual of Systematic Bacteriology, 2nd ed.; Springer: New York, NY, USA, 2004; pp. 112–113. [Google Scholar]
- Austin, B.; Austin, D.A. Bacterial Fish Pathogens: Disease of Farmed and Wild Fish, 4th ed.; Springer: Berlin, Germany, 2007. [Google Scholar]
- Sack, R.B. A search for canine carriers of Vibrio. J. Infect. Dis. 1973, 127, 709–712. [Google Scholar] [CrossRef]
- Izquierdo-Rodríguez, E.; Martín-Carrillo, N.; Valladares, B.; Foronda, P. Study of zoonotic enteric pathogens of Atelerix algirus in Tenerife, Canary Islands, Spain. Front. Vet. Sci. 2020, 7, 579602. [Google Scholar] [CrossRef]
- Sanyal, S.C.; Singh, S.J.; Tiwari, P.C.; Sen, I.C.; Marwah, S.M.; Hazarika, U.R.; Singh, H.; Shimada, T.; Sakazaki, R. Role of household animals in maintenance of cholerae infection in a community. J. Infect. Dis. 1974, 130, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.G. Non-O group 1 Vibrio cholerae: A look at the epidemiology of an occasional pathogen. Epidemiol. Rev. 1990, 12, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Day, M.J.; Rodríguez, I.; van Essen-Zandbergen, A. Diversity of STs, plasmids and ESBL genes among Escherichia coli from humans, animals and food in Germany, the Netherlands and the UK. J. Antimicrob. Chemother. 2016, 71, 1178–1182. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, V.; Mock, R.; Burgkhardt, E.; Junghanns, A.; Ortlieb, F.; Szabo, I. Cloacal aerobic bacterial flora and absence of viruses in free-living slow worms (Anguis fragilis), grass snakes (Natrix natrix) and European Adders (Vipera berus) from Germany. EcoHealth 2014, 11, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.M.; Cowling, A. The distribution and genetic structure of Escherichia coli in Australian vertebrates: Host and geographic effects. Microbiol. Read. Engl. 2003, 149, 3575–3586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopee, N.V.; Adesiyun, A.A.; Caesar, K. A longitudinal study of Escherichia coli strains isolated from captive mammals, birds, and reptiles in Trinidad. J. Zoo Wildl. Med. Off. Publ. Am. Assoc. Zoo Vet. 2000, 31, 353–360. [Google Scholar]
