Helminth Community Structure of Tits Cyanistes caeruleus and Parus major (Paridae) during Their Autumn Migration on the Southern Baltic Coast
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Sampling
2.2. Sample Processing and Parasite Identification
2.3. Data Analysis
3. Results
3.1. Faunistic and Molecular Analyses
3.2. Structure of the Component Communities and Infracommunities of Helminths
3.3. Helminth Infection According to Host Age and Sex
4. Discussion
4.1. Similarities and Differences in Infection of Eurasian Blue Tits and Great Tits: Different Parasite Composition but Similar Helminth Community Structure
- -
- -Both species of birds prefer similar biotopes, such as forests, parks, and gardens, and they can both be found in the same areas.
- -
- -The birds’ food and feeding behaviour are very similar, and their nesting and breeding strategy are essentially identical.
- -
- -At their wintering grounds, the two species form mixed flocks and winter in exactly the same places.
- -
- -The origins of the captured populations and their flight routes are the same, except that, in general, that of Eurasian blue tits is statistically shorter.
- -
- -Nematodes C. pavlovskyi and trematodes L. paradoxum have broad host specificity and are known as parasites of many birds of the order Passeriformes.
- -
- The larger number of helminth species found in the Great tit was linked to the larger sample size; more than twice as many Great tits were examined as Eurasian blue tits.
- -
- Although both species prefer similar biotopes, the Great tit shows greater ecological tolerance regarding habitat conditions, which means that it may also inhabit poorer environments, e.g., coniferous forests or urban areas. The Eurasian blue tit is more closely associated with broadleaf forests and parks. A key to understanding the differences may be their feeding sites. The Great tit prefers feeding on the ground, while the Eurasian blue tit avoids foraging on the ground and prefers to penetrate tree crowns and shrubs.
- -
- The Great tit’s higher tolerance for habitat conditions may result in more frequent contact with parasites present in the environment, as well as with their intermediate hosts, which may partly explain the significantly higher prevalence of helminths, mainly L. paradoxum (but also U. macrostomus), and the significantly higher mean abundance of helminths in the Great tit population than in the Eurasian blue tit population.
4.2. Presence of Parasites in the Geographical Distribution of C. careuleus and P. major
4.3. Similarities and Differences in Juvenile and Adult Tits and in Males and Females
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hahn, S.; Bauer, S.; Liechti, F. The natural link between Europe and Africa—2.1 billion birds on migration. Oikos 2009, 118, 624–626. [Google Scholar] [CrossRef]
- Fuller, T.; Bensch, S.; Müller, I.; Novembre, J.; Pérez-Tris, J.; Ricklefs, R.E.; Smith, T.B.; Waldenström, J. The ecology of emerging infectious diseases in migratory birds: An assessment of the role of climate change and priorities for future research. Ecohealth 2012, 9, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Garamszegi, L.Z. Climate change increases the risk of malaria in birds. Glob. Chang. Biol. 2011, 17, 1751–1759. [Google Scholar] [CrossRef]
- López, G.; Muñoz, J.; Soriguer, R.; Figuerola, J. Increased Endoparasite Infection in Late-Arriving Individuals of a Trans-Saharan Passerine Migrant Bird. PLoS ONE 2013, 8, e61236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilgūnas, M.; Palinauskas, V.; Platonova, E.; Iezhova, T.; Valkiūnas, G. The experimental study on susceptibility of common European songbirds to Plasmodium elongatum (lineage pGRW6), a widespread avian malaria parasite. Malar. J. 2019, 18, 290. [Google Scholar] [CrossRef]
