Sensitivity of High Conservation Value Birds to Para-Aminopropiophenone (PAPP) Determined by Sub-Lethal Dose–Response Assay
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experimental Population
2.3. Instruments
2.4. General Procedures
2.5. Anaesthesia
2.6. Blood Sampling
2.7. Analysis of Acute and Protracted Outcomes
3. Results
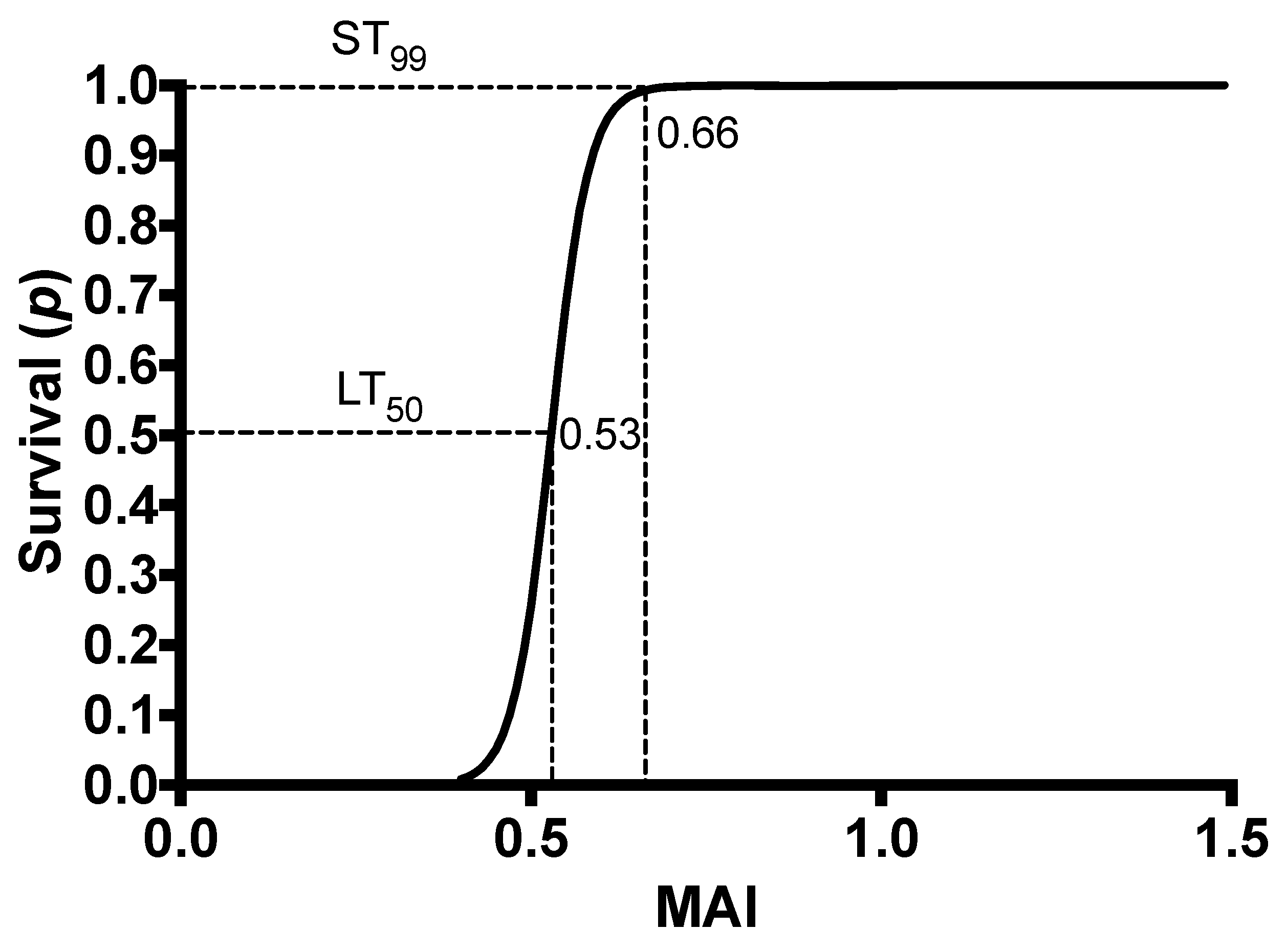
3.1. Establishing the LT50 and ST99
3.2. Protracted Toxicity
3.3. NOEL and LOAEL Estimates
4. Discussion
4.1. Haemoglobin Giveth and Taketh Away
4.2. Acute Sensitivity
4.3. Protracted Toxicity
4.4. NOEL and LOAEL Estimates
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marrs, T.; Inns, R.; Bright, J.; Wood, S. The formation of methaemoglobin by 4-aminopropiophenone (PAPP) and 4-(n-hydroxy) aminopropiophenone. Hum. Exp. Toxicol. 1991, 10, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Marks, C.; Gigliotti, F.; Busana, F.; Johnston, M.; Lindeman, M. Fox control using a para-aminopropiophenone formulation with the M-44 ejector. Anim. Welf. 2004, 13, 401–407. [Google Scholar] [CrossRef]
- Murphy, E.; Shapiro, L.; Hix, S.; MacMorran, D.; Eason, C.T. Control and eradication of feral cats: Field trials of a new toxin. In Island Invasives: Eradication and Management; Veitch, C.R.C.M.N., Towns, D.R., Eds.; IUCN: Gland, Switzerland, 2011; pp. 213–216. [Google Scholar]
- Johnston, M.; Algar, D.; O’Donoghue, M.; Morris, J.; Buckmaster, T.; Quinn, J. Efficacy and welfare assessment of an encapsulated para-aminopropiophenone (PAPP) formulation as a bait-delivered toxicant for feral cats (Felis catus). Wildl. Res. 2020, 47, 686–697. [Google Scholar] [CrossRef]
