Non-Lethal Dose-Response Models Replace Lethal Bioassays for Predicting the Hazard of Para-Aminopropiophenone to Australian Wildlife
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Target and Non-Target Populations
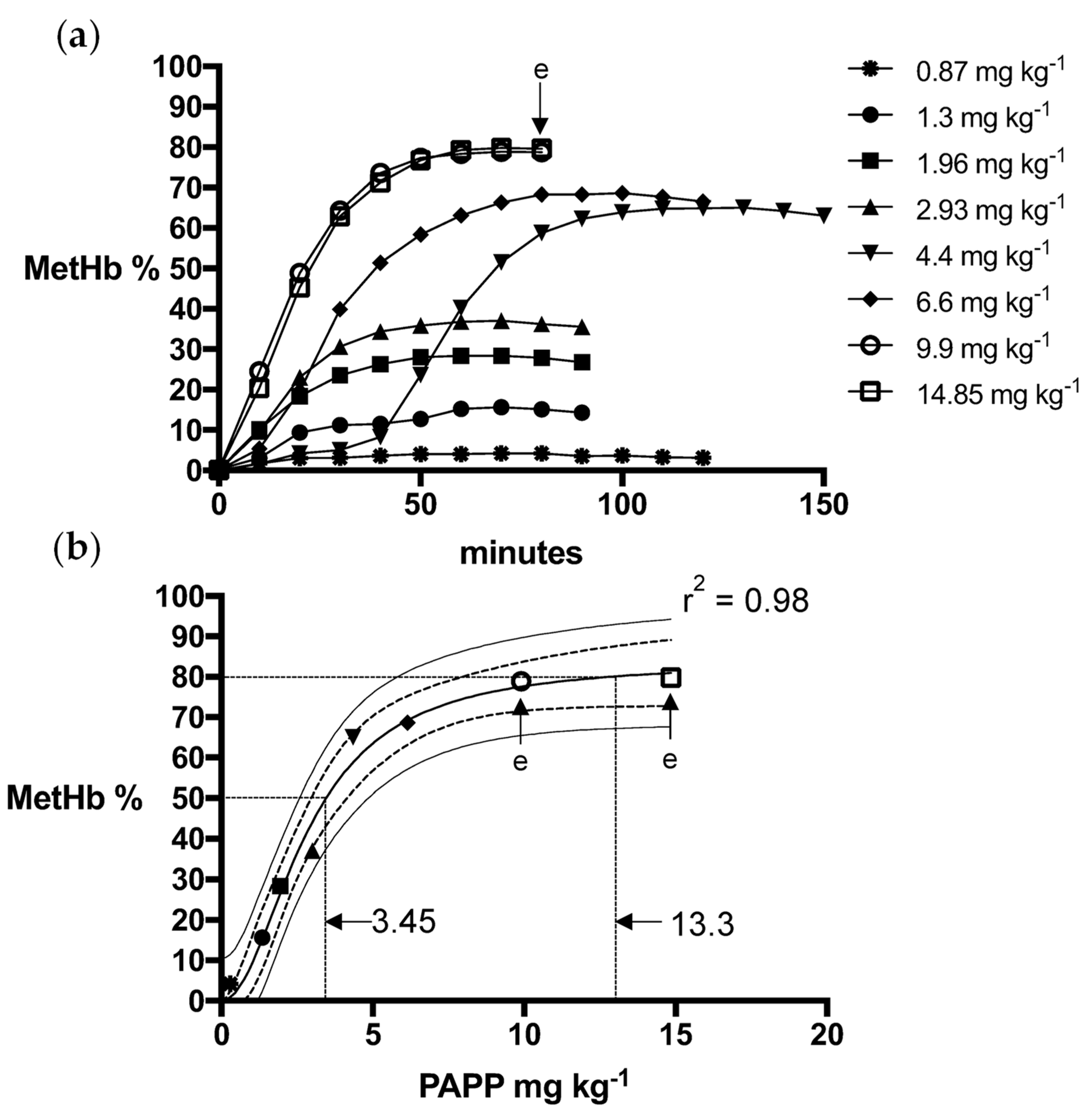
2.2. Dosage Methods
2.3. Blood Sampling and MetHb Determination
2.4. Recovery, Release and Monitoring
2.5. Statistical Analysis
3. Results
3.1. Dose-Response Models for Red Foxes and Wild Dogs
3.2. Comparative Species Sensitivity to PAPP
3.3. Comparison with Published LD50 Data
3.4. 10-Day Survival Assessment
4. Discussion
4.1. Non-Lethal Data to Model Lethal End-Points
4.2. Assessing Survival in the Field
4.3. Re-Thinking How the Hazard of Bait Toxicants Is Assessed in Non-Target Wildlife
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rolls, E.C. They All Ran Wild; Angus and Robertson: Sydney, NSW, Australia, 1969. [Google Scholar]
- Armstrong, R. Baiting operations: Western shield review—February 2003. Conserv. Sci. West. Aust. 2004, 5, 31–50. [Google Scholar]
- Glen, A.; Gentle, M.; Dickman, C. Non-target impacts of poison baiting for predator control in Australia. Mamm. Rev. 2007, 37, 191–205. [Google Scholar] [CrossRef]
- Stobo-Wilson, A.M.; Murphy, B.P.; Legge, S.M.; Caceres-Escobar, H.; Chapple, D.G.; Crawford, H.M.; Dawson, S.J.; Dickman, C.R.; Doherty, T.S.; Fleming, P.A. Counting the bodies: Estimating the numbers and spatial variation of Australian reptiles, birds and mammals killed by two invasive mesopredators. Divers. Distrib. 2022, 28, 976–991. [Google Scholar] [CrossRef]
- Cox, P.; Smith, R. Rodenticide ecotoxicology: Assessing non-target population effects. Funct. Ecol. 1990, 4, 315–320. [Google Scholar] [CrossRef]
- Eason, C.; Wickstrom, M.; Henderson, R.; Milne, L.; Arthur, D. Non-target and secondary poisoning risks associated with cholecalciferol. N. Z. Plant Prot. 2000, 53, 299–304. [Google Scholar]
- Eason, C.T.; Murphy, E.C.; Wright, G.R.; Spurr, E.B. Assessment of risks of brodifacoum to non-target birds and mammals in New Zealand. Ecotoxicology 2002, 11, 35–48. [Google Scholar] [CrossRef]
- Hoare, J.M.; Hare, K.M. The impact of brodifacoum on non-target wildlife: Gaps in knowledge. N. Z. J. Ecol. 2006, 30, 157–167. [Google Scholar]
- Langford, K.H.; Reid, M.; Thomas, K.V. The occurrence of second generation anticoagulant rodenticides in non-target raptor species in Norway. Sci. Total Environ. 2013, 450, 205–208. [Google Scholar] [CrossRef]