- Hauptmanová, K.; Benedikt, V.; Literak, I. Blood Parasites in Passerine Birds in Slovakian East Carpathians. Acta Protozool. 2006, 45, 105–109. [Google Scholar]
- Bandelj, P.; Blagus, R.; Trilar, T.; Vengust, M.; Vergles Rataj, A. Influence of phylogeny, migration and type of diet on the presence of intestinal parasites in the faeces of European passerine birds (Passeriformes). Wildl. Biol. 2015, 21, 227–233. [Google Scholar] [CrossRef]
- Gruianu, A.; Ionita, M.; Fasola-Matasaru, L.; Tibu, P.L.; Mitera, I.L. A survey on ecto- and endoparasites in some migratory birds in the Danube Delta, Romania. Vet. Med. C 2017, 63, 109–114. [Google Scholar]
- Tomiałojć, L. An East-West gradient in the breeding distribution and species richness of the European woodland avifauna. Acta Ornithol. 2000, 35, 3–17. [Google Scholar]
- Kuczyński, L.; Chylarecki, P. Atlas of Common Breeding Birds in Poland: Distribution, Habitat Preferences and Population Trends; GIOŚ: Warsaw, Poland, 2012. [Google Scholar]
- Nowakowski, J.K. Catch numbers at ringing stations in a reflection of bird migration intensity, as exemplified by autumn movements of the great tit (Parus major). Ring 2003, 25, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Nowakowski, J.K.; Chruściel, J. Speed of autumn migration of the Blue Tit (Parus caeruleus) along the eastern and southern Baltic coast. Ring 2004, 26, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Busse, P.; Meissner, W. Bird Ringing Station Manual; De Gruyter Open Ltd.: Berlin, Germany, 2015. [Google Scholar]
- Okulewicz, A.; Okulewicz, J. Great Counting—Do We Know about Bird Parasites of Poland? In Ornitologia Polska na Progu XXI Stulecia–Dokonania i Perspektywy; Sekcja Ornitologiczna PTZool; Uniwersytet Warmińsko-Mazurski w Olsztynie: Olsztyn, Poland, 2006; pp. 135–145. [Google Scholar]
- Awad, S.; Rząd, I. Jericho (Palestine) spring 2014 ornithological and parasitological research results. Ring 2014, 36, 33–43. [Google Scholar] [CrossRef] [Green Version]
- Sitko, J.; Zaleśny, G. The effect of urbanzation on helminth communities in the Eurasian blackbird (Turdus merula L.) from the eastern part of the Czech Republic. J. Helminthol. 2014, 88, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Kalisińska, E.; Rząd, I.; Sitko, J.; Kavetska, K.M.; Królaczyk, K.; Budis, H. Digenea of Haliaeetus albicilla (Linnaeus, 1758) and Pandion haliaetus (Linnaeus, 1758) from central and northwestern Poland. Ann Parasitol. 2008, 54, 349–351. [Google Scholar]
- Hromada, M.; Dudiňák, V.; Yosef, R. An inside perspective of the true shrikes—A revive of the helminthofauna. Ring 2000, 22, 85–294. [Google Scholar]
- Sepulveda, M.S.; Kinsella, J.M. Helminth Collection and Identification from Wildlife. J. Vis. Exp. 2013, 82, e51000. [Google Scholar] [CrossRef] [Green Version]
- Gibson, D.I.; Jones, A.; Bray, R.A. Keys to the Trematoda; CAB International Publishing and The Natural History Museum: Wallingford, UK, 2002; Volume 1. [Google Scholar]
- Anderson, R.C.; Chabaud, A.G.; Willmott, S. Keys to the Nematode Parasites of Vertebrates; Archival Volume (Nos. 1–10); CAB International Publishing and The Natural History Museum: Wallingford, UK, 2009. [Google Scholar]
- Yamaguti, S. Systema Helminthum; Interscience Publishers Inc.: New York, NY, USA, 1958; Volume I, Parts 1 and 2. [Google Scholar]
- Yamaguti, S. Synopsis of Digenetic Treamatodes of Verebrates; Keiagu Publishing Company: Tokyo, Japan, 1971; Volumes I and II. [Google Scholar]