- Vandenbelt, J.; Pfeiffer, C.; Kaiser, M.; Sibert, M. Methemoglobinemia after administration of p-aminoacetophenone and p-aminopropiophenone. J. Pharmacol. Exp. Ther. 1944, 80, 31–38. [Google Scholar]
- Savarie, P.J.; Pan, H.P.; Hayes, D.J.; Roberts, J.D.; Dasch, G.J.; Felton, R.; Schafer, E.W. Comparative acute oral toxicity of para-aminopropiophenone (PAPP) in mammals and birds. Bull. Environ. Contam. Toxicol. 1983, 30, 122–126. [Google Scholar] [CrossRef]
- McLennan, J.; Potter, M.; Robertson, H.; Wake, G.; Colbourne, R.; Dew, L.; Joyce, L.; McCann, A.; Miles, J.; Miller, P. Role of predation in the decline of kiwi, Apteryx spp., in New Zealand. N. Z. J. Ecol. 1996, 20, 27–35. [Google Scholar]
- Watts, J.O.; Moore, A.; Palmer, D.; Molteno, T.C.; Recio, M.R.; Seddon, P.J. Trial reintroduction of buff weka to an unfenced mainland site in central south island, New Zealand. Austral. Ecol. 2017, 42, 198–209. [Google Scholar] [CrossRef]
- Kemp, J.R.; Young, L.; Mosen, C.; Bolitho, L.; Orr-Walker, T.; Yockney, I.; Elliott, G. Irruptive dynamics of invasive carnivores and prey populations, and predator control, affect kea survivorship across the Southern Alps. N. Z. J. Zool. 2022, 1–26. [Google Scholar] [CrossRef]
- Marks, C.A.; Allen, L.; Lindeberg, H. Non-lethal dose-response models replace lethal bioassays for predicting the acute toxicity of PAPP to Australian wildlife. Animals 2023, in press. [Google Scholar]
- Eason, C.; Murphy, E.; Hix, S.; Henderson, R.; MacMorran, D. Susceptibility of Four Bird Species to Para-Aminopropiophenone (PAPP); Department of Conservation: Wellington, New Zealand, 2010; pp. 1–15.
