Genetic Parameters of Growth Traits and Quantitative Genetic Metrics for Selection and Conservation of Mecheri Sheep of Tamil Nadu
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data and Farm Management
2.2. Data Analysis
3. Results
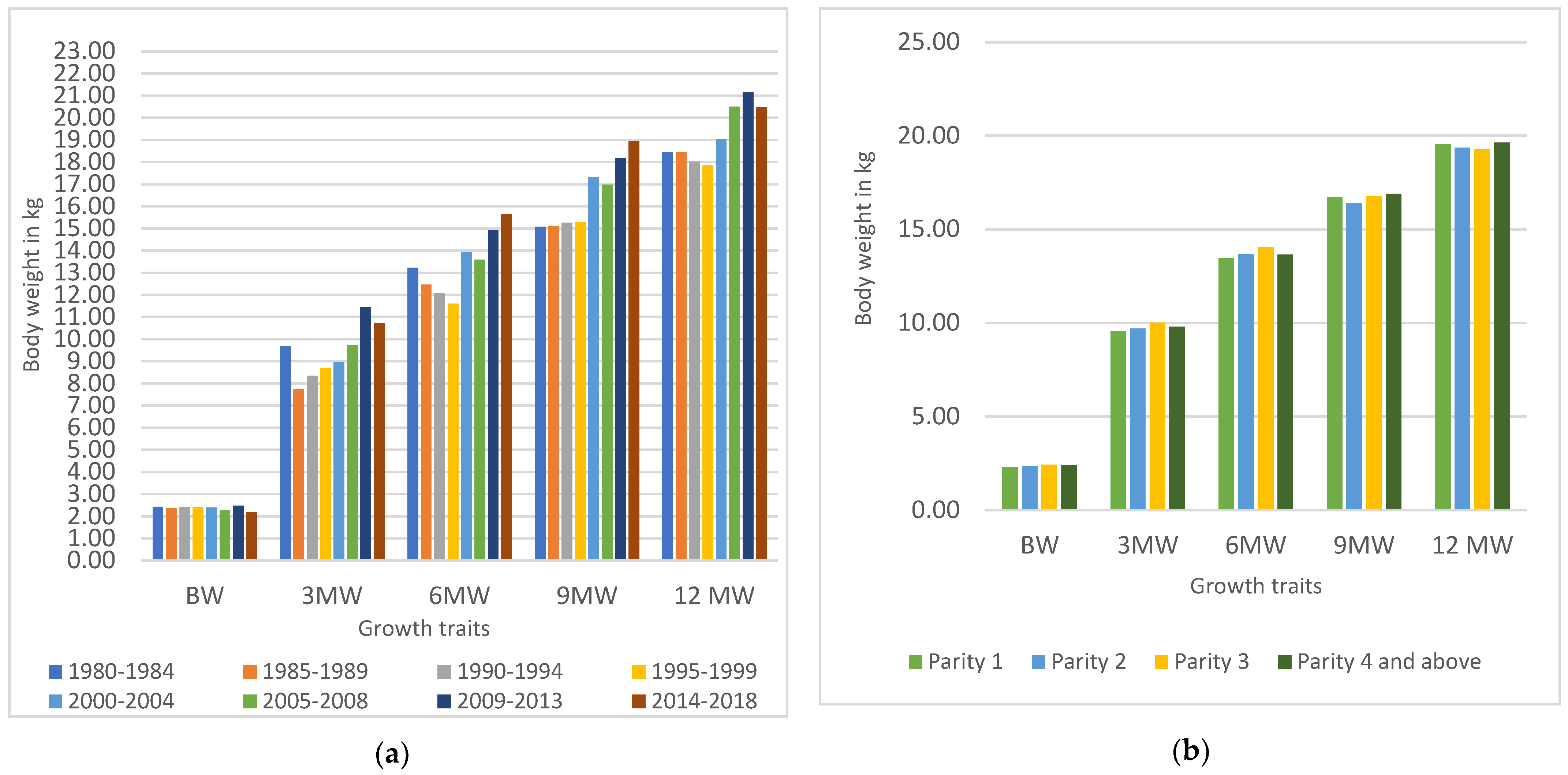
3.1. Growth Performance and Influence of Non-Genetic Factors
3.2. (Co)variance Components and Heritability
3.3. Genetic Correlation
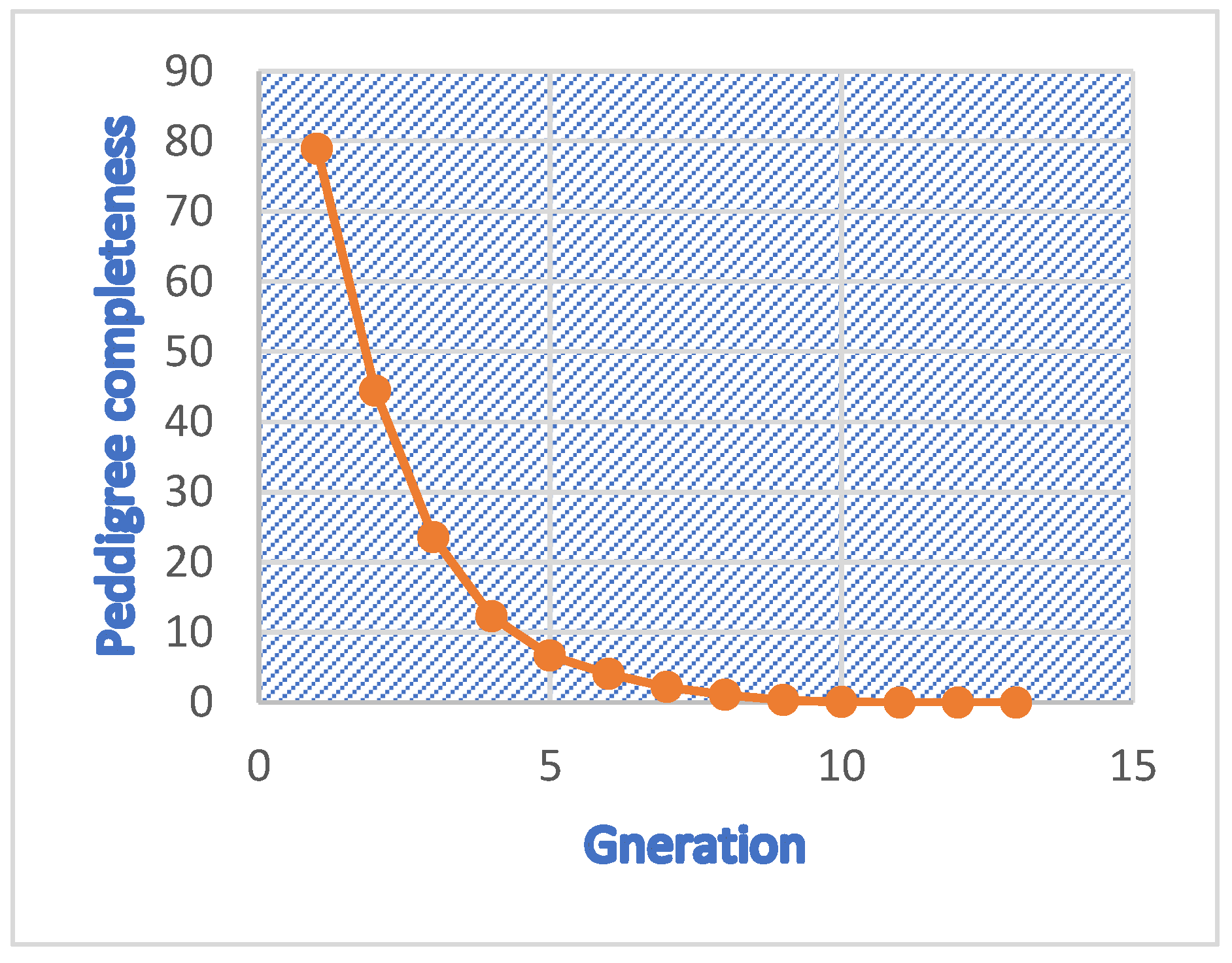
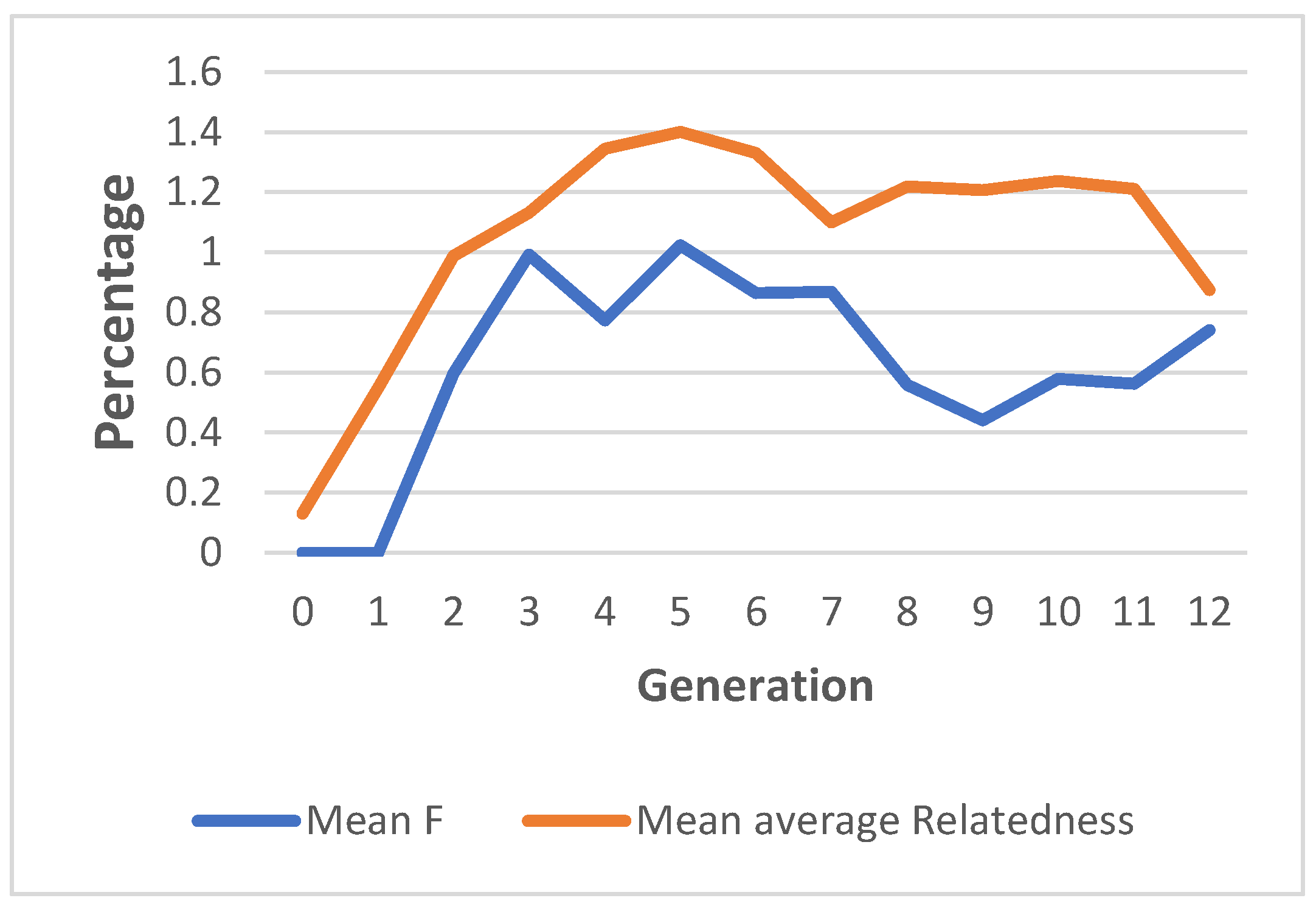
3.4. Pedigree Analysis and Inbreeding
4. Discussion
4.1. Growth Performance and Influence of Non-Genetic Factors
4.2. (Co)variance Components and Heritability
4.3. Genetic Correlation
4.4. Pedigree Analysis and Inbreeding
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thiruvenkadan, A.K.; Karunanithi, K.; Purushothaman, M.R. Socio-economic status of the Mecheri Sheep farmers and economics of rearing under farmer’s management. Indian J. Small Rumin. 2004, 10, 117–122. [Google Scholar]
- Satish Kumar, I.; Gangaraju, G.; Kumar, C.V.; Nath, S. Genetic parameters for growth rate and Kleiber ratios of Nellore sheep. Indian J. Anim. Res. 2018, 52, 1405–1408. [Google Scholar] [CrossRef]
- Neser, F.W.C.; Erasmus, G.J.; van Wyk, J.B. Genetic parameter estimates for preweaning weights in Dorper sheep. Small Rumin. Res. 2001, 40, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Safari, E.; Fogarty, N.M.; Gilmour, A.R. A review of genetic parameter estimates for wool, growth, meat and reproduction traits in sheep. Livest. Prod. Sci. 2005, 92, 271–289. [Google Scholar] [CrossRef]
- Rafter, P.; McHugh, N.; Pabiou, T.; Berry, D.P. Inbreeding trends and genetic diversity in purebred sheep populations. Animal 2022, 16, 100604. [Google Scholar] [CrossRef] [PubMed]
- Robin, W.; Jörn, B. Key genetic parameters for population management. Front. Genet. 2019, 10, 667. [Google Scholar] [CrossRef]
- Boujenane, I.; and Diallo, I.T. Estimates of genetic parameters and genetic trends for pre-weaning growth traits in Sardi sheep. Small Rumin. Res. 2017, 146, 61–68. [Google Scholar] [CrossRef]
- Meyer, K. Restricted maximum likelihood to estimate variance components for animal models with several random effects using a derivative-free algorithm. Genet. Sel. Evol. 1989, 21, 317–340. [Google Scholar] [CrossRef]
- Meyer, K. WOMBAT—A tool for mixed model analyses in quantitative genetics by restricted maximum likelihood (REML). J. Zhejiang Univ. Sci. B 2007, 8, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Snyman, M.A.; Erasmus, G.J.; Van Wyk, J.B.; Olivier, J.J. Direct and maternal (co)variance components and heritability estimates for body weight at different ages and fleece traits in Afrino sheep. Livest. Prod. Sci. 1995, 44, 229–235. [Google Scholar] [CrossRef]
- Van Wyk, J.B.; Fair, M.D.; Cloete, S.W.P. Revised models and genetic parameter estimates for production and reproduction traits in the Elsenburg Dormer sheep stud. S. Afr. J. Anim. Sci. 2003, 33, 213–222. [Google Scholar] [CrossRef]