- Niewiadomska, K.; Pojmańska, T. Przywry Trematoda. Część Systematyczna Digenea: Plagiorchiida; Wydawnictwo Uniwersysteu Łódzkiego: Łódź, Poland, 2018. [Google Scholar]
- Kornyushin, V.V. Fauna of Ukraine. Volume 33. Monogenea and Cestoda. Part 3. Davaineoidea. Biuterinoidea. Paruterinoidea; Naukova Dumka: Kyiv, Ukraine, 1989; 252p. [Google Scholar]
- Komisarovas, J.; Georgiev, B.B. Redescriptions of Monosertum parinum (Dujardin, 1845) and M. mariae (Mettrick, 1958) n. comb. from European passerine birds, with an amended generic diagnosis of Monosertum Bona, 1994 (Cestoda: Dilepididae). Syst. Parasitol. 2007, 66, 43–53. [Google Scholar] [CrossRef]
- Bowles, J.; Blair, D.; McManus, D.P. Genetic variants within the genus Echinococcus identified by mitochondrial DNA sequencing. Mol. Biochem. Parasit. 1992, 54, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Heneberg, P.; Sitko, J.; Bizos, J. Molecular and comparative morphological analysis of central European parasitic flatworms of the superfamily Brachylaimoidea Allison, 1943 (Trematoda: Plagiorchiida). Parasitology 2016, 143, 455–474. [Google Scholar] [CrossRef] [PubMed]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [Green Version]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamura, K.; Dudley, J.; Nei, M.; Kumar, S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007, 24, 1596–1599. [Google Scholar] [CrossRef]
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology meets ecology on its terms: Margolis et al. revisited. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef]
- Magurran, A.E. Measuring Biological Diversity; Blackwell: Oxford, UK, 2004. [Google Scholar]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleonthological Statistics softwere package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. Available online: http://palaeo-electronica.org (accessed on 1 October 2022).
- Okulewicz, A.; Sitko, J. Parasitic helminthes—Probable cause of death of birds. Helminthologia 2012, 49, 241–246. [Google Scholar] [CrossRef] [Green Version]
- Schoenaer, E.R.; Alley, M.R.; Howe, L.; Charleston, T.; Castro, I. Helminths in endemic, native and introduced passerines in New Zeeland. N. Zealand J. Zool. 2012, 39, 245–256. [Google Scholar] [CrossRef] [Green Version]
- Pojmańska, T.; Niewiadomska, K.; Okulewicz, A. Pasożytnicze Helminty Polski. Gatunki, Żywiciele, Białe Plamy; Polskie Towarzystwo Parazytologiczne: Warszawa, Poland, 2007; p. 360. [Google Scholar]
- Freeland, W.J. Parasites and the Coexistence of Animal Host Species. Am. Nat. 1983, 121, 223–236. [Google Scholar] [CrossRef]
- Okulewicz, A. Pasożyty Ptaków. Nicienie—Nematoda. Katalog fauny Pasożytniczej Polski; Zeszyt 2B: Warszawa, Poland, 1997; p. 149. [Google Scholar]
- Saparov, K.; Akramova, F.; Azimov, D.; Golovanov, V.; Kuchboev, A. Biodiversity of filarie (Nematoda: Filariata), parasites of birds in Uzbekistan. Turk. Zool. Derg. 2013, 37, 746–752. [Google Scholar] [CrossRef]
- Syrota, Y.Y.; Korol, E.M.; Kuzmin, Y.I. New records of helminths of the corncrake, Crex crex (Aves, Rallidae) from Ukraine. Zoodiversity 2020, 54, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Anderson, R.C. Nematode Parasites of Vertebrates. Their Development and Transmission, 2nd ed.; CABI Publishing: Wallingford, Oxon, UK, 2000. [Google Scholar]
- Rietschel, G. Untersuchungen über Farbmuster-Typen bei Leucochloridium—Sporozysten (Trematoda, Brachylaimiidae) und deren Zugehörigkeit zu bestimmten Arten. Z. FüR Parasitenkd. 1972, 40, 61–68. [Google Scholar] [CrossRef]
- Bakke, T.A. A revision of the family Leucochloridiidae Poche (Digenea) and studies of the morphology of Leucochloridium paradoxum Carus, 1835. Syst. Parasitol. 1980, 1, 189–202. [Google Scholar] [CrossRef]
