Effect of Air Exposure and Re-Submersion on the Histological Structure, Antioxidant Response, and Gene Expression of Procambarus Clarkii
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Materials
2.2. Experimental Design of Air Exposure and Re-Submersion
2.3. Histopathological Analysis
2.4. Determination of Antioxidant Enzyme Parameters
2.5. Gene Expression Analysis
2.5.1. RNA Extraction and cDNA Synthesis
2.5.2. Real-Time Quantitative PCR
2.6. Statistical Analysis
3. Results
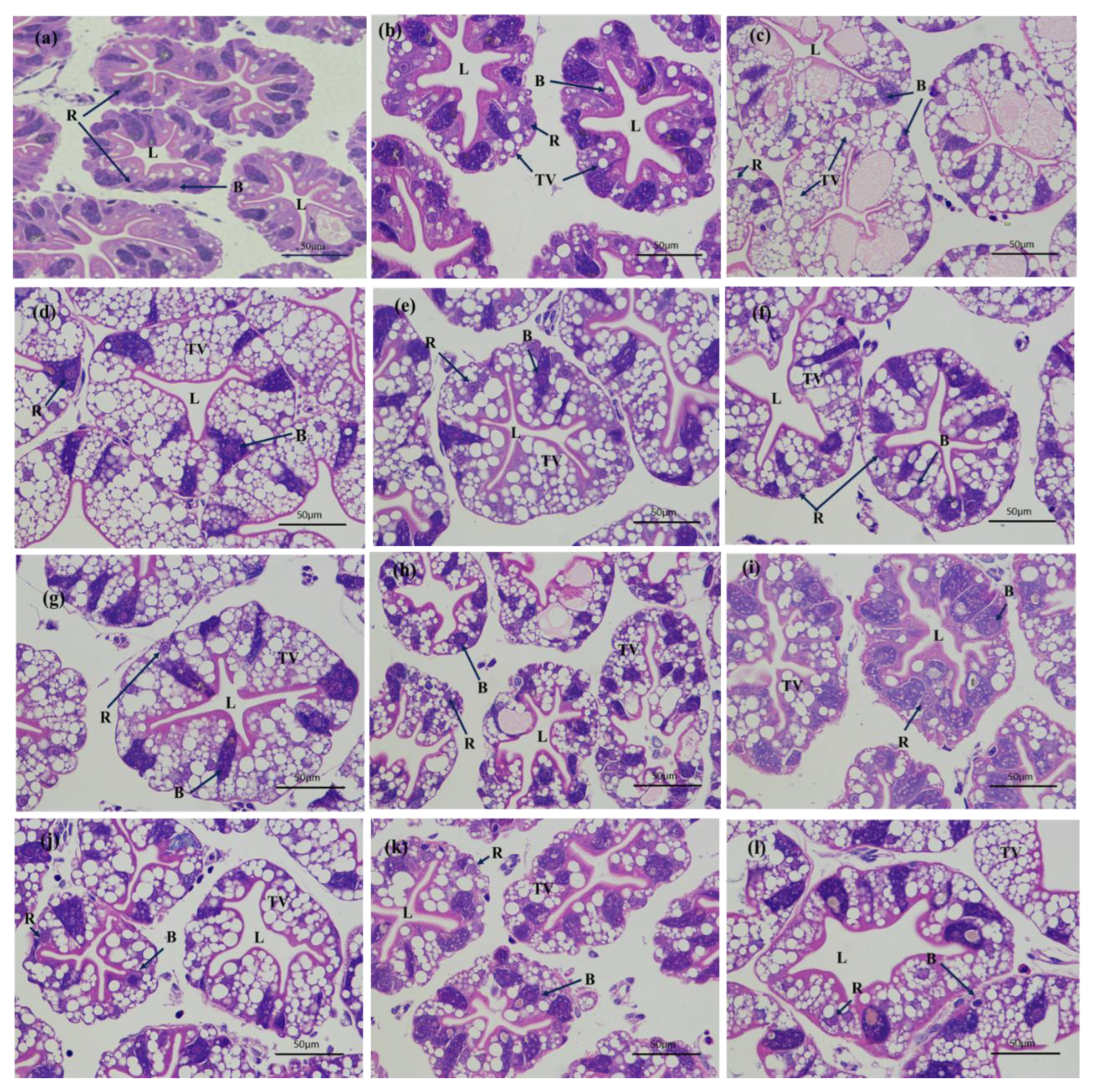
3.1. Histological Changes of Hepatopancreas and Gills after Air Exposure and Re-Submersion
3.2. Antioxidant and Metabolic Enzyme Changes after Air Exposure and Re-Submersion
3.3. Gene Expression in the Hepatopancreas and Gills
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barbaresi, S.; Santini, G.; Tricarico, E.; Gherardi, F. Ranging behaviour of the invasive crayfish, Procambarus clarkia (Girard). J. Nat. Hist. 2004, 38, 2821–2832. [Google Scholar] [CrossRef]
- Shen, C.; Tang, D.; Bai, Y.; Luo, Y.; Wu, L.; Zhang, Y.; Wang, Z. Comparative transcriptome analysis of the gills of Procambarus clarkii provide novel insights into the response mechanism of ammonia stress tolerance. Mol. Biol. Rep. 2021, 48, 2611–2618. [Google Scholar] [CrossRef] [PubMed]
- Bonvillain, C.P.; Rutherford, D.A.; Kelso, W.E. Effects of environmental hypoxia on population characteristics of red swamp crayfish Procambarus clarkii in the Atchafalaya River Basin, Louisiana. Hydrobiologia 2014, 743, 309–319. [Google Scholar] [CrossRef]
- Gherardi, F. Crayfish invading Europe: The case study of Procambarus clarkii. Mar. Freshw. Behav. Physiol. 2006, 39, 175–191. [Google Scholar] [CrossRef]
- Jin, S.; Jacquin, L.; Huang, F.; Xiong, M.; Li, R.; Lek, S.; Li, W.; Liu, J.; Zhang, T. Optimizing reproductive performance and embryonic development of red swamp crayfish Procambarus clarkii by manipulating water temperature. Aquaculture 2019, 510, 32–42. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, H.; Duan, G.; Song, G.; Ling, J.; Pan, T.; Hu, Y.; Zhou, H.; Yang, M. The Genetic Diversity of the Rice-crayfish Eco-farming Procambarus clarkii in Anhui Province, China. Turk. J. Fish. Aquat. Sci. 2021, 22, 105552. [Google Scholar] [CrossRef]
- Lorenzo, R.A.; Tapella, F.; Romero, M.C. Pre-cooling effect on live transport of the Southern king crab, Lithodes santolla. Fish. Res. 2020, 227, 105552. [Google Scholar] [CrossRef]
- Lorenzo, R.A.; Tapella, F.; Romero, M.C. Transportation methods for Southern king crab: From fishing to transient storage and long-haul packaging. Fish. Res. 2020, 223, 105441. [Google Scholar] [CrossRef]
- Paital, B. Antioxidant and oxidative stress parameters in brain of Heteropneustes fossilis under air exposure condition; role of mitochondrial electron transport chain. Ecotoxicol. Environ. Saf. 2013, 95, 69–77. [Google Scholar] [CrossRef]