- Martínez, R.; Sánchez, S.; Alonso, J.M.; Herrera-León, S.; Rey, J.; Echeita, M.A.; Morán, J.M.; García-Sánchez, A. Salmonella spp. and Shiga toxin-producing Escherichia coli prevalence in an ocellated lizard (Timon lepidus) research center in Spain. Foodborne Pathog. Dis. 2011, 8, 1309–1311. [Google Scholar] [CrossRef]
- Bautista-Trujillo, G.U.; Gutiérrez-Micele, F.A.; Mandujano-García, L.; Oliva-Llaven, M.A.; Ibarra-Martínez, C.; Mendoza-Nazar, P.; Ruiz-Sesma, B.; Tejeda-Cruz, C.; Pérez-Vázquez, L.C.; Pérez-Batrez, E.; et al. Captive green iguana is a reservoir of diarrheogenic Escherichia coli pathotypes. bioRixv 2019, 624544. [Google Scholar] [CrossRef] [Green Version]
- Dec, M.; Stepien-Pysniak, D.; Szczepaniak, K.; Turchi, B.; Urban-Chmiel, R. Virulence Profiles and Antibiotic Susceptibility of Escherichia coli Strains from Pet Reptiles. Pathogens 2022, 11, 127. [Google Scholar] [CrossRef]
- Abreu Acosta, N.; Martín Delgado, M.; Ortega Rivas, A.; del Castillo Remiro, A.; Aguiar González, E.; Valladares Hernández, B. Giardia lamblia and Cryptosporidium spp. presence in treated wastewater reutilised for irrigation in Tenerife Island, Spain. Long-distance transport effects in the reutilised water quality. Rev. Salud Ambient. 2002, 2, 2–7. [Google Scholar]
- Blanch, A.R.; García-Aljaro, C.; Muniesa, M.; Jofre, J. Detection, enumeration and isolation of strains carrying the stx2 gene from urban sewage. Water Sci. Technol. 2003, 47, 109–116. [Google Scholar] [CrossRef]
- García-Aljaro, C.; Muniesa, M.; Jofre, J.; Blanch, A. Prevalence of the stx2 gene in coliform populations from aquatic environments. Appl. Environ. Microbiol. 2004, 70, 3535–3540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taghadosi, R.; Shakibaie, M.R.; Alizade, H.; Hosseini-Nave, H.; Askari, A.; Ghanbarpour, R. Serogroups, subtypes and virulence factors of shiga toxin-producing Escherichia coli isolated from human, calves and goats in Kerman, Iran. Gastroenterol. Hepatol. Bed. Bench 2018, 11, 60–67. [Google Scholar] [PubMed]