- McIlroy, J.C. The sensitivity of Australian animals to 1080 poison. IX. Comparisons between the major groups of animals, and the potential danger non-target species face from 1080 poisoning campaigns. Aust. Wildl. Res. 1986, 13, 39–48. [Google Scholar] [CrossRef]
- Spurr, E.B.; Berben, P.H. Assessment of non-target impact of 1080-poisoning for vertebrate pest control on weta (Orthoptera: Anostostomatidae and Rhaphidophoridae) and other invertebrates in artificial refuges. N. Z. J. Ecol. 2004, 28, 63–72. [Google Scholar]
- Weeks, A.R.; Heinze, D.; Perrin, L.; Stoklosa, J.; Hoffmann, A.A.; Van Rooyen, A.; Kelly, T.; Mansergh, I. Genetic rescue increases fitness and aids rapid recovery of an endangered marsupial population. Nat. Commun. 2017, 8, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crees, J.J.; Collins, A.C.; Stephenson, P.; Meredith, H.M.; Young, R.P.; Howe, C.; Price, M.R.S.; Turvey, S.T. A comparative approach to assess drivers of success in mammalian conservation recovery programs. Conserv. Biol. 2016, 30, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Erhirhie, E.O.; Ihekwereme, C.P.; Ilodigwe, E.E. Advances in acute toxicity testing: Strengths, weaknesses and regulatory acceptance. Interdiscip. Toxicol. 2018, 11, 5–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calver, M.; McIlroy, J.; King, D.; Bradley, J.; Gardner, J. Assessment of an approximate lethal dose technique for determining the relative susceptibility of non-target species to 1080-toxin. Wildl. Res. 1989, 16, 33–40. [Google Scholar] [CrossRef]
- Organization for Economic Co-operation and Development. OECD Guidelines for Testing of Chemicals, Acute Oral Toxicity-Fixed Dose Procedure; OECD: Paris, France, 2001. [Google Scholar]
- Rennie, A.; Buchanan-Smith, H. Refinement of the use of non-human primates in scientific research. Part III: Refinement of procedures. Anim. Welfare 2006, 15, 239–261. [Google Scholar] [CrossRef]
- Scholz, S.; Sela, E.; Blaha, L.; Braunbeck, T.; Galay-Burgos, M.; García-Franco, M.; Guinea, J.; Klüver, N.; Schirmer, K.; Tanneberger, K. A European perspective on alternatives to animal testing for environmental hazard identification and risk assessment. Regul. Toxicol. Pharmacol. 2013, 67, 506–530. [Google Scholar] [CrossRef]