- Okulewicz, A. Parasitic nematodes of birds from family Paridae in Poland. Wiad. Parazytol. 1991, 37, 491–498. [Google Scholar] [PubMed]
- Nowak, M.A.; May, R.M. Superinfection and the evolution of parasite virulence. Proc. R Soc. Lond. B 1994, 255, 81–89. [Google Scholar] [CrossRef]
- Sitko, J.; Faltýnková, A.; Scholz, T. Checklist of the Trematodes (Digenea) of Birds of the Czech and Slovak Republics; Academia: Praha, Czech Republic, 2006; p. 112. [Google Scholar]
- Rząd, I.; Sitko, J.; Wysocki, D.; Stępniewski, K. Digenean trematodes from six species of birds (Passeriformes, Piciformes and Strigiformes) from north-western Poland. Ann. Parasitol. 2011, 57, 271–276. [Google Scholar]
- Rząd, I.; Hofsoe, P.; Panicz, R.; Nowakowski, J.K. Morphological and molecular characterization of adult worms of Leucochloridium paradoxum Carus, 1835 and L. perturbatum Pojmańska, 1969 (Digenea: Leucochloridiidae) from the great tit, Parus major L., 1758 and similarity with the sporocyst stages. J. Helminthol. 2014, 88, 506–510. [Google Scholar] [CrossRef]
- Iskova, N.I.; Sharpilo, L.D.; Tkach, V.V. Catalogue of Helminths of the Ukraine: Trematodes of Terrestrial Vertebrates; Naukova Dumka: Kiev, Ukraine, 1995; pp. 3–93. [Google Scholar]
- Bykhovskaya-Pavlovskaya, I.E. Trematodes of Birds in the Fauna of the USSR; Izdatel’stvo Akademii Nauk: Moscow and Leningrad, Russia, 1962; p. 407. [Google Scholar]
- Kirillowa, N.Y.U.; Kirillow, A.A. Structure and seasonal dynamics of helminth fauna of the great tit Parus major (Passeriformes, Paridae) from Samarskaya Luka. Russ. J. Parasitol. 2018, 12, 35–40. [Google Scholar] [CrossRef]
- Hildebrand, J.; Pyrka, E.; Sitko, J.; Jeżewski, W.; Zaleśny, G.; Tkach, V.; Laskowski, Z. Molecular phylogeny provides new insights on the taxonomy and composition of Lyperosomum Looss, 1899 (Digenea, Dicrocoeliidae) and related genera. Int. J. Parasitol. Parasites Wildl. 2019, 9, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Heneberg, P.; Sitko, J.; Bizos, J. Integrative taxonomy of central European parasitic flatworms of the family Prosthogonimidae Lühe, 1909 (Trematoda: Plagiorchiida). Parasitol. Int. 2015, 64, 264–273. [Google Scholar] [CrossRef]
- Helminthological Collection of the Institute of Parasitology; Biology Centre CAS: České Budějovice, Czech Republic, 2022; Available online: https://www.paru.cas.c (accessed on 1 October 2022).
- Haukisalmi, V. Checklist of tapeworms (Platyhelminthes, Cestoda) of vertebrates in Finland. ZooKeys 2015, 533, 1–61. [Google Scholar] [CrossRef] [Green Version]
- Sitko, J.; Okulewicz, A. Checklist of the Nematodes in Birds in the Czech Republic and the Slovak Republic; Comenius Museum Přerov: Přerov, Czech Republic, 2010; 104p. [Google Scholar]
- Skryabin, K.I. (Ed.) Principles of Nematodology; Volumes I–XXIX; Izdatelstvo Akademii Nauk SSR: Moscow, Russia, 1949–1979. (In Russian) [Google Scholar]
- Königová, A.; Molnár, L.; Hrčková, G.; Várady, M. The first report of serratospiculiasis in Great Tit (Parus major) in Slovakia. Helminthologia 2013, 50, 254–260. [Google Scholar] [CrossRef] [Green Version]
- Dogiel, V.A. General Parasitology; Izdatelstvo Leningradskogo Universiteta: Saint Petersburg, Russia, 1962; 464p. (In Russian) [Google Scholar]
- Rząd, I.; Sitko, J.; Sałamatin, R.; Wysocki, D. Helminth community structure study on urban and forest blackbird (Turdus merula L.) populations in relation to seasonal bird migration on the south Baltic Sea coast (NW Poland). Helminthologia 2014, 51, 117–129. [Google Scholar] [CrossRef] [Green Version]
- Fauna Europaea. Available online: https://fauna-eu.org/ (accessed on 29 December 2022).