- Gutiérrez, J.P.; Goyache, F. A note on ENDOG: A computer program for analysing pedigree information. J. Anim. Breed. Genet. 2005, 120, 357–360. [Google Scholar] [CrossRef]
- Thiruvenkadan, A.K.; Karunanithi, K.; Murugan, M.; Arunachalam, K.; Babu, R. A comparative study on growth performance of crossbred and purebred Mecheri sheep raised under dry land farming conditions. S. Afr. J. Anim. Sci. 2009, 39, 121–125. [Google Scholar] [CrossRef]
- Jeichitra, V.; Rajendran, R.; Karunanithi, K.; Rahumathulla, P.S. Genetic analysis of growth traits in Mecheri sheep. Indian J. Anim. Res. 2016, 50, 430–433. [Google Scholar] [CrossRef]
- Balasubramanyam, D.; Raja, T.V.; Kumarasamy, P.; Sivaselvam, S.N. Estimation of genetic parameters and trends for body weight traits in madras red sheep. Indian J. Small Rumin. 2012, 18, 173–179. [Google Scholar]
- Devendran, P.; Cauveri, D.; Murali, N.; Kumarasamy, P. Growth profile of Madras Red sheep in farmer’s flocks. Indian J. Small Rumin. 2014, 20, 20–23. [Google Scholar]
- Mane, P.M.; Pachpute, S.T.; Nimase, R.G. Growth and reproductive performance of Deccani sheep in an organised farm. Indian J. Small Rumin. 2014, 20, 23–27. [Google Scholar]
- Bhakthavatchalam, S. Genetic Analysis of Productive and Reproductive Traits in Farm Bred Nellore Jodipi Sheep. Ph.D. Thesis, Sri Venkateswara Veterinary University, Tirupati, India, 2019. [Google Scholar]
- El Bouyahiaoui, R.; Belkheir, B.; Moulla, F.; Ahmed, N.B.B.; Djaout, A.; Arbouche, F.; Ghozlane, F. Reproduction and Growth Performance of the Algerian Tazegzawt Sheep Breed. Genet. Biodivers. J. 2019, 3, 50–62. [Google Scholar] [CrossRef]
- Areb, E.; Getachew, T.; Kirmani, M.A.; Abate, Z.; Haile, A. Estimation of (co)variance components, genetic parameters, and genetic trends of growth traits in community-based breeding programs of Bonga sheep. Animal 2021, 15, 100202. [Google Scholar] [CrossRef]
- Meka, Z.I.; Martin, A.; Meutchieye, F.; Tadakeng, Y.; Fonteh, F. Biometric Assessment of Blackbelly Sheep in Central Africa. Genet. Biodivers. J. 2021, 5, 149–163. [Google Scholar] [CrossRef]
- Thiruvenkadan, A.K.; Karunanithi, K.; Muralidharan, J.; Babu, R.N. Genetic analysis of pre-weaning and post-weaning growth traits of Mecheri Sheep under dry land farming conditions. Asian-Australas. J. Anim. Sci. 2011, 24, 1041–1047. [Google Scholar] [CrossRef]
- Ekambaram, B.; Alexander, G.; Chakravarthi, M.K. Performance of Nellore sheep (Jodipi) under farm conditions. Indian Vet. J. 2013, 90, 35–37. [Google Scholar]
- Kumar, I.S.; Gangaraju, G.; Kumar, C.V.; Nath, S. Study on growth and reproductive traits of Nellore sheep in semi-arid region of Andhra Pradesh. Indian J. Small Rumin. 2017, 23, 163–167. [Google Scholar] [CrossRef]
- Venkataramanan, R.; Subramanian, A.; Sivaselvam, S.N.; Sivakumar, T.S.K.; Sreekumar, C.; Iyue, M. Direct and maternal components of variance for growth traits in Nilagiri and Sandyno sheep of South India. Indian J. Small Rumin. 2015, 21, 204–210. [Google Scholar] [CrossRef]
- Swenson, M.J.; Reece, W.O. Dukes’ Physiology of Domestic Animals, 11th ed.; Cornell University Press: New York, NY, USA, 1993. [Google Scholar]