- Zheng, C.; Zhao, Q.; Li, E.; Zhao, D.; Sun, S. Role of hypoxia in the behaviour, physiology, immunity and response mechanisms of crustaceans: A review. Rev. Aquac. 2021, 14, 676–687. [Google Scholar] [CrossRef]
- Yan, M.; Miao, F.; Zhang, X.; Guo, H.; Gul, Y.; Li, Q.; Song, J.; Wang, Y.; Hu, M. ‘Delayed’ interference effects of air exposure on adult Chinese horseshoe crab Tachypleus tridentatus. Aquac. Res. 2019, 50, 3633–3642. [Google Scholar] [CrossRef]
- Duan, Y.; Dong, H.; Wang, Y.; Li, Z.; Zhang, J. Effects of air exposure on respiratory metabolic enzyme activities and RNA/DNA ratios of Marsupenaeus japonicus. . Mar. Fish. 2016, 38, 42–50. [Google Scholar] [CrossRef]
- Mo, N.; Zhu, D.-D.; Liu, J.-X.; Feng, T.; Cui, Z. Metabolic responses to air-exposure stress of the Chinese mitten crab (Eriocheir sinensis) revealed by a combined analysis of metabolome and transcriptome. Aquaculture 2022, 548, 737710. [Google Scholar] [CrossRef]
- Nogueira, L.; Mello, D.F.; Trevisan, R.; Garcia, D.; Acosta, D.D.S.; Dafre, A.L.; de Almeida, E.A. Hypoxia effects on oxidative stress and immunocompetence biomarkers in the mussel Perna perna (Mytilidae, Bivalvia). Mar. Environ. Res. 2017, 126, 109–115. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.-P.; Zhang, X.-X.; Zheng, P.-H.; Zhang, Z.-L.; Li, J.-T.; Wang, D.-M.; Xian, J.-A.; Wang, A.-L.; Wang, L. Effects of air exposure on survival, histological structure, non-specific immunity and gene expression of red claw crayfish (Cherax quadricarinatus). Aquac. Rep. 2021, 21, 100898. [Google Scholar] [CrossRef]
- Duan, Y.; Zhang, Y.; Dong, H.; Zhang, J. Effect of desiccation on oxidative stress and antioxidant response of the black tiger shrimp Penaeus monodon. Fish Shellfish. Immunol. 2016, 58, 10–17. [Google Scholar] [CrossRef]
- Gao, J.; Xu, G.; Xu, P. Effect of addition of salt on oxidant activity and apoptosis of Coilia nasus juveniles under air exposure stress. Aquac. Rep. 2021, 20, 100696. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.; Li, J.T.; Pan, L.Q.; Yang, A.G. Effects of air exposure on the expression of HSP70 and ferritin genes of Exopalaemon carinicauda. Oceanol. Et. Limnol. Sin. 2013, 44, 409–414. [Google Scholar]
- Bao, J.; Xing, Y.-N.; Jiang, H.-B.; Li, X.-D. Identification of immune-related genes in gills of Chinese mitten crabs (Eriocheir sinensis) during adaptation to air exposure stress. Fish Shellfish. Immunol. 2018, 84, 885–893. [Google Scholar] [CrossRef]
- Liu, H.-L.; Yang, S.-P.; Wang, C.-G.; Chan, S.-M.; Wang, W.-X.; Feng, Z.-H.; Sun, C.-B. Effect of Air Exposure and Resubmersion on the Behavior and Oxidative Stress of Pacific White Shrimp Litopenaeus vannamei. N. Am. J. Aquac. 2014, 77, 43–49. [Google Scholar] [CrossRef]