- Orth, D.; Grif, K.; Khan, A.B.; Naim, A.; Dierich, M.P.; Würzner, R. The Shiga toxin genotype rather than the amount of Shiga toxin or the cytotoxicity of Shiga toxin in vitro correlates with the appearance of the hemolytic uremic syndrome. Diagn. Microbiol. Infect. Dis. 2007, 59, 235–242. [Google Scholar] [CrossRef]
- Louie, M.; de Azavedo, J.C.; Handelsman, M.Y.; Clark, C.G.; Ally, B.; Dytoc, M.; Sherman, P.; Brunton, J. Expression and characterization of the eaeA gene product of Escherichia coli serotype O157:H7. Infect Immun. 1993, 61, 4085–4092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EFSA Panel on Biological Harzards (BIOHAZ). Scientific Opinion on VTEC-seropathotype and scientific criterio regarding pathogenicity assessment. EFSA J. 2013, 11, 3138. [Google Scholar] [CrossRef]
- Lazić, M.M.; Carretero, M.A.; Mihailov-Krstev, T.; Lazarecvić-Macanović, M.; Krstić, N.; Crnobrnja-Isailović, J. Incidence patterns of ectodermic lesions in wild populations of common wall lizard (Podarcis muralis). Ambiphia-Reptil. 2012, 33, 327–336. [Google Scholar] [CrossRef] [Green Version]
- Naldo, J.L.; Libanan, N.L.; Samour, J.H. Health assessment of a spiny-tailed lizard (Uromastyx spp.) population in Abu Dhabi, United Arab Emirates. J. Zoo Wildl. Med. 2009, 40, 445–452. [Google Scholar] [CrossRef]
- Al-Taii, N.A.; Khalil Hoff, N.K.; Abd Al-Rudha, A.M.H. Pathogenic bacteria isolated from Hemidactylus turcicus in Baghdad Province, Iraq. J. Entomol. Zool. Stud. 2017, 5, 1348–1350. [Google Scholar]
- Martínez-Silvestre, A.; Silveira, L.; Mateo, J.A.; Urioste, J.; Rodríguez-Domínguez, M.A.; Pether, J. Microbiología cloacal en lagartos gigantes amenazados de las Islas Canarias (género Gallotia) en cautividad. Rev. Esp. Herp. 2003, 17, 29–37. [Google Scholar]
- Piette, A.; Verschraegen, G. Role of coagulase-negative staphylococci in human disease. Vet. Microbiol. 2009, 134, 45–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, K.; Heilmann, C.; Peters, G. Coagulase-negative staphylococci. Clin. Microbiol. Rev. 2014, 27, 870–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaplan, M.; Jereb, R. Ulcerative stomatitis (Mouthrot) in reptiles. J. Wildl. Rehabil. 1995, 18, 13. [Google Scholar]