- Devendran, P.; Cauveri, D.; Gajendran, K. Comparative performance of farm and field bred rams of Madras Red sheep. Indian Vet. J. 2007, 84, 1002–1003. [Google Scholar]
- Mahala, S.; Saini, S.; Kumar, A.; Prince, L.L.L.; Gowane, G.R. Effect of non-genetic factors on growth traits of Avikalin sheep. Small Rumin. Res. 2019, 174, 47–52. [Google Scholar] [CrossRef]
- Norouzian, M.A. Effects of lambing season, birth type and sex on early performance of lambs. N. Z. J. Agric. Res. 2015, 58, 84–88. [Google Scholar] [CrossRef]
- Gowane, G.R.; Prince, L.L.L.; Lopes, F.B.; Paswan, C.; Sharma, R.C. Genetic and phenotypic parameter estimates of live weight and daily gain traits in Malpura sheep using Bayesian approach. Small Rumin. Res. 2015, 128, 10–18. [Google Scholar] [CrossRef]
- Mokhtari, M.S.; Rashidi, A.; Mohammadi, Y. Estimation of genetic parameters for post-weaning traits of Kermani sheep. Small Rumin. Res. 2008, 80, 22–27. [Google Scholar] [CrossRef]
- Shahdadi, A.R.; Saghi, D.A. Estimating genetic parameters of body weight traits in Kourdi sheep. Iran. J. Appl. Anim. Sci. 2016, 6, 657–663. [Google Scholar]
- Ahmad, S.F.; Khan, N.N.; Ganai, N.A.; Shanaz, S.; Rather, M.A.; Alam, S. Multivariate quantitative genetic analysis of body weight traits in Corriedale sheep. Trop. Anim. Health Prod. 2021, 53, 197. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, B.P.; Mandal, A.; Arora, A.L.; Kumar, R.; Kumar, S.; Notter, D.R. Direct and maternal (co)variance components and heritability estimates for body weights in Chokla sheep. J. Anim. Breed. Genet. 2009, 126, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Prince, L.L.L.; Gowane, G.R.; Chopra, A.; Arora, A.L. Estimates of (co) variance components and genetic parameters for growth traits of Avikalin sheep. Trop. Anim. Health Prod. 2010, 42, 1093–1101. [Google Scholar] [CrossRef]
- Singh, H.; Pannu, U.; Narula, H.K.; Chopra, A.; Naharwara, V.; Bhakar, S.K. Estimates of (co) variance components and genetic parameters of growth traits in Marwari sheep. J. Appl. Anim. Res. 2016, 44, 27–35. [Google Scholar] [CrossRef]
- Abegaz, S.; van Wyk, J.B.; Olivier, J.J. Model comparisons and genetic and environmental parameter estimates of growth and the Kleiber ratio in Horro sheep. S. Afr. J. Anim. Sci. 2005, 35, 30–40. [Google Scholar] [CrossRef]
- Mokhtari, M.S.; Shahrebabak, M.M.; Shahrebabk, H.M.; Sadeghi, M. Estimation of (co) variance components and genetic parameters for growth traits in Arman sheep. J. Livest. Sci. Technol. 2012, 1, 38–47. [Google Scholar]
- Gowane, G.R.; Chopra, A.; Paswan, C.; Arora, A.L. Estimates of (co)variance components and genetic parameters for body weights and first greasy fleece weight in Malpura sheep. Livest. Sci. 2010, 131, 94–101. [Google Scholar] [CrossRef]
- Prakash, V.; Prince, L.L.L.; Gowane, G.R.; Arora, A.L. Estimation of (co)variance components and genetic parameters for growth traits and Kleiber ratios for Malpura sheep in India. Small Rumin. Res. 2012, 108, 54–58. [Google Scholar] [CrossRef]