- Trevan, J.W. The error of determination of toxicity. Proc. R. Soc. London. Ser. B Contain. Pap. A Biol. Character 1927, 101, 483–514. [Google Scholar]
- Cox, P.; Smith, R. Rodenticide ecotoxicology: Assessing non-target population effects. Funct. Ecol. 1990, 3, 315–320. [Google Scholar] [CrossRef]
- Eason, C.; Wickstrom, M.; Henderson, R.; Milne, L.; Arthur, D. Non-Target and Secondary Poisoning Risks Associated with Cholecalciferol; New Zealand Plant Protection Society: Rotorua, New Zealand, 1998; pp. 299–304. [Google Scholar]
- Eason, C.T.; Murphy, E.C.; Wright, G.R.; Spurr, E.B. Assessment of risks of brodifacoum to non-target birds and mammals in New Zealand. Ecotoxicology 2002, 11, 35–48. [Google Scholar] [CrossRef]
- Hoare, J.M.; Hare, K.M. The impact of brodifacoum on non-target wildlife: Gaps in knowledge. N. Z. J. Ecol. 2006, 30, 157–167. [Google Scholar]
- Langford, K.H.; Reid, M.; Thomas, K.V. The occurrence of second generation anticoagulant rodenticides in non-target raptor species in norway. Sci. Total Environ. 2013, 450, 205–208. [Google Scholar] [CrossRef]
- McIlroy, J.C. The sensitivity of Australian animals to 1080 poison. IX. Comparisons between the major groups of animals, and the potential danger non-target species face from 1080 poisoning campaigns. Aust. Wildl. Res. 1986, 13, 39–48. [Google Scholar] [CrossRef]
- Spurr, E.B.; Berben, P.H. Assessment of non-target impact of 1080-poisoning for vertebrate pest control on weta (orthoptera: Anostostomatidae and Rhaphidophoridae) and other invertebrates in artificial refuges. N. Z. J. Ecol. 2004, 28, 63–72. [Google Scholar]
- Pillai, S.K.; Kobayashi, K.; Mathews, M.; Mathai, T.; Sivakumar, B.; Sadasivan, P. John William Trevan’s concept of median lethal dose (LD50/LD50)–more misused than used. J. Pre-Clin. Clin. Res. 2021, 15, 137. [Google Scholar] [CrossRef]
- WHO. Principles and Methods for the Risk Assessment of Chemicals in Food; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Dorato, M.A.; Engelhardt, J.A. The no-observed-adverse-effect-level in drug safety evaluations: Use, issues, and definition(s). Regul. Toxicol. Pharmacol. 2005, 42, 265–274. [Google Scholar] [CrossRef]
- Kroes, R.; Kleiner, J.; Renwick, A. The threshold of toxicological concern concept in risk assessment. Toxicol. Sci. 2005, 86, 226–230. [Google Scholar] [CrossRef]