- Cercenado, E. Staphylococcus lugdunensis: Un estafilococo coagulasa negativo diferente de los demás. Enferm. Infecc. 2009, 27, 139–142. [Google Scholar] [CrossRef]
- Peix, A.; Ramirez-Bahena, M.H.; Velazquez, E. Historical evolution and current status of the taxonomy of genus Pseudomonas. Infect. Genet. Evol. 2009, 9, 1132–1147. [Google Scholar] [CrossRef] [PubMed]
- Spiers, A.J.; Buckling, A.; Rainey, P.B. The causes of Pseudomonas diversity. Microbiology 2000, 146, 2345–2350. [Google Scholar] [CrossRef] [Green Version]
- Palamthodi, S.M.; Gaikwad, V.J.; Ghasghase, N.V.; Patil, S.S. Antibacterial targets in Pseudomonas aeruginosa. Int. J. Pharmacol. 2011, 2, 159–164. [Google Scholar]
- Cushing, A.; Pinborough, M.; Stanford, M. Review of bacterial and fungal culture and sensitivity results from reptilian samples submitted to a UK laboratory. Vet. Rec. 2011, 169, 390. [Google Scholar] [CrossRef]
- Ajayi, J.O.; Ogunleye, A.O.; Happi, A.N.; Okunlade, A.O. Bacteria Isolated from the Oral and Cloaca Swabs of Lizards co-habitating with Poultry in Some Poultry Farms in Ibadan, Oyo State, Nigeria. Afr. J. Biomed. Res. 2015, 18, 211–215. [Google Scholar]
- Dipineto, L.; Raia, P.; Varriale, L.; Borrelli, L.; Botta, V.; Serio, C.; Capasso, M.; Rinaldi, L. Bacteria and parasites in Podarcis sícula and P. sícula klemmerii. BMC Vet. Res. 2018, 14, 392–398. [Google Scholar] [CrossRef] [Green Version]
- Graves, S.R.; Rawlinson, P.A.; Kennelly-Merrit, S.A.; McLaren, D.A.; Harvey, K.J.; Thornton, W.B. Enteric bacteria of reptiles on Java and Krakatau Islands. Phil. Trans. R. Soc. Lond. B 1988, 322, 355–361. [Google Scholar]
- Abreu-Acosta, N. Protozoos Emergentes en Canarias. Ph. D. Thesis, Universidad de La Laguna, La Laguna, Spain, 2009. [Google Scholar]