- Pojmanska, T. European species of Leucochloridium Carus. Acta Parasitol. Pol. 1969, 16, 193–205. [Google Scholar]
- Richner, H.; Christe, P.; Opplinger, A. Parental investment affects prevalence of malaria. Proc. Natl. Acad. Sci. USA 1995, 92, 1192–1194. [Google Scholar] [CrossRef] [Green Version]
- Norris, K.; Anvar, A.F. Reproductive effort influences the prevalence of hematozoon parasites in great tits. J. Anim. Ecol. 1995, 63, 601–610. [Google Scholar] [CrossRef]
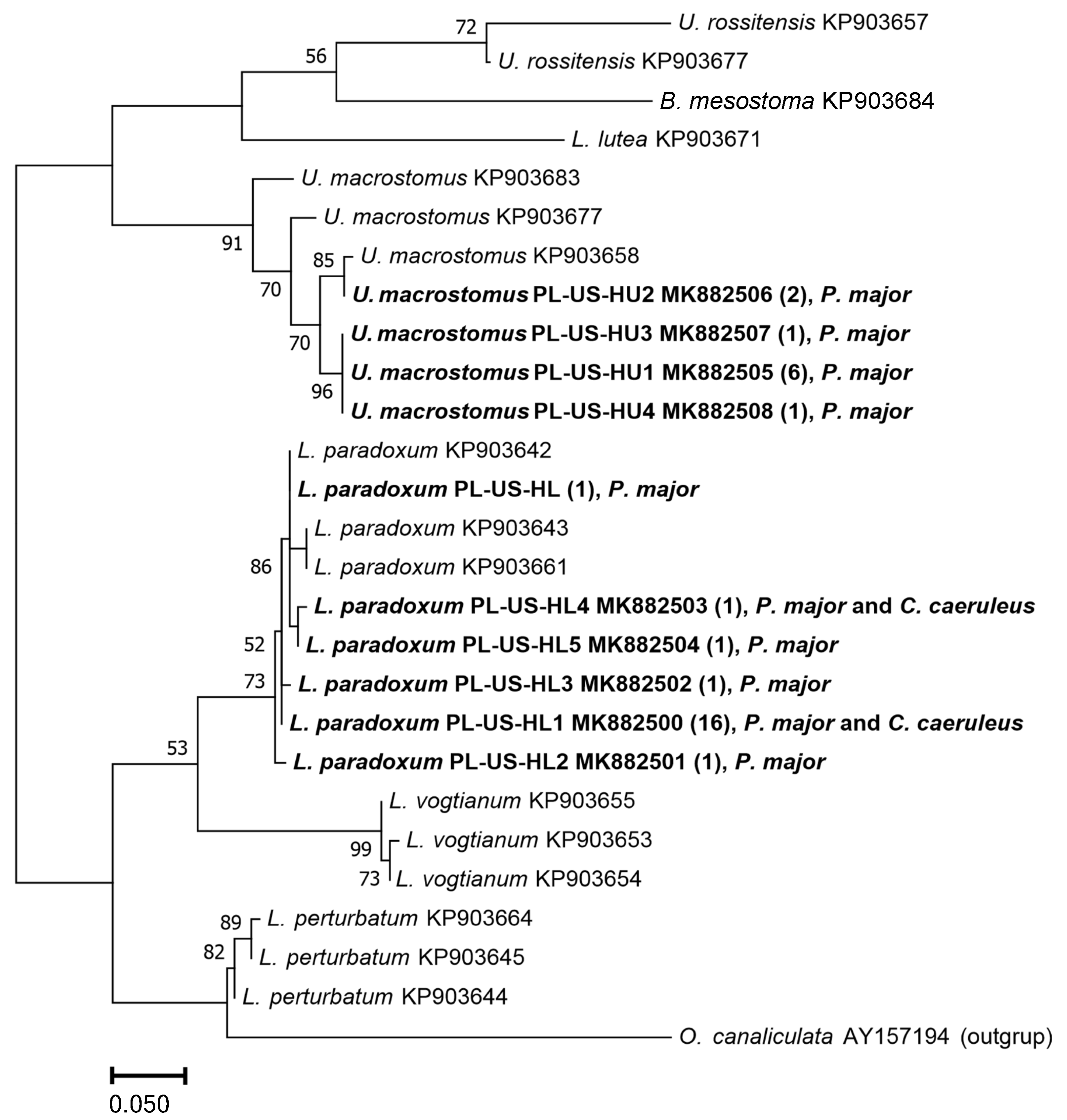
| Helminth | Eurasian Blue Tit Cyanistes caeruleus (67) | Great Tit Parus major (166) | ||||||
|---|---|---|---|---|---|---|---|---|
| Prevalence [%]/n | Mean Intensity | Range of Intensity | Mean Abundance | Prevalence [%] | Mean Intensity | Range of Intensity | Relative Abundance | |
| Leucochloridium paradoxum | 6.0/4 * | 81.7 | 5–154 | 4.9 | 16.9/28 * | 56.5 | 2–220 | 9.5 |
| Urogonimus macrostomus | 0 | - | - | - | 2.4/4 NH | 18 | 1–60 | 0.4 |
| Plagiorchis maculosus | 1.5/1 | 1 | - | 0.0 | 0/0 | - | - | - |
| Digenea—total: | 7.5/5 * | 65.6 | 1–154 | 4.9 | 19.3/32 * | 51.7 | 1–220 | 10.0 |
| Anonchotaenia globata | 0 | - | - | - | 0.6/1 NGR, NH | 2 | - | 0.0 |
| Monosertum parinum | 1.5/1 NH | 10 | - | 0.1 | 0.6/1 NH | 5 | - | 0.0 |
| Cestoda—total: | 6.0/4 | 3.5 | 1–10 | 0.2 | 11.4/19 | 3.7 | 1–10 | 0.1 |
| Cardiofilaria pavlovskyi | 32.8/22 NH | 2.1 | 1–6 | 0.7 | 44.0/73 | 2.6 | 1–9 | 1.1 |
| Capillaria tridens | 0 | - | - | - | 0.6/1 | 1 | - | 0.0 |
| Diplotriaena henryi | 1.5/1 NH | 2 | - | 0.0 | 0.6/1 | 6 | - | 0.0 |
| Diplotriaena obtusa | 0 | - | - | - | 0.6/1 NH | 1 | - | 0.0 |
| Nematoda—total: | 37.3/25 | 2.3 | 1–6 | 0.8 | 45.8/76 | 2.7 | 1–14 | 1.2 |
| Component Community | Infracommunity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Host | Prevalence [%]/n | Mean Intensity of Infection | Range of Intensity | Mean Abundance | Number of Parasite Species | Simpson’s Index (1-D) | Berger–Parker Index | Dominant Species | Brillouin Index | Mean Number of Helminth Species in Infracommunity Min–Max |