- Ganesan, R.; Dhanavanthan, P.; Balasubramanyam, D.; Kumarasamy, P. Estimates of genetic parameters of growth traits in Madras Red sheep. J. Agric. Vet. Sci. 2013, 3, 69–73. [Google Scholar] [CrossRef]
- Li, W.H.; Li, G.Y.; Zhang, J.; Wang, X.J.; Zhang, A.W.; Zhao, J.T.; Wang, L.J.; Yang, J.F.; Luo, T.Z.; Shen, K.Z. Estimates of (co) variance components and phenotypic and genetic parameters of growth traits and wool traits in Alpine Merino sheep. J. Anim. Breed. Genet. 2022, 139, 351–365. [Google Scholar] [CrossRef]
- Sallam, A.M.; Ibrahim, A.H.; Alsheikh, S.M. Estimation of genetic parameters and variance components of pre-weaning growth traits in Barki lambs. Small Rumin. Res. 2018, 173, 94–100. [Google Scholar] [CrossRef]
- Jawasreh, K.; Ismail, Z.B.; Iya, F.; Castaneda-Bustos, V.J.; Valencia-Posadas, M. Genetic parameter estimation for preweaning growth traits in Jordan Awassi sheep. Vet. World. 2018, 11, 254. [Google Scholar] [CrossRef]
- Tesema, Z.; Alemayehu, K.; Getachew, T.; Kebede, D.; Deribe, B.; Taye, M.; Tilahun, M.; Lakew, M.; Kefale, A.; Belayneh, N.; et al. Estimation of genetic parameters for growth traits and Kleiber ratios in Boer x Central Highland goat. Trop. Anim. Health Prod. 2020, 52, 3195–3205. [Google Scholar] [CrossRef] [PubMed]
- Ghaderi–Zefrehei, M.; Safari, A.; Moridi, M.; Khanzadeh, H.; Rashidi Dehsaraei, A.R. Bayesian estimate of genetic parameters for growth traits in Lori Bakhtiari sheep. Trop. Anim. Health Prod. 2021, 53, 457. [Google Scholar] [CrossRef]
- Habtegiorgis, K.; Haile, A.; Getachew, T.; Kirmani, M.A.; Gemiyo, D. Analysis of genetic parameters and genetic trends for early growth and reproductive traits of Doyogena sheep managed under community-based breeding program. Heliyon 2022, 18, e09749. [Google Scholar] [CrossRef]
- Ehsaninia, J. Estimates of (co) variance components and genetic parameters for pre-weaning body weight traits and Kleiber ratio in Sangsari sheep breed. Ital. J. Anim. Sci. 2021, 20, 918–927. [Google Scholar] [CrossRef]
- Hanford, K.J.; Van Vleck, L.D.; Snowder, G.D. Estimates of genetic parameters and genetic change for reproduction, weight, and wool characteristics of Columbia sheep. J. Anim. Sci. 2002, 80, 3086–3098. [Google Scholar] [CrossRef]
- Oyieng, E.; Mrode, R.; Ojango, J.M.K.; Ekine-Dzivenu, C.C.; Audho, J.; Okeyo, A.M. Genetic parameters and genetic trends for growth traits of the Red Maasai sheep and its crosses to Dorper sheep under extensive production system in Kenya. Small Rumin. Res. 2022, 206, 106588. [Google Scholar] [CrossRef]
- Saghi, D.A.; Shahdadi, A.R.; Borzelabad, F.K.; Mohammadi, K. Estimates of covariance functions for growth of Kordi sheep in Iran using random regression models. Small Rumin. Res. 2018, 162, 69–76. [Google Scholar] [CrossRef]
- Mohammadi, K.; Abdollahi-Arpanahi, R.; Amraei, F.; Mohamadi, E.M.; Rashidi, A. Genetic parameter estimates for growth and reproductive traits in Lori sheep. Small Rumin. Res. 2015, 131, 35–42. [Google Scholar] [CrossRef]