- Colinon, C.; Jocktane, D.; Brothier, E.; Rossolini, G.M.; Cournoyer, B.; Nazaret, S. Genetic analyses of Pseudomonas aeruginosa isolated from healthy captive snakes: Evidence of high inter-and intrasite dissemination and occurrence of antibiotic resistance genes. Environ. Microbiol. 2010, 12, 716–729. [Google Scholar] [CrossRef] [PubMed]
- Frye, F.L. Biomedical and Surgical Aspects of Captive Reptile Husbandry; Krieger Publishing Co.: Malabar, FL, USA, 1991; Volume 1. [Google Scholar]
- Girling, S.J.; Fraser, M. Listeriosis in an Inland Bearded Dragon, Pogona vitticeps. J. Herpetol. Med. Surg. 2014, 14, 6–9. [Google Scholar] [CrossRef] [Green Version]
- Vancraeynest, D.; Pasmans, F.; De Graef, E.; Hermans, K.; Decostere, A. Listeria monocytogenes associated myocardial perforation in a bearded dragon (Pogona vitticeps). Vlaams Diergeneeskd. Tijdschr. 2006, 75, 232–234. [Google Scholar]
- Soldati, G.; Lu, Z.H.; Vaughan, L.; Polkinghorne, A.; Zimmermann, D.R.; Huder, J.B.; Pospischil, A. Detection of Mycobacteria and Chlamydiae in granulomatous inflammation of reptiles: A retrospective study. Vet. Pathol. 2004, 41, 388–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slany, M.; Knotek, Z.; Skoric, M.; Knotkova, Z.; Svobodova, J.; Mrlik, V.; Moravkova, M.; Pavlik, I. Systemic mixed infection in a brown caiman (Caiman crocodilus fuscus) caused by Mycobacterium szulgai and M. chelonae: A case report. Vet. Med. 2010, 55, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Reil, I.; Špičić, S.; Kompes, G.; Duvnjak, S.; Zdelar-Tuk, M.; Stojević, D.; Cvetnić, Ž. Nontuberculous mycobacteria in captive and pet reptiles. Acta Vet. BRNO 2017, 86, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Ebani, V.V.; Fratini, F.; Bertelloni, F.; Cerri, D.; Tortoli, E. Isolation and identification of mycobacteria from captive reptiles. Res. Veter. Sci. 2012, 93, 1136–1138. [Google Scholar] [CrossRef]
- Girling, S.; Fraser, M. Systemic mycobacteriosis in an inland bearded dragon (Pogona vitticeps). Vet. Rec. 2007, 160, 526–528. [Google Scholar] [CrossRef]