| Eurasian blue tit Cyanistes careuleus (67) | 44.8 */30 | 13.7 | 1–157 | 6.0 * | 5 T– 2, C–1, N–2 | 0.4 | 0.8 | Leucochloridium paradoxum | 0.02 | 1.0 1–2 |
| Great tit Parus major (166) | 62.7 */104 | 19.4 | 1–222 | 11.4 * | 8 T–2, C–2, N–4 | 0.3 | 0.9 | Leucochloridium paradoxum | 0.03 | 1.1 1–3 |
| Juveniles | Adults | Females | Males | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prevalence [%]/n | Mean Intensity | Mean Abundance | Prevalence [%]/n | Mean Intensity | Mean Abundance | Prevalence [%]/n | Mean Intensity | Mean Abundance | Prevalence [%]/n | Mean Intensity | Mean Abundance | ||
| Cyanistes caeruleus | (16) | (14) | (17) | (13) | |||||||||
| L. paradoxum | 6.25/1 | 5 | 0.3 | 7.1/1 | 92 | 6.6 | 11.8/2 | 48.5 | 5.7 | 0.0 | - | - | |
| M. parinum | 0.0 | - | - | 0.0/0 | - | - | 0.0/0 | 0.0/0 | 0.0 | 0.0/0 | - | - | |
| Cestoda gen. sp. | 0.0 | - | - | 7.1/1 | 1 | 0.1 | 5.9 | 1 | 0.2 | 0.0/0 | - | - | |
| Total Cestoda | 0.0/0 | 7.1/1 | 1 | 0.1 | 5.9/1 | 1 | 0.2 | 0.0/0 | - | - | |||
| C. pavlovskyi | 50.0/8 | 1.8 | 0.9 | 35.7/5 | 1.8 | 0.6 | 41.2/7 | 2.1 | 0.9 | 46.2/6 | 1.3 | 0.6 | |
| Nematoda gen. sp. | 0.0 | - | - | 7.1/1 | 4 | 0.3 | 5.9/1 | 4 | 0.2 | 0.0 | - | - | |
| Total Nematoda | 50.0/8 | 1.8 | 0.9 | 35.7/5 | 2.6 | 0.9 | 41.2/7 | 2.7 | 1.1 | 46.2 | 1.3 | 0.6 | |
| Total helminths | 50.0/8 | 2.4 | 1.2 | 42.9(6) | 17.7 | 7.6 | 47.1/8 | 14.6 | 6.9 | 46.2/6 | 1.3 | 0.6 | |
| Parus major | (73) | (22) | (58) | (43) | |||||||||
| L. paradoxum | 12.3/9 | 62.1 | 7.7 | 9.1/2 | 32.5 | 2.9 | 19.0/11 | 63.5 | 12.1 | 11.6/5 | 30.2 | 3.5 | |
| U. macrostomus | 2.7/2 | 30.5 | 0.8 | 4.5/1 | 10 | 0.4 | 1.7/1 | 10 | 0.2 | 7.0/3 | 20.7 | 1.4 | |
| Digenea gen sp. | 1.4/1 | 1.0 | 0.0 | 0.0/0 | - | - | 1.7/1 | 1 | 0.0 | 0.0/0 | - | - | |
| Total Digenea | 16.4/12 | 51.8 | 8.5 | 13.6/3 | 25 | 3.4 | 22.4/13 | 54.6 | 12.2 | 16.3/7 | 30.4 | 5.0 | |
| A. globata | 0.0 | - | - | 4.5/1 | 2 | 0.1 | 1.7/1 | 2 | 0.0 | 0.0 | - | - | |