- Marks, C.A.; Trought, K.; Brown, S.; Arrow, J.; Hopkins, B. Monitoring methaemoglobinaemia in birds using 5 µl of whole blood. PLoS ONE 2023. in review. [Google Scholar]
- Votey, S.R.; Bosse, G.M.; Bayer, M.J.; Hoffman, J.R. Flumazenil: A new benzodiazepine antagonist. Ann. Emerg. Med. 1991, 20, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Yap, B.W.; Sim, C.H. Comparisons of various types of normality tests. J. Stat. Comput. Simul. 2011, 81, 2141–2155. [Google Scholar] [CrossRef]
- Klawonn, F.; Hoffmann, G.; Orth, M. Quantitative laboratory results: Normal or lognormal distribution? J. Lab. Med. 2020, 44, 143–150. [Google Scholar] [CrossRef]
- Harvey, J.W. Introduction to veterinary hematology. Vet. Hematol. 2012, 1–10. [Google Scholar] [CrossRef]
- Hoffman, J.I. Biostatistics for Medical and Biomedical Practitioners; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Konishi, S.; Kitagawa, G. Generalised information criteria in model selection. Biometrika 1996, 83, 875–890. [Google Scholar] [CrossRef]
- Brody, T. FDA’s Drug Review Process and the Package Label: Strategies for Writing Successful FDA Submissions; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Minias, P. The use of haemoglobin concentrations to assess physiological condition in birds: A review. Conserv. Physiol. 2015, 3, cov007. [Google Scholar] [CrossRef]
- Senior, A. ATP synthesis by oxidative phosphorylation. Physiol. Rev. 1988, 68, 177–231. [Google Scholar] [CrossRef]
- Rodnan, G.P.; Ebaugh, F.G., Jr.; Fox, M.S.; Chambers, D.M. The life span of the red blood cell and the red blood cell volume in the chicken, pigeon and duck as estimated by the use of na2cr51o4: With observations on red cell turnover rate in the mammal, bird and reptile. Blood 1957, 12, 355–366. [Google Scholar] [CrossRef]
- Hawkey, C.; Bennett, P.; Gascoyne, S.; Hart, M.; Kirkwood, J. Erythrocyte size, number and haemoglobin content in vertebrates. Br. J. Haematol. 1991, 77, 392–397. [Google Scholar] [CrossRef]
- Boutilier, R.G. Mechanisms of cell survival in hypoxia and hypothermia. J. Exp. Biol. 2001, 204, 3171–3181. [Google Scholar] [CrossRef]
- Bishop, C.M. The maximum oxygen consumption and aerobic scope of birds and mammals: Getting to the heart of the matter. Proc. R. Soc. Lond. B. Biol. Sci. 1999, 266, 2275–2281. [Google Scholar] [CrossRef]