- Mohammadi, Y.; Rashidi, A.; Mokhtari, M.S.; Esmailizadeh, A.K. Quantitative genetic analysis of growth traits and Kleiber ratios in Sanjabi sheep. Small Rumin. Res. 2010, 93, 88–93. [Google Scholar] [CrossRef]
- Javed, K.; Iram, A.; Abdullah, M.; Sattar, M.A.; Akhtar, M. Genetic trends for some productive traits of Lohi sheep in Pakistan. Pak. J. Sci. 2013, 65, 492–495. [Google Scholar]
- Gamasaee, V.A.; Hafezian, S.H.; Ahmadi, A.; Baneh, H.; Farhadi, A.; Mohamadi, A. Estimation of genetic parameters for body weight at different ages in Mehraban sheep. Afr. J. Biotechnol. 2010, 9, 5218–5223. [Google Scholar]
- Venkataramanan, R. Genetic Evaluation of Growth Performance of Farmbred Nilagiri and Sandyno Sheep. Ph.D. Thesis, Tamil Nadu Veterinary and Animal Sciences University, Tamil Nadu, India, 2013. [Google Scholar]
- Pedrosa, V.B.; Santana, M.L.; Oliveira, P.S.; Eler, J.P.; Ferraz, J.B.S. Population structure and inbreeding effects on growth traits of Santa Inês sheep in Brazil. Small Rumin. Res. 2010, 93, 135–139. [Google Scholar] [CrossRef]
- Mallick, P.K.; Chauhan, I.; Thirumaran, S.M.K.; Pourouchttamane, R.; Kumar, A. Genetic Variability of Bharat Merino Sheep Derived from Pedigree Information. Indian J. Anim. Res. 2020, 54, 1324–1331. [Google Scholar] [CrossRef]
- Gowane, G.R.; Prakash, V.; Chopra, A.; Prince, L.L.L. Population structure and effect of inbreeding on lamb growth in Bharat Merino sheep. Small Rumin. Res. 2013, 114, 72–79. [Google Scholar] [CrossRef]
- Venkataramanan, R.; Subramanian, A.; Sivaselvam, S.N.; Sivakumar, T.; Sreekumar, C.; Iyue, M. Effect of inbreeding and individual increase in inbreeding on growth in Nilagiri and Sandyno breeds of sheep. Anim. Genet. Resour. Inf. 2016, 58, 63–71. [Google Scholar] [CrossRef]
- Ghafouri-Kesbi, F. Analysis of genetic diversity in a close population of Zandi sheep using genealogical information. J. Genet. 2010, 89, 479–483. [Google Scholar] [CrossRef]
- Alderson, G.L.H. A System to Maximize the Maintenance of Genetic Variability in Small Populations; CABI: Wallingford, UK, 1992; pp. 18–29. [Google Scholar]
- Rieman, B.E.; Allendorf, F.W. Effective population size and genetic conservation criteria for bull trout. N. Am. J. Fish. Manag. 2001, 21, 756–764. [Google Scholar] [CrossRef]
- Meuwissen, T.H.E. Operation of conservation schemes. In Genebanks and the Conservation of Farm Animal Genetic Resources; Oldenbroek, J.K., Ed.; DLO Institute for Animal Science and Health: Lelystad, The Netherlands, 1999; pp. 91–112. [Google Scholar]
- Mokhtari, M.S.; Shahrbabak, M.M.; Esmailizadeh, A.K.; Shahrbabak, H.M.; Gutierrez, J.P. Pedigree Analysis of Iran-black Sheep and Inbreeding Effects on Growth and Reproduction Traits. Small Rumin. Res. 2014, 116, 14–20. [Google Scholar] [CrossRef]
- Mokhtari, M.S.; Miraei-Ashtiani, S.R.; Jafaroghli, M.; Gutiérrez, J.P. Studying genetic diversity in Moghani sheep using pedigree analysis. J. Agric. Sci. Technol. 2015, 17, 1151–1160. [Google Scholar]