| M. parinum | 0.0 | - | - | 0.0 | - | - | 0.0 | - | - | 2.3/1 | 5 | 0.1 | |
| Cestoda gen. sp. | 2.7/2 | - | - | 27.3/6 | - | - | 12.1/7 | - | - | 4.7/3 | - | - | |
| Total Cestoda | 2.7/2 * | - | - | 31.8/7 * | - | - | 12.1/7 | - | - | 7.0/3 | - | - | |
| C. pavlovskyi | 52.1/38 | 2.8 | 1.4 | 36.4/8 | 3.6 | 1.2 | 44.8/26 | 2.6 | 1.1 | 51.2/22 | 3.1 | 1.6 | |
| D. obtusa | 0.0 | - | - | 4.5/1 | - | - | 1.7/1 | 1 | 0.0 | 0.0/0 | - | - | |
| Nematoda gen sp. | 1.4/1 | 1.0 | 0.0 | 0.0/0 | - | - | 0.0/0 | - | - | 0.0/0 | - | - | |
| Total Nematoda | 53.4/39 | 2.7 | 1.5 | 36.4/8 | 4.9 | 1.8 | 48.3/28 | 3.0 | 1.4 | 51.2/22 | 3.1 | 1.6 | |
| Total helminths | 60.3/44 | 16.5 | 10.1 | 68.2/15 | 3.6 | 1.9 | 70.7/41 | 20.8 | 13.9 | 55.8/26 | 11.3 | 6.6 | |
| Cyanistes caeruleus | Parus major |
|---|---|
| DIGENEA | |
| Leucochloridium paradoxum Carus 1835 [36,48,49,50] and this study Lutztrema attenuatum/ Brachylecithum attenuatum (Dujardin, 1845) [48,51,52,53] Plagiorchis maculosus (Rudolphi, 1802) [48,49] and this study Plagiorchis elegans (Rudolphi, 1802) [48,51,52,53] Prosthogonimus ovatus (Rudolphi, 1803) [48,51,52,53,54,55] | |
| Hypoderaeum sp. Duetz, 1909 [56] | Collyriclum faba (Bremser, 1831) [51,52,53] Cortrema magnicaudata/ Renicola magnicaudata (Bykhovskaya-Pavlovskaya, 1950) [51,52,53] Leucochloridium actitis /L. perturbatum Pojmańska, 1969 [50] Lyperosomum petiolatum /Lyperosomum platynosoides (Railliet, 1900) [51,52,53,54] Leyogonimus postgonoporos/ Maycella postgonoporus Neiland, 1951 [48] Plagiorchis laricola Skrjabin, 1924 [51,52,53] Plagiorchis notabilis Nicoll, 1909 [51,52,53] Plagiorchis multiglandularis Semenov, 1927 [48] Urogonimus macrostomus /Leucochloridium macrostomum (Rudolphi, 1802) [48,51,52,53] and this study |
| CESTODA | |
| Anonchotaenia globata (von Linstow, 1879) [25,36,53] and this study Emberizotaenia reductorhyncha (Spasskaya 1957) [53] Monosertum parinum (Dujardin, 1845) [26,57] and this study | |