- Yang, H.; Gu, W.; Qiu, H.; Sun, G.; Liang, J.; Li, K.; Xiao, Y.; Duan, R.; Jing, H.; Wang, X. Comparison of Growth and the Cytokines Induced by Pathogenic Yersinia enterocolitica Bio-Serotypes 3/O: 3 and 2/O: 9. Front. Cell. Infect. Microbiol. 2017, 7, 158. [Google Scholar] [CrossRef] [Green Version]
- Risco, D.; García, A.; Serrano, E.; Fernández-Llario, P.; Benítez, J.M.; Martínez, R.; García, W.L.; de Mendoza, J.H. High-density dependance but low impact of selected reproduction parameters of Brucella suis Biovar 3 in wild boar Hunting Estates from South-Western Spain. Transbound Emerg. Dis. 2013, 61, 555–6224. [Google Scholar] [CrossRef] [PubMed]
- Buendia, A.; Fallon, P.G.; Del Rio, L.; Ortega, N.; Caro, M.R.; Gallego, M.C.; Salinas, J. Previous infection with the nematode Nippostrongylus brasiliensis alters the immune specific response against Chlamydophila abortus infection. Microb. Pathog. 2002, 33, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Dalla Valle, L.; Benato, F.; Maistro, S.; Quinzani, S.; Alibardi, L. Bioinformatic and molecular characterization of betadefensins-like peptides isolated from the green lizard Anolis carolinensis. Dev. Comp. Immunol. 2012, 36, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.R.; Singh, V.; Ebibeni, N.; Singh, R.K. Maternal transfer of bacteria to eggs of common house gecko (Hemidactylus frenatus). J. Micro. Res. 2014, 4, 78–85. [Google Scholar]
- Jenssen, T.A.; Greenberg, N.; Hovde, K.A. Behavioral profile of free-ranging male lizards, Anolis carolinensis, across breeding and post-breeding seasons. Herpetol. Monogr. 1995, 9, 41–62. [Google Scholar] [CrossRef]
- Nunez, S.C.; Jenssen, T.A.; Ersland, K. Female activity profile of a polygynous lizard (Anolis carolinensis): Evidence of intersexual asymmetry. Behaviour 1997, 134, 205–223. [Google Scholar] [CrossRef]
- Losos, J. Lizards in an Evolutionary Tree: Ecology and Adaptive Radiation of Anoles; University of California Press: Berkeley, CA, USA, 2009; p. 528. [Google Scholar]
- Otokunefor, T.V.; Kindzeka, B.I.; Ubitey, I.O.; Osuji, G.U.; Obi, F.O.; Jack, A.W.K. Salmonella in gut and droppings of three pest lizards in Nigeria. World J. Micro. Biotech. 2003, 19, 545–548. [Google Scholar] [CrossRef]