- Barros, E.A.; Brasil, L.H.A.; Tejero, J.P.; Delgado-Bermejo, J.V.; Ribeiro, M.N. Population structure and genetic variability of the Segureña sheep breed through pedigree analysis and inbreeding effects on growth traits. Small Rumin. Res. 2017, 149, 128–133. [Google Scholar] [CrossRef]
- Vatankhah, M.; Sigdel, A.; Abdollahi-Arpanahi, R. Population structure of Lori-Bakhtiari sheep in Iran by pedigree analysis. Small Rumin. Res. 2019, 174, 148–155. [Google Scholar] [CrossRef]
| Trait | Total Records | Number of Ewes | Number of Rams | Mean Records Per | |
|---|---|---|---|---|---|
| Ewe | Ram | ||||
| Birth | 2616 | 1044 | 226 | 2.50 | 11.57 |
| Weaning | 2286 | 961 | 208 | 2.37 | 10.99 |
| Six months | 1578 | 814 | 183 | 1.93 | 8.62 |
| Nine months | 1203 | 701 | 167 | 1.71 | 7.20 |
| One year old | 1019 | 638 | 160 | 1.59 | 6.36 |
| Effect | BW | 3MW | 6MW | 9MW | 12MW |
|---|---|---|---|---|---|
| Overall | 2.35 ± 0.11 (2616) | 9.76 ± 0.06 (2286) | 13.72 ± 0.10 (1578) | 16.68 ± 0.11 (1203) | 19.46 ± 0.12 (1019) |
| Sex | * | ** | |||
| Male | 2.38 ± 0.01 (1410) | 9.90 ± 0.08 (1212) | 14.16 ± 0.14 (682) | 17.48 b ± 0.17 (469) | 20.82 b ± 0.20 (352) |
| Female | 2.32 ± 0.01 (1206) | 9.62 ± 0.09 (1074) | 13.30 ± 0.13 (896) | 15.92 a ± 0.14 (734) | 18.18 a ± 0.15 (667) |
| Period | ** | ** | ** | ** | ** |
| 1980–1984 | 2.42 a ± 0.02 (286) | 9.68 c ± 0.13 (255) | 13.22 bc ± 0.21 (172) | 15.07 a ± 0.25 (147) | 18.45 b ± 0.30 (123) |
| 1985–1989 | 2.35 c ± 0.04 (195) | 7.75 a ± 0.25 (141) | 12.47 ab ± 0.39 (104) | 15.09 a ± 0.43 (77) | 18.45 a ± 0.44 (75) |
| 1990–1994 | 2.43 b ± 0.03 (179) | 8.34 b ± 0.14 (156) | 12.08 a ± 0.19 (134) | 15.25 a ± 0.22 (120) | 18.02 ab ± 0.25 (97) |
| 1995–1999 | 2.41 bd ±0.02 (167) | 8.69 ab ± 0.22 (151) | 11.61 a ± 0.30 (127) | 15.28 a ± 0.24 (100) | 17.87 a ± 0.29 (74) |
| 2000–2004 | 2.39 cd ±0.02 (312) | 8.98 b ± 0.16 (266) | 13.93 b ± 0.22 (253) | 17.31 b ± 0.28 (196) | 19.04 ab ± 0.33 (158) |
| 2005–2008 | 2.26 b ± 0.02 (635) | 9.74 b ± 0.15 (596) | 13.59 c ± 0.20 (449) | 16.98 b ± 0.23 (389) | 20.50 c ± 0.24 (345) |
| 2009–2013 | 2.47 e ± 0.02 (585) | 11.44 e ± 0.15 (558) | 14.91 d ± 0.25 (300) | 18.18 c ± 0.36 (146) | 21.16 d ± 0.39 (127) |
| 2014–2018 | 2.17 ac ± 0.03 (257) | 10.72 e ± 0.22 (163) | 15.64 d ± 0.44 (39) | 18.93 c ± 0.51 (28) | 20.48 cd ± 0.58 (20) |
| Season | ** | * | |||
| Main | 2.36 ± 0.01 (1810) | 9.09 a ± 0.06 (1572) | 13.20 ± 0.09 (1161) | 16.48 ± 0.12 (899) | 18.98 a ± 0.13 (750) |
| Off | 2.35 ± 0.02 (806) | 10.60 b ± 0.12 (714) | 14.44 ± 0.19 (417) | 17.03 ± 0.22 (304) | 20.24 b ± 0.25 (269) |
| Birth type | ** | ||||
| Single | 2.42 b± 0.09 (2577) | 9.60 ± 0.05 (2257) | 13.67 ± 0.09 (1569) | 16.63 ± 0.11 (1200) | 19.39 ± 0.12 (1016) |
| Twin | 1.98 a ± 0.04 (39) | 10.86 ± 0.34 (29) | 14.46 ± 0.76 (9) | 18.26 ± 1.30 (3) | 21.57 ± 1.37 (3) |
| Parity | * | * | |||
| One | 2.28 a ± 0.02 (948) | 9.56 ± 0.11 (786) | 13.46 a ± 0.17 (564) | 16.70 ± 0.19 (435) | 19.54 ± 0.24 (351) |
| Two | 2.34 b ± 0.02 (685) | 9.69 ± 0.13 (594) | 13.69 c ± 0.17 (405) | 16.39 ± 0.22 (311) | 19.37 ± 0.24 (268) |