- Perkins, E.J.; Ankley, G.T.; Crofton, K.M.; Garcia-Reyero, N.; LaLone, C.A.; Johnson, M.S.; Tietge, J.E.; Villeneuve, D.L. Current perspectives on the use of alternative species in human health and ecological hazard assessments. Environ. Health Perspect. 2013, 121, 1002–1010. [Google Scholar] [CrossRef] [Green Version]
- Clewell, R.A.; McMullen, P.D.; Adeleye, Y.; Carmichael, P.L.; Andersen, M.E. Pathway based toxicology and fit-for-purpose assays. In Validation of Alternative Methods for Toxicity Testing; Springer: Cham, Switzerland, 2016; pp. 205–230. [Google Scholar]
- Andersen, M.E.; Krewski, D. Toxicity testing in the 21st century: Bringing the vision to life. Toxicol. Sci. 2008, 107, 324–330. [Google Scholar] [CrossRef] [Green Version]
- Marino, M.T.; Urquhart, M.R.; Sperry, M.L.; Bredow, J.; Brown, L.D.; Lin, E.; Brewer, T.G. Pharmacokinetics and Kinetic-Dynamic Modelling of Aminophenones as Methaemoglobin Formers. J. Pharm. Pharmacol. 1997, 49, 282–287. [Google Scholar] [CrossRef]
- Graffe, W.; Kiese, M.; Rauscher, E. The formation in vivo of p-hydroxylaminopropiophenone from p-aminopropiophenone and its action in vivo and in vitro. Naunyn-Schmiedebergs Arch. Exp. Pathol. Und Pharmakol. 1964, 249, 168–175. [Google Scholar] [CrossRef]
- Senior, A. ATP synthesis by oxidative phosphorylation. Physiol. Rev. 1988, 68, 177–231. [Google Scholar] [CrossRef]
- Boutilier, R.G. Mechanisms of cell survival in hypoxia and hypothermia. J. Exp. Biol. 2001, 204, 3171–3181. [Google Scholar] [CrossRef]
- Vandenbelt, J.; Pfeiffer, C.; Kaiser, M.; Sibert, M. Methemoglobinemia after administration of p-aminoacetophenone and p-aminopropiophenone. J. Pharmacol. Exp. Ther. 1944, 80, 31–38. [Google Scholar]
- Savarie, P.J.; Pan, H.P.; Hayes, D.J.; Roberts, J.D.; Dasch, G.J.; Felton, R.; Schafer, E.W. Comparative acute oral toxicity of para-aminopropiophenone (PAPP) in mammals and birds. Bull. Environ. Contam. Toxicol. 1983, 30, 122–126. [Google Scholar] [CrossRef]
- Marks, C.; Gigliotti, F.; Busana, F.; Johnston, M.; Lindeman, M. Fox control using a para-aminopropiophenone formulation with the M-44 ejector. Anim. Welfare 2004, 13, 401–407. [Google Scholar] [CrossRef]
- APVMA. The Reconsideration of Registrations of Products Containing Sodium Fluoroacetate (1080) and Their Associated Labels; Australian Government Publisher: Canberra, NSW, Australia, 2005.