- Han, S.Y.; Wang, M.-Q.; Liu, M.; Wang, B.-J.; Jiang, K.-Y.; Wang, L. Comparative sensitivity of the hepatopancreas and midgut in the white shrimp Litopenaeus vannamei to oxidative stress under cyclic serious/medium hypoxia. Aquaculture 2018, 490, 44–52. [Google Scholar] [CrossRef]
- Chenga, W.; Liub, C.H.; Hsuc, J.P.; Chend, J.C. Effect of hypoxia on the immune response of giant freshwater prawn Macrobrachium rosenbergii and its susceptibility to pathogen Enterococcus. Fish Shellfish. Immunol. 2002, 13, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Direkbusarakom, S.; Danayadol, Y. Effect of oxygen depletion on some parameters of the immune system in black tiger shrimp (Penaeus monodon). Adv. Shrimp Biotechnol. 1998, 5, 147–149. [Google Scholar]
- Lorenzon, S.; Giulianini, P.; Libralato, S.; Martinis, M.; Ferrero, E. Stress effect of two different transport systems on the physiological profiles of the crab Cancer pagurus. Aquaculture 2008, 278, 156–163. [Google Scholar] [CrossRef]
- Kim, C.W.; Kang, H.S. The Expression of Hsp70 and GST Genes in Mytilus coruscus during Air Exposure and Starvation. Korean J. Malacol. 2016, 32, 73–81. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Ma, H.-L.; Deng, Y.-Q.; Feng, J.; Chen, X.-L.; Guo, Z.-X. Transcriptome analysis and histopathology of the mud crab (Scylla paramamosain) after air exposure. Comp. Biochem. Physiol. Part CToxicol. Pharmacol. 2019, 228, 108652. [Google Scholar] [CrossRef]
- Eriksson, S.P.; Baden, S.P. Behaviour and tolerance to hypoxia in juvenile Norway lobster (Nephrops norvegicus) of different ages. Mar. Biol. 1997, 128, 49–54. [Google Scholar] [CrossRef]
- Duan, Y.; Zhang, J.; Dong, H.; Wang, Y.; Liu, Q.; Li, H. Effect of desiccation and resubmersion on the oxidative stress response of the kuruma shrimp Marsupenaeus japonicus. Fish Shellfish. Immunol. 2016, 49, 91–99. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Xu, W.B.; Cheng, Y.X.; Chen, D.Y.; Lin, C.Y.; Li, B.Z.; Dong, W.N.; Shu, M.A. Effects of air exposure stress on crustaceans: Histopathological changes, antioxidant and immunity of the red swamp crayfish Procambarus clarkii. Dev. Comp. Immunol. 2022, 135, 104480. [Google Scholar] [CrossRef]
- Luo, Y.; Shen, C.; Tang, D.; Bai, Y.; Wu, L.; Zhang, Y.; Wu, Y.; Wang, Z. The effects of ammonia exposure on immune response, oxidative stress, and apoptosis in Procambarus clarkii. Aquac. Int. 2022, 30, 533–546. [Google Scholar] [CrossRef]
- Stara, A.; Kouba, A.; Velisek, J. Biochemical and histological effects of sub-chronic exposure to atrazine in crayfish Cherax destructor. Chem. Interactions 2018, 291, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cheng, Y.; Li, J.; Xi, Y.; Li, J. Effect of air exposure and resubmersion on resistance to oxidative stress of Procambarus clarkii. South China Fish. Sci. 2019, 15, 69–76. [Google Scholar] [CrossRef]
- Guo, K.; Zhao, Z.; Luo, L.; Wang, S.; Zhang, R.; Xu, W.; Qiao, G. Immune and intestinal microbiota responses to aerial exposure stress in Chinese mitten crab (Eriocheir sinensis). Aquaculture 2021, 541, 736833. [Google Scholar] [CrossRef]