- Butler, P.J. The physiological basis of bird flight. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150384. [Google Scholar] [CrossRef]
- Voss, M.; Shutler, D.; Werner, J. A hard look at blood sampling of birds. Auk 2010, 127, 704–708. [Google Scholar] [CrossRef]
- Newell, G.W.; Shaffner, C. Blood volume determinations in chickens. Poult. Sci. 1950, 29, 78–87. [Google Scholar] [CrossRef]
- Minias, P.; Włodarczyk, R.; Piasecka, A.; Kaczmarek, K.; Janiszewski, T. Ecological, physiological, and morphological correlates of blood hemoglobin concentration in a migratory shorebird. Physiol. Biochem. Zool. 2014, 87, 771–781. [Google Scholar] [CrossRef]
- Gladden, L.B. Lactic acid: New roles in a new millennium. Proc. Natl. Acad. Sci. USA 2001, 98, 395–397. [Google Scholar] [CrossRef]
- Le Maho, Y.; Karmann, H.; Briot, D.; Handrich, Y.; Robin, J.-P.; Mioskowski, E.; Cherel, Y.; Farni, J. Stress in birds due to routine handling and a technique to avoid it. Am. J. Physiol. -Regul. Integr. Comp. Physiol. 1992, 263, R775–R781. [Google Scholar] [CrossRef]
- Bauman, R.A.; Widholm, J.J. Operant leverpressing and wheelrunning were differentially reduced by PAPP (p-aminopropiophenone) -induced methemoglobinemia. Pharmacol. Biochem. Behav. 2007, 87, 444–452. [Google Scholar] [CrossRef]
- Peters, G.W.; Steiner, D.A.; Rigoni, J.A.; Mascilli, A.D.; Schnepp, R.W.; Thomas, S.P. Cardiorespiratory adjustments of homing pigeons to steady wind tunnel flight. J. Exp. Biol. 2005, 208, 3109–3120. [Google Scholar] [CrossRef]
- Ustinova, O.I. Academician I.V. Davydovskiy on pathology, physiology and biological fitness of the organism for adaptation, ecology and environmental fitness of the functional systems involved in the process of adaptation. Life Sci. J. 2014, 11, 579–582. [Google Scholar]
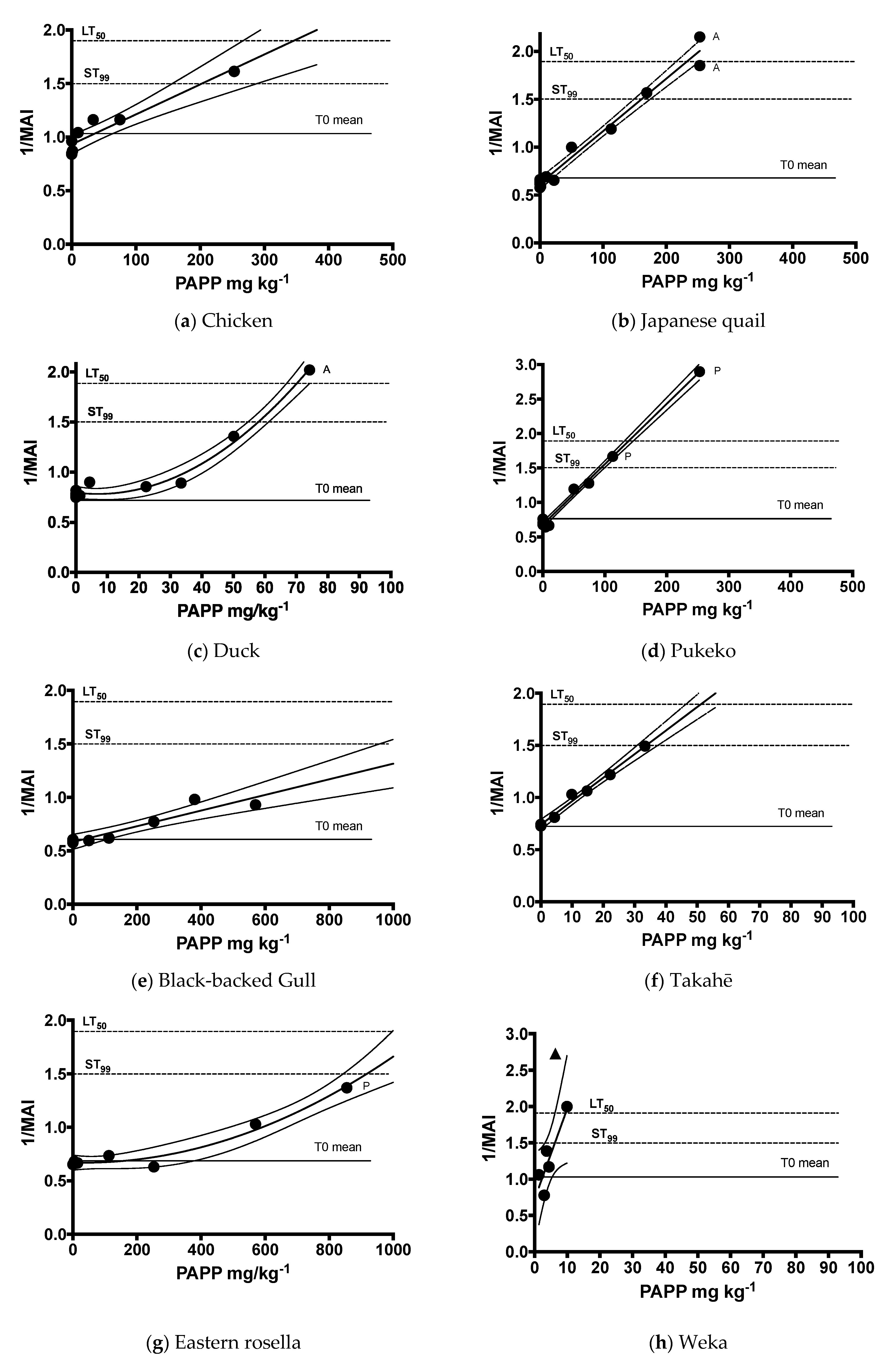
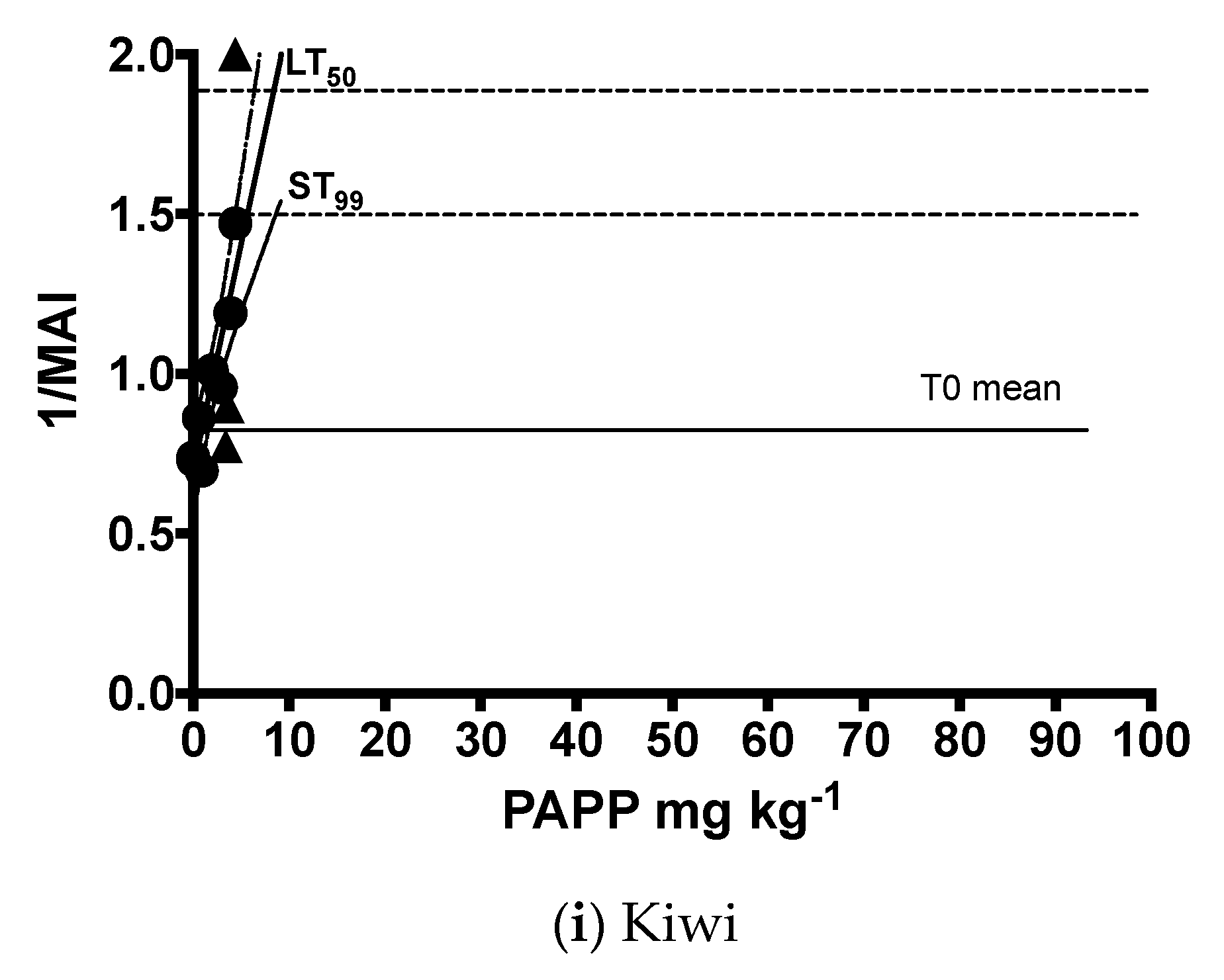
| Common Name | Equation | n | d.f. | R2 | F | p |
|---|---|---|---|---|---|---|
| Chicken | y = 0.002799x + 0.932 | 8 | 1, 6 | 0.9 | 52.96 | <0.0003 |
| Japanese quail | y = 0.005474x + 0.6203 | 13 | 1, 11 | 0.98 | 522.8 | <0.0001 |
| Duck | y = 0.7945 − 0.00383x + 0.0002763x2 | 10 | 7 | 0.98 | 174.5 | <0.0001 |
| Pukeko | y = 0.008767x + 0.6769 | 11 | 1, 9 | 0.99 | 1411 | <0.0001 |
| Black-backed gull | y = 0.0007323x + 0.5831 | 8 | 1, 6 | 0.88 | 45.5 | <0.0005 |
| Takahē | y = 0.02254x + 0.7399 | 7 | 1, 5 | 0.99 | 337.3 | <0.0001 |