- Marks, C.A. Bait-delivered cabergoline for the reproductive control of the red fox (Vulpes vulpes): Estimating mammalian non-target risk in south-eastern Australia. Reprod. Fertil. Dev. 2001, 13, 499–510. [Google Scholar] [CrossRef]
- Hem, A.; Smith, A.J.; Solberg, P. Saphenous vein puncture for blood sampling of the mouse, rat, hamster, gerbil, guineapig, ferret and mink. Lab. Anim. 1998, 32, 364–368. [Google Scholar] [CrossRef]
- Marks, C.A.; (Victorian Institute of Animal Science, Melbourne, Victoria, Australia). Sensitivity of feral cat to para-aminiopropiophenone (PAPP) assessed by sub-lethal dose-response trials. Unpublished work.
- Konishi, S.; Kitagawa, G. Generalised information criteria in model selection. Biometrika 1996, 83, 875–890. [Google Scholar] [CrossRef] [Green Version]
- Norušis, M.J. SPSS 14.0 Guide to Data Analysis; Prentice Hall Press: Upper Saddle River, NJ, USA, 2006. [Google Scholar]
- Scawin, J.; Swanston, D.; Marrs, T. The acute oral and intravenous toxicity of p-aminopropiophenone (PAPP) to laboratory rodents. Toxicol. Lett. 1984, 23, 359–365. [Google Scholar] [CrossRef]
- Marks, C.A.; (Victorian Institute of Animal Science, Melbourne, Victoria, Australia). Sensitivity of feral cat to para-aminiopropiophenone (PAPP) assessed by lethal-dose bioassay. Unpublished work.
- Coleman, M.D.; Coleman, N.A. Drug-induced methaemoglobinaemia. Drug Saf. 1996, 14, 394–405. [Google Scholar] [CrossRef]
- Fisher, P.; O’Connor, C.; Morriss, G. Oral toxicity of p-aminopropiophenone to brushtail possums (Trichosurus vulpecula), dama wallabies (Macropus eugenii), and mallards (Anas platyrhynchos). J. Wildl. Dis. 2008, 44, 655–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loomis, T.; Hayes, A. Toxicologic testing methods. In Loomis’s Essentials of Toxicology; Academic Press, Inc.: San Diego, CA, USA, 1996; pp. 205–248. [Google Scholar]
- Pillai, S.K.; Kobayashi, K.; Mathews, M.; Mathai, T.; Sivakumar, B.; Sadasivan, P. John William Trevan’s concept of Median Lethal Dose (LD50/LC50)–more misused than used. J. Pre-Clin. Clin. Res. 2021, 15, 137–141. [Google Scholar] [CrossRef]
- Dorato, M.A.; Engelhardt, J.A. The no-observed-adverse-effect-level in drug safety evaluations: Use, issues, and definition (s). Regul. Toxicol. Pharmacol. 2005, 42, 265–274. [Google Scholar] [CrossRef]
- Kroes, R.; Kleiner, J.; Renwick, A. The threshold of toxicological concern concept in risk assessment. Toxicol. Sci. 2005, 86, 226–230. [Google Scholar] [CrossRef] [Green Version]
- Baskurt, O.K.; Meiselman, H.J. Blood Rheology and Hemodynamics; Semin. Thromb. Hemost.; Stratton Intercontinental Medical Book Corporation: New York, NY, USA, 2003; pp. 435–450. [Google Scholar]
- French, C.; Yaun, S.S.; Baldwin, L.; Leonard, D.; Zhao, X.; Calabrese, E. Potency ranking of methemoglobin-forming agents. J. Appl. Toxicol. 1995, 15, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Bosch-Marce, M.; Shimoda, L.A.; Tan, Y.S.; Baek, J.H.; Wesley, J.B.; Gonzalez, F.J.; Semenza, G.L. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J. Biol. Chem. 2008, 283, 10892–10903. [Google Scholar] [CrossRef] [Green Version]
- Bauman, R.A.; Widholm, J.J. Operant leverpressing and wheelrunning were differentially reduced by PAPP (p-aminopropiophenone)-induced methemoglobinemia. Pharmacol. Biochem. Behav. 2007, 87, 444–452. [Google Scholar] [CrossRef]