- Cervellione, F.; McGurk, C.; Eriksen, T.B.; Broeck, W.V.D. Use of computer-assisted image analysis for semi-quantitative histology of the hepatopancreas in whiteleg shrimp Penaeus vannamei (Boone). J. Fish Dis. 2016, 40, 1223–1234. [Google Scholar] [CrossRef] [PubMed]
- Han, S.Y.; Wang, B.J.; Liu, M.; Wang, M.Q.; Jiang, K.Y.; Qi, C.C.; Wang, L. Effect of cyclic serious/medium hypoxia stress on the survival, growth performance and resistance against Vibrio parahemolyticus of white shrimp Litopenaeus vannamei. Invertebr. Surviv. J. 2017, 14, 259–270. [Google Scholar] [CrossRef]
- Li, E.; Chen, L.; Zeng, C.; Yu, N.; Xiong, Z.; Chen, X.; Qin, J.G. Comparison of digestive and antioxidant enzymes activities, haemolymph oxyhemocyanin contents and hepatopancreas histology of white shrimp, Litopenaeus vannamei, at various salinities. Aquaculture 2008, 274, 80–86. [Google Scholar] [CrossRef]
- Tao, Y.; Qiang, J.; Wang, H.; Xu, P.; Ma, X.; Zhao, W. Effect of low pH on enzyme activity and histological structure of gill and hepatopancreas in the crayfish Procambarus clarkii. J. Fish. Sci. China 2016, 23, 1279–1289. [Google Scholar] [CrossRef]
- Yang, M.; Sun, S.; Fu, H.; Qiao, H.; Zhang, W.; Gong, Y.; Jiang, S.; Xiong, Y.; Xu, L.; Zhao, C.; et al. Hypoxia and reoxygenation on antioxidant enzyme activities and histological structure of Macrobrachium nipponense. J. Fish. Sci. China 2019, 26, 493–503. [Google Scholar] [CrossRef]
- Lahiri, S. Historical perspectives of cellular oxygen sensing and responses to hypoxia. J. Appl. Physiol. 2000, 88, 1467–1473. [Google Scholar] [CrossRef] [Green Version]
- Morel, Y.; Barouki, R. Repression of gene expression by oxidative stress. Biochem. J. 1999, 342, 481–496. [Google Scholar] [CrossRef]
- Welker, A.F.; Moreira, D.C.; Campos, G.; Hermes-Lima, M. Role of redox metabolism for adaptation of aquatic animals to drastic changes in oxygen availability. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2013, 165, 384–404. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Duan, J.; Wang, Y.; Wang, L.; Ouyang, L.; Gao, H.; Lai, X.; Zhang, Q.; Yan, B. Effect of air exposure on survival and oxidative stress response of Exopalaemon carinicauda. Fish. Sci. 2021, 40, 244–249. [Google Scholar] [CrossRef]
- Sollid, J.; Nilsson, G.E. Plasticity of respiratory structures—Adaptive remodeling of fish gills induced by ambient oxygen and temperature. Respir. Physiol. Neurobiol. 2006, 154, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-L.; Xi, Q.-Y.; Yang, L.; Li, H.-Y.; Jiang, Q.-Y.; Shu, G.; Wang, S.-B.; Gao, P.; Zhu, X.-T.; Zhang, Y.-L. The effect of dietary Panax ginseng polysaccharide extract on the immune responses in white shrimp, Litopenaeus vannamei. Fish Shellfish. Immunol. 2011, 30, 495–500. [Google Scholar] [CrossRef]