| Three | 2.42 c ± 0.01 (473) | 10.01 ± 0.12 (440) | 14.07 b ± 0.21 (329) | 16.76 ± 0.23 (266) | 19.28 ± 0.24 (232) |
| Four and above | 2.40 c ± 0.02 (510) | 9.80 ± 0.15 (466) | 13.66 b ± 0.23 (280) | 16.9 ± 0.26 (191) | 19.64 ± 0.28 (168) |
| BWD | ** | ** | ** | ** | ** |
| Model | Component | BW | 3MW | 6MW | 9MW | 12MW |
|---|---|---|---|---|---|---|
| Model 1 | σ2a | 0.08 | 0.8 | 0.69 | 0.95 | 0.88 |
| σ2e | 0.25 | 2.14 | 5.67 | 6.01 | 7.77 | |
| σ2p | 0.33 | 2.94 | 6.36 | 6.96 | 8.65 | |
| h2 | 0.24 ± 0.04 | 0.27 ± 0.04 | 0.11± 0.03 | 0.14 ± 0.04 | 0.10 ± 0.06 | |
| Model 2 * | σ2a | 0.07 | 0.72 | 0.56 | 0.98 | 0.78 |
| σ2m | 0.04 | 0.14 | 0.24 | 0.24 | 0.34 | |
| σ2e | 0.23 | 2.12 | 4.98 | 5.25 | 7.53 | |
| σ2p | 0.34 | 2.98 | 5.78 | 6.47 | 8.65 | |
| h2 | 0.21 ± 0.05 | 0.24 ± 0.05 | 0.10 ± 0.04 | 0.15 ± 0.04 | 0.09 ± 0.06 | |
| m2 | 0.12 ± 0.05 | 0.05 ± 0.02 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.02 | |
| Model 3 | σ2a | 0.04 | 0.84 | 0.93 | 0.98 | 0.92 |
| σ2m | 0.03 | 0.23 | 0.24 | 0.25 | 0.26 | |
| σam | −0.85 | −1.42 | −2.27 | −3.32 | −3.28 | |
| σ2e | 1.04 | 3.78 | 7.36 | 8.68 | 10.59 | |
| σ2p | 0.26 | 3.43 | 6.26 | 6.59 | 8.49 | |
| h2 | 0.15 ± 0.05 | 0.24 ± 0.07 | 0.15 ± 0.05 | 0.15 ± 0.04 | 0.11 ± 0.05 | |
| m2 | 0.12 ± 0.05 | 0.07 ± 0.02 | 0.04 ± 0.02 | 0.04 ± 0.01 | 0.03 ± 0.01 | |
| ram | −0.987 | −0.993 | −0.993 | −0.967 | −0.957 | |
| Model 4 | σ2a | 0.082 | 0.86 | 0.87 | 0.98 | 0.79 |
| σ2c | 0.043 | 0.29 | 0.32 | 0.28 | 0.34 | |
| σ2e | 0.243 | 2.154 | 5.23 | 5.84 | 7.14 | |
| σ2p | 0.368 | 3.304 | 6.42 | 7.1 | 8.27 | |
| h2 | 0.22 ± 0.05 | 0.26 ± 0.07 | 0.14 ± 0.03 | 0.14 ± 0.06 | 0.10 ± 0.05 | |
| c2 | 0.117 | 0.088 | 0.049 | 0.039 | 0.041 | |
| Model 5 | σ2a | 0.08 | 0.85 | 0.67 | 0.943 | 0.824 |
| σ2m | 0.06 | 0.189 | 0.231 | 0.234 | 0.143 | |
| σ2c | 0.02 | 0.167 | 0.28 | 0.27 | 0.18 | |
| σ2e | 0.212 | 2.234 | 5.46 | 5.83 | 7.85 | |
| σ2p | 0.372 | 3.44 | 6.641 | 7.277 | 8.997 | |
| h2 | 0.22 ± 0.06 | 0.25 ± 0.04 | 0.10 ± 0.06 | 0.13 ± 0.04 | 0.00 ± 0.05 | |
| m2 | 0.16 ± 0.04 | 0.05 ± 0.02 | 0.03 ± 0.01 | 0.03 ± 0.02 | 0.02 ± 0.01 | |
| c2 | 0.053 | 0.048 | 0.042 | 0.037 | 0.020 | |
| Model 6 | σ2a | 0.06 | 0.734 | 0.598 | 0.854 | 0.814 |
| σ2m | 0.03 | 0.189 | 0.183 | 0.284 | 0.283 | |
| σam | −0.68 | −2.34 | −2.85 | −3.12 | −3.28 | |
| σ2c | 0.024 | 0.227 | 0.267 | 0.252 | 0.34 | |
| σ2e | 0.945 | 4.234 | 8.56 | 7.89 | 9.89 | |
| σ2p | 0.379 | 3.044 | 6.758 | 6.16 | 8.047 | |
| h2 | 0.16 ± 0.06 | 0.24 ± 0.04 | 0.09 ± 0.06 | 0.14 ± 0.04 | 0.10 ± 0.05 | |
| m2 | 0.08 ± 0.04 | 0.06 ± 0.02 | 0.03 ± 0.01 | 0.05 ± 0.02 | 0.04 ± 0.01 | |
| ram | −0.967 | −0.987 | −0.998 | −0.978 | −0.979 | |
| c2 | 0.063 | 0.074 | 0.039 | 0.040 | 0.042 |
| Trait | BW | 3MW | 6MW | 9MW | 12MW |
|---|---|---|---|---|---|
| BW | 0.34 ± 0.12 | 0.26 ± 0.11 | 0.24 ± 0.20 | 0.20 ± 0.21 | |
| 3MW | 0.66 ± 0.11 | 0.64 ± 0.12 | 0.46 ± 0.14 | 0.45 ± 0.16 | |
| 6MW | 0.28 ± 0.11 | 0.73 ± 0.15 | 0.80 ± 0.07 | 0.70 ± 0.12 | |
| 9MW | 0.27 ± 0.21 | 0.60 ± 0.17 | 0.94 ± 0.05 | 0.81 ± 0.06 | |