- Haymond, S.; Cariappa, R.; Eby, C.S.; Scott, M.G. Laboratory assessment of oxygenation in methemoglobinemia. Clin. Chem. 2005, 51, 434–444. [Google Scholar] [CrossRef]
- Hall, A.H.; Kulig, K.W.; Rumack, B.H. Drug-and chemical-induced methaemoglobinaemia. Med. Toxicol. 1986, 1, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, A.; Nakayama, Y.; Kitayama, T.; Tomita, M. Simulation study of methemoglobin reduction in erythrocytes. Fed. Eur. Biochem. Soc. J. 2007, 274, 1449–1458. [Google Scholar] [CrossRef]
- Bodansky, O.; Gutmann, H. Treatment of methemoglobinemia. J. Pharmacol. Exp. Ther. 1947, 90, 46–56. [Google Scholar]
- Marks, C.A.; Trought, K.; Brown, S.; Arrow, J.; Hopkins, B. Sensitivity of high conservation value birds to para-aminopropiophenone (PAPP) determined by sub-lethal dose-response assay. Animals 2023, 13, 433. [Google Scholar]
- Trevan, J.W. The error of determination of toxicity. Proc. R. Soc. Lond. Ser. B 1927, 101, 483–514. [Google Scholar]
- Oliver, J. Opportunities for using fewer animals in acute toxicity studies. In Chemicals Testing and Animal Welfare; The National Chemicals Inspectorate: Solna, Sweden, 1986. [Google Scholar]
- Millar, J.S.; Hickling, G.J. Fasting endurance and the evolution of mammalian body size. Funct. Ecol. 1990, 4, 5–12. [Google Scholar] [CrossRef]
- Crawshaw, L.I. Temperature regulation in vertebrates. Annu. Rev. Physiol. 1980, 42, 473–491. [Google Scholar] [CrossRef]
- Fischer, C.P.; Romero, L.M. Chronic captivity stress in wild animals is highly species-specific. Conserv. Physiol. 2019, 7, coz093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mason, G.J. Species differences in responses to captivity: Stress, welfare and the comparative method. Trends Ecol. Evol. 2010, 25, 713–721. [Google Scholar] [CrossRef] [Green Version]
- Calisi, R.M.; Bentley, G.E. Lab and field experiments: Are they the same animal? Horm. Behav. 2009, 56, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Clingerman, K.J. The LD50 (Median Lethal Dose) and LC50 (Median Lethal Concentration) Toxicity Tests, January 1980–August 1990; National Agricultural Library: Beltsville, MD, USA, 1990.
- Clark, M.; Steger-Hartmann, T. A big data approach to the concordance of the toxicity of pharmaceuticals in animals and humans. Regul. Toxicol. Pharmacol. 2018, 96, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Dearden, J.C.; Hewitt, M. Prediction of human lethal doses and concentrations of MEIC chemicals from Rodent LD50 values: An attempt to make some reparation. Altern. Lab. Anim. 2021, 49, 10–21. [Google Scholar] [CrossRef]
- Božina, N.; Bradamante, V.; Lovrić, M. Genetic polymorphism of metabolic enzymes P450 (CYP) as a susceptibility factor for drug response, toxicity, and cancer risk. Arch. Ind. Hyg. Toxicol. 2009, 60, 217–242. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Qian, J.; Zhang, Z.-B.; Wan, J.-X.; Wu, F.; Jin, X.-P.; Fan, W.-W.; Lu, D.-R.; Zhao, N.-Q.; Christiani, D.C. Polymorphisms in phase I and phase II metabolism genes and risk of chronic benzene poisoning in a Chinese occupational population. Carcinogenesis 2008, 29, 2325–2329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rümke, C.L. Some Limitations of Animal Tests. In Evaluation of Drug Activities: Pharmacometrics; Elsevier: Amsterdam, The Netherlands, 2013; p. 125. [Google Scholar]