- Jia, X.-Y.; Zhong, D.-S.; Zhang, D.; Wang, F.; Zhou, W.-L. Energy metabolic enzyme responses of Litopenaeus vannamei to thermal stress: A comparative study in freshwater and seawater conditions. Aquac. Int. 2018, 26, 1067–1081. [Google Scholar] [CrossRef]
- Kaja, S.; Payne, A.J.; Singh, T.; Ghuman, J.K.; Sieck, E.G.; Koulen, P. An optimized lactate dehydrogenase release assay for screening of drug candidates in neuroscience. J. Pharmacol. Toxicol. Methods 2015, 73, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Lian, C.; Li, J.; Li, J.; Feng, Y. Effects of air exposure on respiratory metabolism related enzymes of Exopalaemon carinicauda. Prog. Fish. Sci. 2017, 38, 53–60. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, J.; Wang, Q.; Zhou, J.; Liang, J.; Zhang, R.; Yuan, H. Effect of air exposure time on the antioxidant activity of Penaeus vannamei. Fish. Sci. 2021, 40, 651–660. [Google Scholar] [CrossRef]
- Roberts, R.J.; Agius, C.; Saliba, C.; Bossier, P.; Sung, Y.Y. Heat shock proteins (chaperones) in fish and shellfish and their potential role in relation to fish health: A review. J. Fish Dis. 2010, 33, 789–801. [Google Scholar] [CrossRef]
- Cha, I.S.; Kwon, J.; Bin Park, S.; Bin Jang, H.; Nho, S.W.; Kim, Y.K.; Hikima, J.-I.; Aoki, T.; Jung, T.S. Heat shock protein profiles on the protein and gene expression levels in olive flounder kidney infected with Streptococcus parauberis. Fish Shellfish. Immunol. 2013, 34, 1455–1462. [Google Scholar] [CrossRef]
- Aishi, K.; Sinnasamy, S.; MacRae, T.H.; Muhammad, T.S.T.; Lv, A.; Sun, J.; Chen, S.; Shi, H.; Pau, T.M.; Abdullah, M.D.-D.; et al. Hsp70 knockdown reduced the tolerance of Litopenaeus vannamei post larvae to low pH and salinity. Aquaculture 2019, 512, 734346. [Google Scholar] [CrossRef]
- Wang, W.; Yang, L.; Yang, E.; Xie, R.; Chen, G.; Huang, J. Effect of hypoxic stress on the immune-related gene transcript level expression of Rachycentron canadum. Haiyang Xuebao 2021, 43, 92–101. [Google Scholar]
- Song, Y.; Wu, M.; Pang, Y.; Song, X.; Shi, A.; Shi, X.; Niu, C.; Cheng, Y.; Yang, X. Effects of melatonin feed on the changes of hemolymph immune parameters, antioxidant capacity, and mitochondrial functions in Chinese mitten crab (Eriocheir sinensis) caused by acute hypoxia. Aquaculture 2021, 535, 736374. [Google Scholar] [CrossRef]
- Li, M. Expression Characteristics of the Ferritin Gene and Heat Shock Protein 70 Gene of Exopalaemon carinicauda under WSSV and Air Exposure Conditions; Shanghai Ocean University: Shanghai, China, 2012. [Google Scholar]
- Chen, J.; Zhao, Y.L.; Wang, D. Present research situation of Ferritin. J. Henan Norm. Univ. 2010, 38, 152–155. [Google Scholar] [CrossRef]