| Rosella | y = 0.6696 + 0.00006619x + 0.000001059x2 | 8 | 5 | 0.97 | 75.6 | <0.0002 |
| Weka | y = 0.1248x + 0.725 | 5 | 1, 3 | 0.79 | 11.3 | 0.045 |
| Brown kiwi | y = 0.1412x + 0.6982 | 8 | 1, 6 | 0.85 | 33.39 | 0.0012 |
| Common Name | 1 SD | 2 SDs | ST99 | LT50 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | |||||||||
| Mean | Upper | Lower | Mean | Upper | Lower | Mean | Upper | Lower | Mean | Upper | Lower | |
| Chicken | 111.6 | 215.80 | 59.74 | 179.7 | 318.3 | 110.7 | 202.9 | 353.4 | 128.1 | 342.3 | 563.3 | 232.4 |
| Japanese quail | 11.9 | 25.5 | 0.8 | 23.5 | 38.2 | 11.3 | 160.7 | 190.1 | 136.5 | 232.0 | 268.9 | 201.5 |
| Duck | 11.1 | 35.8 | - | 22.3 | 43.0 | 13.2 | 51.0 | 71.1 | 41.7 | 63.3 | 84.9 | 52.8 |
| Pukeko | 33.1 | 40.8 | 26.2 | 56.2 | 65.4 | 48.0 | 93.9 | 105.5 | 83.6 | 138.4 | 152.9 | 125.5 |
| Black-backed gull | 78.3 | 156.5 | - | 118.1 | 334.5 | 25.4 | 1252.1 | 1878.2 | 958.3 | 1784.7 | - | 1358.0 |
| Takahē | 3.9 | 7.0 | 1.5 | 8.5 | 12.3 | 5.5 | 33.7 | 41.5 | 30.6 | 51.0 | 61.4 | 43.1 |
| Rosella | 292.8 | 471.0 | 188.6 | 393.9 | 536.4 | 274.1 | 886.1 | 1068.5 | 841.5 | 1074.0 | - | 995.5 |
| Weka | 2.1 | - | 0.5 | 3.2 | - | 0.6 | 6.2 | - | 0.9 | 9.3 | 21.0 | 2.2 |
| Brown kiwi | 1.8 | - | 0.5 | 2.7 | - | 1.2 | 5.7 | 8.7 | 3.3 | 8.4 | 16.4 | 5.2 |
| 1 SD | 2 SDs | ST99 | LT50 | |||||
|---|---|---|---|---|---|---|---|---|
| HbFe2+ (g dL−1) | Ratio | HbFe2+ (g dL−1) | Ratio | HbFe2+ (g dL−1) | Ratio | HbFe2+ (g dL−1) | Ratio | |
| Chicken | 12.10 | 0.12 | 11.09 | 0.19 | 10.81 | 0.21 | 9.51 | 0.31 |
| Japanese quail | 20.25 | 0.04 | 19.32 | 0.08 | 14.26 | 0.32 | 13.22 | 0.37 |
| Duck | 17.29 | 0.09 | 16.79 | 0.11 | 14.45 | 0.24 | 13.58 | 0.28 |
| Pukeko | 19.28 | 0.09 | 17.64 | 0.16 | 15.92 | 0.24 | 14.67 | 0.30 |
| Black-backed gull | 20.00 | 0.15 | 19.66 | 0.16 | 15.62 | 0.33 | 14.95 | 0.36 |
| Takahē | 20.38 | 0.03 | 19.49 | 0.07 | 16.80 | 0.20 | 15.89 | 0.24 |
| Rosella | 17.93 | 0.09 | 17.15 | 0.13 | 13.37 | 0.32 | 12.37 | 0.37 |
| Weka | 19.16 | <0.01 | 17.70 | 0.06 | 15.07 | 0.20 | 13.47 | 0.29 |
| Brown kiwi | 17.48 | 0.08 | 16.52 | 0.13 | 14.53 | 0.23 | 13.47 | 0.29 |
| Mean decline | 0.08 | 0.12 | 0.26 | 0.32 | ||||
| p < 0.05 | ±0.030 | ±0.029 | ±0.036 | ±0.030 | ||||
| Common Name | 24 h | 48 h | 72 h | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | p | mg kg−1 | p < 0.05 | R2 | p | mg kg−1 | p < 0.05 | R2 | p | mg kg−1 | p < 0.05 | ||||