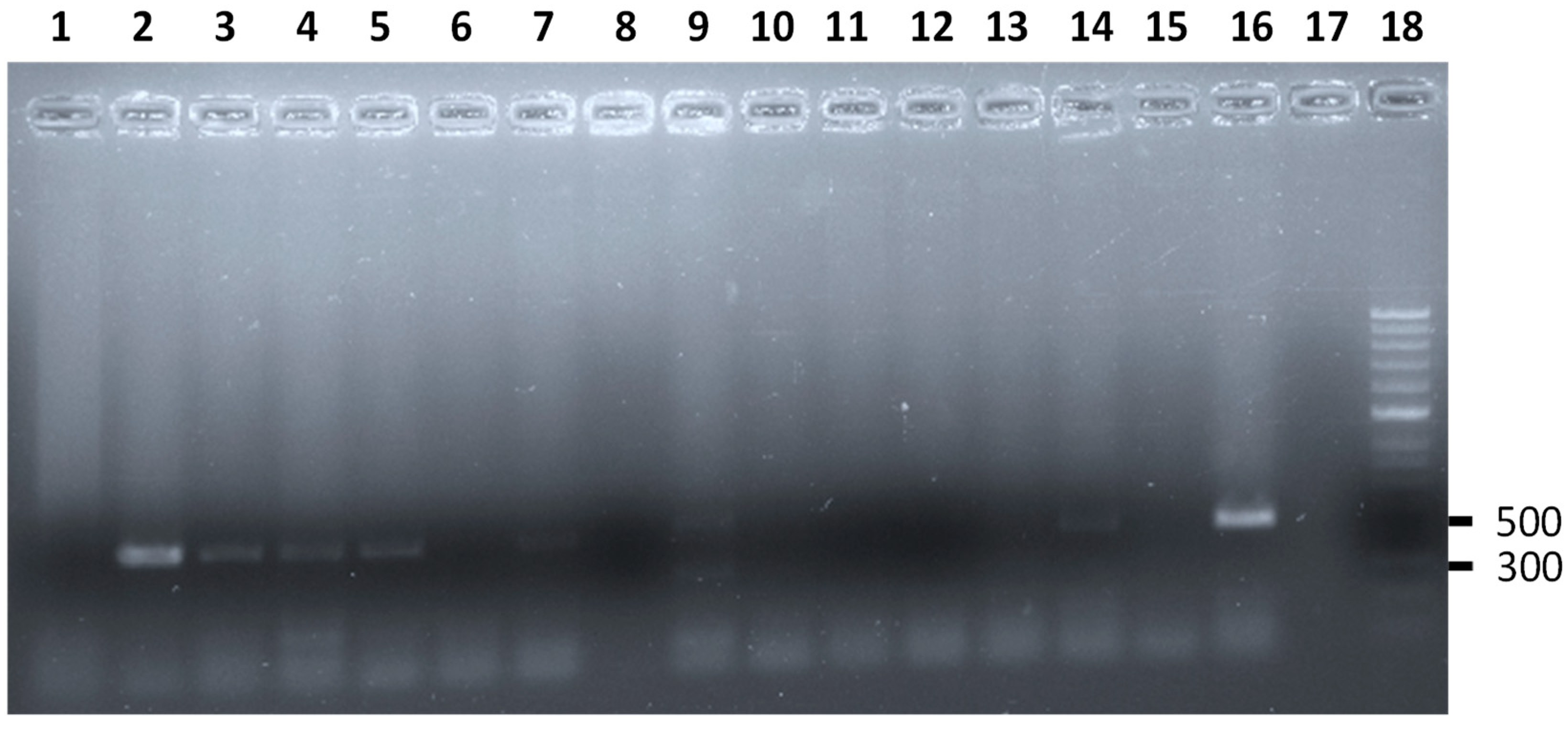
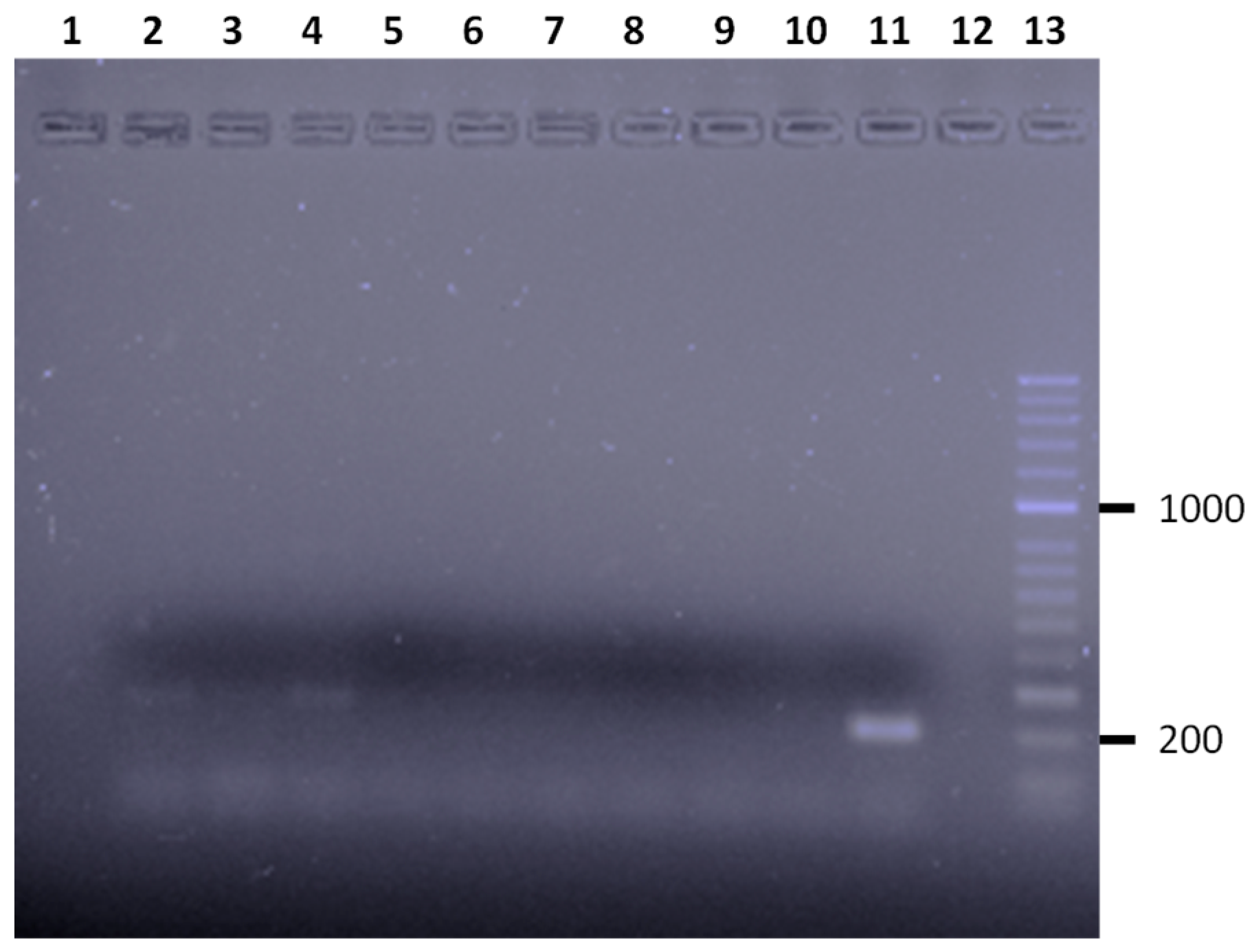
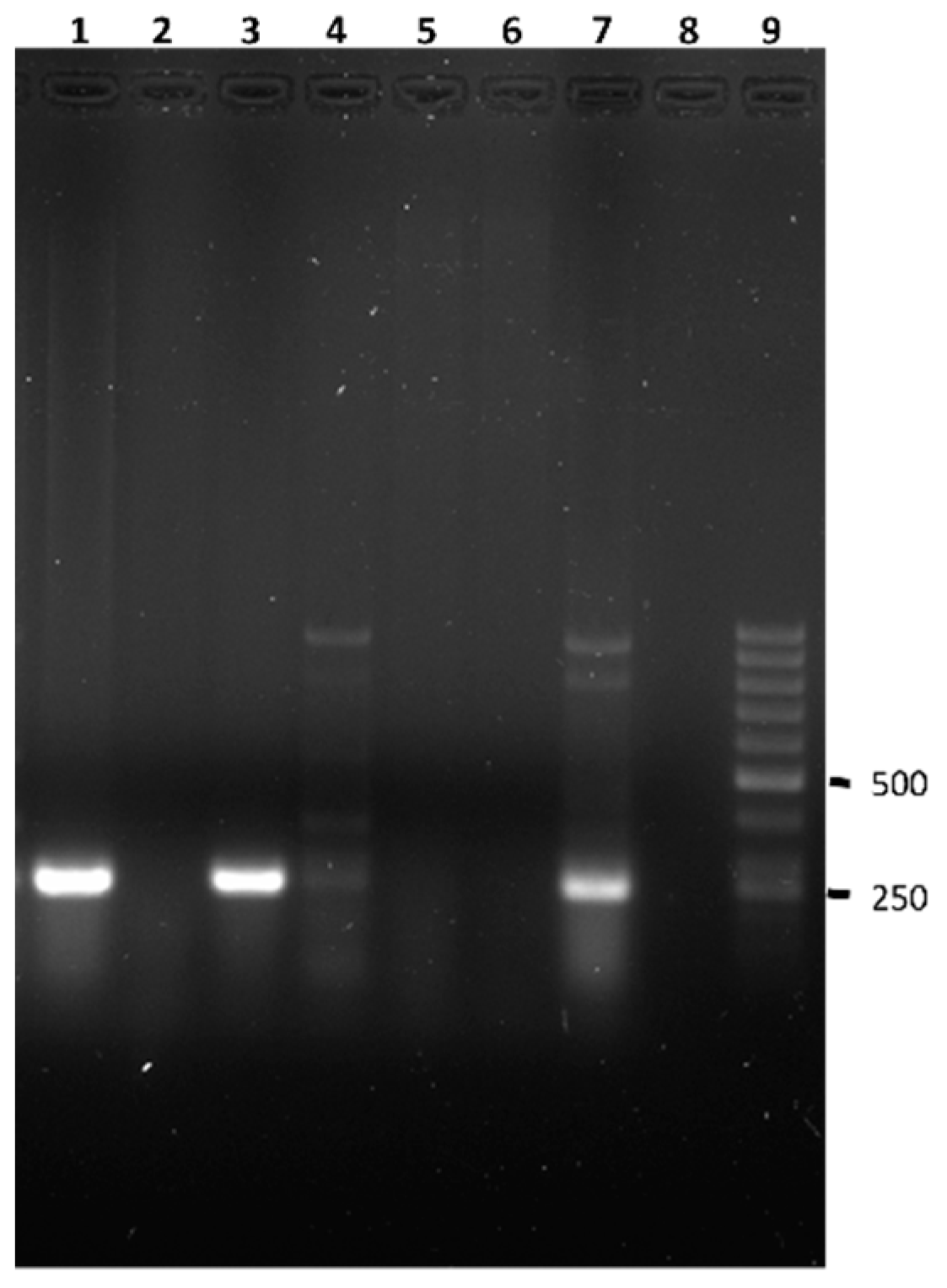
| Bacteria Type | No. of Isolates (n) | Prevalence (%) (CI 95%) |
|---|---|---|
| Pseudomonas spp. | 18 (28) | 64.3 (45.5–82.1) |
| E. coli (stx1/stx2/eae genes) | 33 (74) | 44.6 (38.8–50.3) |
| Campylobacter spp. | 24 (74) | 32.4 (21.4–42.6) |
| Staphylococcus lugdunensis | 11 (74) | 14.9 (10.7–19.1) |
| Vibrio spp. | 8 (74) | 10.8 (3.7–17.8) |
| Salmonella spp. | 4 (74) | 5.4 (4.4–6.6) |
| Listeria monocytogenes | 0 (74) | 0.00 |
| Yersinia enterocolitica | 0 (74) | 0.00 |
| Mycobacterium spp. | 0 (74) | 0.00 |
| Campylobacter Species | Positive Males (Prevalence) (CI 95%) n = 25 | Positive Females (Prevalence) (CI 95%) n = 45 | Positive Juveniles (Prevalence) (CI 95%) n = 4 | Total of Positive Individuals (Prevalence) (CI 95%) n = 74 |
|---|---|---|---|---|
| Campylobacter fetus | 2 (8.0%) (2.6–18.6) | 2 (4.4%) (1.6–10.4) | 2 (50.00%) (1.0–99.0) | 6 (8.1%) (1.9–14.3) |
| Campylobacter coli | 0 | 5 (11.1%) (1.9–20.3) | 0 | 5 (6.8%) (1.0–12.5) |
| Campylobacter jejuni | 3 (12.0%) (0.7–24.7) | 1 (2.2%) (0.0–6.5) | 0 | 4 (5.4%) (0.2–10.5) |
| Campylobacter upsaliensis | 2 (8.0%) (2.6–18.6) | 1 (2.2%) (0.0–6.5) | 0 | 3 (4.1%) (0.0–8.5) |
| Campylobacter lari | 0 | 0 | 0 | 0 |
| Virulence Genes | Positive Males (Prevalence) (CI 95%) n = 25 | Positive Females (Prevalence) (CI 95%) n = 45 | Positive Juveniles (Prevalence) (CI 95%) n = 4 | Total Positive Individuals (Prevalence) (CI 95%) n = 74 |
|---|---|---|---|---|
| stx1 | 4 (16.0%) (1.6–30.3) | 4 (0.5–17.1) | 0 | 8 (10.8%) (3.7–17.9) |
| stx2 | 6 (24.0%) (7.3–40.7) | 14 (17.6–44.6) | 1 (25.0%) (0.0–67.4) | 21 (28.8%) (18.1–38.7) |
| eae | 1 (4.0%) (3.0–11.7) | 2 (4.4%) (0.0–5.9) | 1 (25.0%) (0.0–67.4) | 4 (5.4%) (0.3–10.5) |
| No. of Individuals | Mixed Infection of Bacteria/Virulence Gen | Percentage % |
|---|---|---|
| 7 | Campylobacter spp. + stx2 gene | 30.4 |
| 6 | Campylobacter spp. + Staphylococcus lugdunensis | 26.1 |