- Andrews, S.C.; Harrison, P.M.; Yewdall, S.J.; Arosio, P.; Levi, S.; Bottke, W.; von Darl, M.; Briat, J.-F.; Laulhère, J.-P.; Lobreaux, S. Structure, function, and evolution of ferritins. J. Inorg. Biochem. 1992, 47, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Orino, K.; Lehman, L.; Tsuji, Y.; Ayaki, H.; Torti, S.V.; Torti, F.M. Ferritin and the response to oxidative stress. Biochem. J. 2001, 357 Pt 1, 241–247. [Google Scholar] [CrossRef]
- Sun, J.-L.; Zhao, L.-L.; Liao, L.; Tang, X.-H.; Cui, C.; Liu, Q.; He, K.; Ma, J.-D.; Jin, L.; Yan, T.; et al. Interactive effect of thermal and hypoxia on largemouth bass (Micropterus salmoides) gill and liver: Aggravation of oxidative stress, inhibition of immunity and promotion of cell apoptosis. Fish Shellfish. Immunol. 2019, 98, 923–936. [Google Scholar] [CrossRef]
- Orino, K.; Watanabe, K. Molecular, physiological and clinical aspects of the iron storage protein ferritin. Vet. J. 2008, 178, 191–201. [Google Scholar] [CrossRef]





| Primer Name | Nucleotide Sequence (5′-3′) |
|---|---|
| 18S-F | TCTTCTTAGAGGGATTAGCGG |
| 18S-R | AAGGGGATTGAACGGGTTA |
| CAT-F | GCTGAGGTGGAACAGATGGCAAT |
| CAT-R | CGATGAGTGTCATTGTAGGCGAAGA |
| SOD-F | GAGGCAGACTACCAAGGA |
| SOD-R | ATGGACAACGATGGCTAG |
| HSP70-F | TCAGCATCAAGTCGGCAGTCTCT |
| HSP70-R | TCCTTCATCTGGTGCTCGTATTCCT |
| Ferritin-F | ATCCGCCAGAACTACCAT |
| Ferritin-R | TTCACGCTCTTCATCACTT |
| 0 h | 1 h | 3 h | 6 h | 9 h | 12 h | 24 h | R-1 h | R-3 h | R-6 h | R-12 h | R-24 h | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hepatopancreas | L | (-) | (+) | (+) | (++) | (++) | (++) | (++) | (++) | (+) | (+) | (+) | (+) |
| B | (-) | (+) | (+) | (++) | (++) | (++) | (++) | (++) | (+) | (+) | (+) | (+) | |
| R | (-) | (-) | (+) | (+) | (+) | (+) | (++) | (++) | (+) | (+) | (+) | (+) | |
| TV | (-) | (+) | (++) | (+++) | (+++) | (+++) | (+++) | (+++) | (++) | (++) | (++) | (++) | |
| Gill | Rec | (-) | (-) | (+) | (+) | (++) | (++) | (+++) | (+++) | (+++) | (++) | (++) | (++) |
| H | (-) | (-) | (-) | (-) | (++) | (++) | (++) | (+++) | (+++) | (++) | (++) | (++) | |
| GM | (-) | (-) | (+) | (+) | (++) | (++) | (++) | (++) | (++) | (++) | (++) | (++) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, X.; Yang, L.; Tan, L.; Yang, Q.; Zhou, F.; Jiang, S.; Huang, J. Effect of Air Exposure and Re-Submersion on the Histological Structure, Antioxidant Response, and Gene Expression of Procambarus Clarkii. Animals 2023, 13, 462. https://doi.org/10.3390/ani13030462
Lei X, Yang L, Tan L, Yang Q, Zhou F, Jiang S, Huang J. Effect of Air Exposure and Re-Submersion on the Histological Structure, Antioxidant Response, and Gene Expression of Procambarus Clarkii. Animals. 2023; 13(3):462. https://doi.org/10.3390/ani13030462
Chicago/Turabian StyleLei, Xiangyu, Lishi Yang, Liqi Tan, Qibin Yang, Falin Zhou, Shigui Jiang, and Jianhua Huang. 2023. "Effect of Air Exposure and Re-Submersion on the Histological Structure, Antioxidant Response, and Gene Expression of Procambarus Clarkii" Animals 13, no. 3: 462. https://doi.org/10.3390/ani13030462