| Upper | Lower | Upper | Lower | Upper | Lower | ||||||||||
| Chicken | n.s. | 0.83 | 0.03 | 252.1 | 148.6 | - | - | - | - | - | - | ||||
| Japanese quail | 0.89 | 0.01 | 194.3 | 228.5 | 144.4 | 0.81 | 0.04 | 158.2 | 51.4 | 219.6 | 0.76 | 0.06 | 168.9 | 59.0 | 245.1 |
| Duck | n.s. | n.s. | n.s. | ||||||||||||
| Pukeko | 0.96 | 0.0005 | 142.2 | 196.9 | 108.5 | 0.96 | 0.0005 | 160.9 | 135.0 | 199.5 | 0.97 | 0.0004 | 71.1 | 45.7 | 92.8 |
| Black-backed gull | 0.94 | 0.004 | 314.8 | 366.9 | 245.1 | n.s. | 0.86 | 0.02 | 216.5 | 371.6 | |||||
| Takahē | 0.96 | 0.04 | 26.6 | 32.1 | 18.7 | 0.95 | 0.05 | 29.0 | - | - | 0.93 | 0.07 | 26.7 | 12.1 | |
| Rosella | 0.95 | 0.002 | 625.0 | 700.6 | 519.3 | 0.97 | 0.006 | 466.6 | 522.1 | 399.1 | n.s. | ||||
| Weka | n.s. | n.s. | n.s. | ||||||||||||
| Brown kiwi | n.s. | n.s. | n.s. | ||||||||||||
| Common Name | 24 h | 48 h | 72 h | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | p | mg kg−1 | p < 0.05 | R2 | p | mg kg−1 | p < 0.05 | R2 | p | mg kg−1 | p < 0.05 | ||||
| Upper | Lower | Upper | Lower | Upper | Lower | ||||||||||
| Chicken | 0.66 | 0.02 | 221.3 | 105.4 | 0.9 | 0.008 | 344.2 | 385.4 | 313.3 | - | - | - | - | - | |
| Japanese quail | 0.58 | 0.05 | 96.4 | - | - | n.s. | 0.57 | 0.05 | 90.6 | - | - | ||||
| Duck | n.s. | n.s. | n.s. | ||||||||||||
| Pukeko | n.s. | 0.80 | 0.0005 | 61.0 | 158.7 | 32.0 | 0.93 | 0.0004 | 29.5 | 42.9 | 18.1 | ||||
| Black-backed gull | 0.95 | 0.05 | 374.9 | 516.5 | 139.8 | n.s. | 0.86 | 0.02 | 103.8 | 446.7 | - | ||||
| Takahē | 0.98 | 0.02 | 29.4 | 34.1 | 25.4 | 0.95 | 0.05 | 29.4 | - | 20.3 | 0.78 | 0.05 | 25.6 | - | 17.7 |
| Rosella | n.s. | n.s. | n.s. | ||||||||||||
| Weka | 0.89 | 0.02 | 10.4 | - | 7.6 | 0.97 | 0.002 | 9.2 | 11.9 | 7.7 | 0.88 | 0.02 | 9.6 | - | 7.1 |
| Brown kiwi | n.s. | n.s. | n.s. | ||||||||||||
| Common Name | 24 h | 48 h | 72 h | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | p | mg kg−1 | p < 0.05 | R2 | p | mg kg−1 | p < 0.05 | R2 | p | mg kg−1 | p < 0.05 | ||||
| Upper | Lower | Upper | Lower | Upper | Lower | ||||||||||
| Chicken | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Japanese quail | 0.59 | 0.04 | 92.5 | 403.0 | - | 0.53 | 0.07 | 109.4 | - | 0.54 | 0.06 | 83.2 | ≈250 | ||
| Duck | n.s. | n.s. | n.s. | ||||||||||||