| 5 | Campylobacter spp. + stx1 gene | 21.7 |
| 3 | Campylobacter spp. + Salmonella spp. | 13.0 |
| 3 | stx2 gene + Vibrio spp. | 13.0 |
| 3 | stx2 gene + Staphylcoccus lugdunensis | 13.0 |
| 2 | Campylobacter spp. + Vibrio spp. | 8.7 |
| 2 | stx1 gene + Staphylcoccus lugdunensis | 8.7 |
| 2 | stx1 gene + stx2 gene | 8.7 |
| 1 | Campylobacter spp. + eae gene | 4.4 |
| 1 | stx1 gene + Salmonella spp. | 4.4 |
| 1 | stx1 gene + Vibrio spp. | 4.4 |
| 1 | Staphylcoccus lugdunensis + Vibrio spp. | 4.4 |
| Mixed Infection of Bacteria/Virulence Gen | Ic | Chi-Square |
|---|---|---|
| Campylobacter spp. + stx2 gen | 0.28 | 0.29 |
| Campylobacter spp. + Staphylococcus lugdunensis | 4.80 | 6.32 * |
| Campylobacter spp. + stx1 gen | 16.92 | 10.2 * |
| Campylobacter spp. + Salmonella spp. | 10.48 | 17.6 * |
| stx2 gen + Vibrio spp. | 7.80 | 7.24 * |
| stx2 gen + Staphylcoccus lugdunensis | 2.86 | 3.98 |
| Campylobacter spp. + Vibrio spp. | −1.96 | 10.2 * |
| stx1 gen + Staphylcoccus lugdunensis | 10.23 | 0.54 |
| stx1 gen + stx2 gen | −1.00 | 7.24 * |
| Campylobacter spp. + eae gen | −0.93 | 17.1 * |
| stx1 gen + Salmonella spp. | 5.18 | 1.44 |
| stx1 gen + Vibrio spp. | 2.26 | 0.00 |
| Staphylcoccus lugdunensis + Vibrio spp. | −1.05 | 0.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abreu-Acosta, N.; Pino-Vera, R.; Izquierdo-Rodríguez, E.; Afonso, O.; Foronda, P. Zoonotic Bacteria in Anolis sp., an Invasive Species Introduced to the Canary Islands (Spain). Animals 2023, 13, 414. https://doi.org/10.3390/ani13030414
Abreu-Acosta N, Pino-Vera R, Izquierdo-Rodríguez E, Afonso O, Foronda P. Zoonotic Bacteria in Anolis sp., an Invasive Species Introduced to the Canary Islands (Spain). Animals. 2023; 13(3):414. https://doi.org/10.3390/ani13030414
Chicago/Turabian StyleAbreu-Acosta, Néstor, Román Pino-Vera, Elena Izquierdo-Rodríguez, Oscar Afonso, and Pilar Foronda. 2023. "Zoonotic Bacteria in Anolis sp., an Invasive Species Introduced to the Canary Islands (Spain)" Animals 13, no. 3: 414. https://doi.org/10.3390/ani13030414