| 12MW | 0.19 ± 0.23 | 0.53 ± 0.19 | 0.82 ± 0.11 | 0.91 ± 0.07 |
| Population Parameter | Value/Estimate |
|---|---|
| Population size | 4168 |
| Reference (pedigreed) population size | 3063 |
| Actual base population size (Assuming one unknown parent = half founder) | 877 |
| Effective number of founder animals in the reference population (fe) | 117 |
| Effective population size of founder | 182.83 |
| Number of ancestors contributing to the reference population | 648 |
| Effective number of ancestor animals for the reference population (fa) | 99 |
| fe/fa ratio | 1.18 |
| Number of ancestors explaining 50% variability | 42 |
| Realized effective population size (Ne) | 128.48 |
| Mean complete generation | 1.00 |
| Mean equivalent generation | 1.74 |
| Proportion of inbred individuals in the population | 0.1312 |
| Proportion of inbred individuals in the population | 0.1312 |
| Generation | Animals (N) | Mean F | % Inbred Individuals | Average F for Inbred Individuals | Mean Average Relatedness | % Pedigree Completeness |
|---|---|---|---|---|---|---|
| 0 | 649 | 0 | 0 | 0 | 0.0013 | 0.00 |
| 1 | 854 | 0 | 0 | 0 | 0.0054 | 78.95 |
| 2 | 460 | 0.0059 | 0.0282 | 0.2115 | 0.0098 | 44.42 |
| 3 | 359 | 0.0099 | 0.0863 | 0.1149 | 0.0113 | 23.57 |
| 4 | 213 | 0.0077 | 0.1361 | 0.0568 | 0.0134 | 12.28 |
| 5 | 209 | 0.0102 | 0.2344 | 0.0436 | 0.0140 | 6.66 |
| 6 | 166 | 0.0086 | 0.2228 | 0.0387 | 0.0133 | 4.03 |
| 7 | 159 | 0.0086 | 0.1194 | 0.0727 | 0.0109 | 2.24 |
| 8 | 206 | 0.0055 | 0.2184 | 0.0256 | 0.0122 | 1.05 |
| 9 | 391 | 0.0044 | 0.2429 | 0.0181 | 0.0120 | 0.31 |
| 10 | 292 | 0.0057 | 0.4246 | 0.0136 | 0.0123 | 0.06 |
| 11 | 179 | 0.0056 | 0.5195 | 0.0108 | 0.0121 | 0.00 |
| 12 | 28 | 0.0074 | 0.4285 | 0.0172 | 0.0087 | 0.00 |
| 13 | 3 | 0 | 0 | 0 | 0.0088 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balasundaram, B.; Thiruvenkadan, A.K.; Murali, N.; Muralidharan, J.; Cauveri, D.; Peters, S.O. Genetic Parameters of Growth Traits and Quantitative Genetic Metrics for Selection and Conservation of Mecheri Sheep of Tamil Nadu. Animals 2023, 13, 454. https://doi.org/10.3390/ani13030454
Balasundaram B, Thiruvenkadan AK, Murali N, Muralidharan J, Cauveri D, Peters SO. Genetic Parameters of Growth Traits and Quantitative Genetic Metrics for Selection and Conservation of Mecheri Sheep of Tamil Nadu. Animals. 2023; 13(3):454. https://doi.org/10.3390/ani13030454
Chicago/Turabian StyleBalasundaram, Balakrishnan, Aranganoor Kannan Thiruvenkadan, Nagarajan Murali, Jaganadhan Muralidharan, Doraiswamy Cauveri, and Sunday Olusola Peters. 2023. "Genetic Parameters of Growth Traits and Quantitative Genetic Metrics for Selection and Conservation of Mecheri Sheep of Tamil Nadu" Animals 13, no. 3: 454. https://doi.org/10.3390/ani13030454
APA StyleBalasundaram, B., Thiruvenkadan, A. K., Murali, N., Muralidharan, J., Cauveri, D., & Peters, S. O. (2023). Genetic Parameters of Growth Traits and Quantitative Genetic Metrics for Selection and Conservation of Mecheri Sheep of Tamil Nadu. Animals, 13(3), 454. https://doi.org/10.3390/ani13030454