| Pukeko | n.s. | 0.55 | 0.06 | >1000 | - | 215.4 | 0.54 | 0.06 | 850.5 | - | 159.3 | ||||
| Black-backed gull | n.s. | n.s. | . | n.s. | |||||||||||
| Takahē | 0.94 | 0.06 | 29.7 | - | 22.1 | 0.97 | 0.04 | 29.6 | - | 22.0 | 0.78 | 0.05 | 25.7 | - | 12.8 |
| Rosella | n.s. | n.s. | n.s. | ||||||||||||
| Weka | 0.9 | 0.01 | 10.3 | - | 7.6 | 0.97 | 0.003 | 9.2 | 12.1 | 7.7 | 0.89 | 0.02 | 9.9 | - | 7.3 |
| Brown kiwi | 0.58 | 0.08 | 5.0 | - | 4.4 | n.s. | n.s. | ||||||||
| Common Name | NOEL (at T1 and T2) | LOAEL (3 Day) | LOAEL–NOEL | NOEL/LOAEL | ||||
|---|---|---|---|---|---|---|---|---|
| mg kg−1 | mg kg−1 | mg kg−1 | % | |||||
| Mean | Upper | Lower | Mean | Upper | Lower | |||
| Chicken | 111.6 | 215.80 | 59.74 | 179.7 | 318.3 | 110.7 | 68.1 | 37.9 |
| Japanese quail | 11.9 | 25.5 | 0.8 | 23.5 | 38.2 | 11.3 | 11.6 | 49.4 |
| Duck | 11.1 | 35.8 | - | 22.3 | 43.0 | 13.2 | 11.2 | 50.2 |
| Pukeko | 33.1* | - | - | 29.5 | 42.9 | 18.1 | −3.6 | −12.2 |
| Black-backed gull | 78.3 | 156.5 | - | 103.8 | 446.7 | - | 25.5 | 24.6 |
| Takahē | 3.9 | 7.0 | 1.5 | 8.5 | 12.3 | 5.5 | 4.6 | 54.1 |
| Rosella | 292.8 | 471.0 | 188.6 | 393.9 | 536.4 | 274.1 | 101.1 | 25.7 |
| Weka | 2.1 | - | 0.5 | 3.2 | - | 0.6 | 1.1 | 34.4 |
| Brown kiwi | 1.8 | - | 0.5 | 2.7 | - | 1.2 | 0.9 | 33.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marks, C.A.; Trought, K.; Brown, S.; Arrow, J.; Hopkins, B. Sensitivity of High Conservation Value Birds to Para-Aminopropiophenone (PAPP) Determined by Sub-Lethal Dose–Response Assay. Animals 2023, 13, 433. https://doi.org/10.3390/ani13030433
Marks CA, Trought K, Brown S, Arrow J, Hopkins B. Sensitivity of High Conservation Value Birds to Para-Aminopropiophenone (PAPP) Determined by Sub-Lethal Dose–Response Assay. Animals. 2023; 13(3):433. https://doi.org/10.3390/ani13030433
Chicago/Turabian StyleMarks, Clive A., Katherine Trought, Samantha Brown, Jane Arrow, and Brian Hopkins. 2023. "Sensitivity of High Conservation Value Birds to Para-Aminopropiophenone (PAPP) Determined by Sub-Lethal Dose–Response Assay" Animals 13, no. 3: 433. https://doi.org/10.3390/ani13030433
APA StyleMarks, C. A., Trought, K., Brown, S., Arrow, J., & Hopkins, B. (2023). Sensitivity of High Conservation Value Birds to Para-Aminopropiophenone (PAPP) Determined by Sub-Lethal Dose–Response Assay. Animals, 13(3), 433. https://doi.org/10.3390/ani13030433