| Anonchotaenia magniuterina (Rysavy, 1957) [25,53] Monopylidium praecox (Krabbe, 1879) [56] | Orthoskrjabinia bobica (Clerc, 1903) [56] Passerilepis passeris (Gmelin, 1970) [53] Passerilepis parina (Fuhrmann, 1907) [57] Passerilepis spasskii (Sudarikov, 1950) [53] Variolepis farciminosa (Goeze, 1782) [53] |
| NEMATODA | |
| Capillaria tridens (Dujardin, 1845) [46,58] and this study Diplotriaena henryi (Blanc, 1919) [46,58] and this study Cardiofilaria pavlovskyi (Strom, 1937) [46,58] and this study | |
| Dispharynx nasuta (Rudolphi, 1819) [46,59] Diplotriaena obtusa (Sonin 1968) [59] and this study Serratospiculum spp. (Skrjabin, 1915) [60] Physocephalus sexalatus (Molin, 1860), larvae [53] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rząd, I.; Okulewicz, A.; Sałamatin, R.; Szenejko, M.; Panicz, R.; Nowakowski, J.K.; Stapf, A. Helminth Community Structure of Tits Cyanistes caeruleus and Parus major (Paridae) during Their Autumn Migration on the Southern Baltic Coast. Animals 2023, 13, 421. https://doi.org/10.3390/ani13030421
Rząd I, Okulewicz A, Sałamatin R, Szenejko M, Panicz R, Nowakowski JK, Stapf A. Helminth Community Structure of Tits Cyanistes caeruleus and Parus major (Paridae) during Their Autumn Migration on the Southern Baltic Coast. Animals. 2023; 13(3):421. https://doi.org/10.3390/ani13030421
Chicago/Turabian StyleRząd, Izabella, Anna Okulewicz, Rusłan Sałamatin, Magdalena Szenejko, Remigiusz Panicz, Jarosław K. Nowakowski, and Agata Stapf. 2023. "Helminth Community Structure of Tits Cyanistes caeruleus and Parus major (Paridae) during Their Autumn Migration on the Southern Baltic Coast" Animals 13, no. 3: 421. https://doi.org/10.3390/ani13030421
APA StyleRząd, I., Okulewicz, A., Sałamatin, R., Szenejko, M., Panicz, R., Nowakowski, J. K., & Stapf, A. (2023). Helminth Community Structure of Tits Cyanistes caeruleus and Parus major (Paridae) during Their Autumn Migration on the Southern Baltic Coast. Animals, 13(3), 421. https://doi.org/10.3390/ani13030421