- Modlinska, K.; Pisula, W. The natural history of model organisms: The Norway rat, from an obnoxious pest to a laboratory pet. Elife 2020, 9, e50651. [Google Scholar] [CrossRef] [PubMed]
- King, D.; Oliver, A.; Mead, R. The adaptation of some Western Australian mammals to food plants containing fluoroacetate. Aust. J. Zool. 1978, 26, 699–712. [Google Scholar] [CrossRef]
- Twigg, L.E.; Martin, G.R.; Lowe, T.J. Evidence of pesticide resistance in medium-sized mammalian pests: A case study with 1080 poison and Australian rabbits. J. Appl. Ecol. 2002, 39, 549–560. [Google Scholar] [CrossRef] [Green Version]
- Hothorn, L.A. Statistical evaluation of toxicological bioassays–a review. Toxicol. Res. 2014, 3, 418–432. [Google Scholar] [CrossRef]




| Common Name | Specific Name | Model * | n | m | r2 | F | p | MetHb50 | MetHb80 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose | p < 0.05 | Dose | p < 0.05 | ||||||||
| mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | ||||||||
| Australian raven | Corvus coronoides | 1 | 7 | 1 | 0.97 | 220 | <0.0001 | 33.82 | 35.1, 33.2 | - | - |
| Brushtail possum | Trichosurus vulpecula | 2 | 9 | 0 | 0.94 | 101 | <0.0001 | 574.8 | 604, 590.1 | 614.9 | 760.0, 604.0 |
| Bush rat | Rattus fuscipes | 3 | 7 | 0 | 0.99 | 433 | <0.0001 | 293 | 205.5, 144.7 | 1035 | 1217.0, 634.0 |
| Eastern quoll | Dasyurus viverrinus | 3 | 9 | 0 | 0.91 | 110 | <0.0001 | 78.3 | 170.5, 25.8 | 277 | 504.5, 107.7 |
| Fat-tailed dunnart | Sminthopsis crassicaudata | 3 | 6 | 1 | 0.99 | 299 | <0.0001 | 40 | 61.9, 22.7 | 101 | 140.5, 67.9 |
| Laboratory rat | Rattus norvegicus | 3 | 8 | 0 | 0.97 | 234 | <0.0001 | 46.5 | 76.5, 25.5 | 182.7 | 257.0, 106.0 |
| Long-nosed potoroo | Potorous tridactylus | 2 | 6 | 0 | 0.95 | 85.1 | <0.001 | 87.0 | 121.8, 54.0 | 164.3 | 201.9, 127.4 |
| Red fox | Vulpes vulpes | 4 | 9 | 3 | 0.99 | 371 | <0.0001 | 4.0 | 5.3, 3.2 | 15.4 | 38.0, 11.48 |
| Silver gull | Chroicocephalus novaehollandiae | 4 | 7 | 0 | 0.97 | 67 | <0.001 | >1000 | - | >1000 | - |
| Brown-bandicoot | Isoodon obesulus | 3 | 5 | 1 | 1 | 397 | <0.001 | 2.96 | 3.3, 2.6 | 6.3 | 6.9, 5.8 |
| Spotted-tailed quoll | Dasyurus maculatus | 2 | 9 | 0 | 0.97 | 346 | <0.0001 | 12.9 | 18.9, 9.7 | 27.1 | 29.3, 20.1 |
| Swamp rat | Rattus lutreolus | 2 | 8 | 0 | 0.96 | 190 | <0.0001 | 14.5 | 18.8, 10.4 | 26.1 | 29.9, 22.4 |
| Tasmanian devil | Sarcophilus harrisii | 4 | 8 | 0 | 0.99 | 960 | <0.0001 | 27.2 | 32.4, 22.9 | 120.3 | 156.0, 71.5 |
| Tasmanian pademelon | Thylogale billardierii | 3 | 7 | 0 | 0.91 | 80 | <0.0003 | 88.5 | 191.0, 127.9 | 334 | 396.0, 103.0 |
| Wild dog | Canis lupus familiaris | 4 | 9 | 2 | 0.99 | 268 | <0.0001 | 1.94 | 2.7, 1.5 | 8.5 | 19.8, 4.4 |
| Total | 114 | ||||||||||
| Common Name | × Dog | p < 0.05 | × Fox | p < 0.05 | |
|---|---|---|---|---|---|
| Australian raven | 17.4 * | 18.1, 17.1 * | 8.5 | 8.8, 8.3 | |
| Brushtail possum | 72.3 | 89.4, 71.1 | 39.9 | 49.4, 39.2 | |
| Bush rat | 121.8 | 143.2, 74.6 | 67.2 | 79.0, 41.2 | |
| Eastern quoll | 32.6 | 59.4, 12.7 | 18.0 | 32.8, 7.0 | |
| Fat-tailed dunnart | 11.9 | 16.5, 8.0 | 6.6 | 9.1, 4.4 | |
| Laboratory rat | 21.5 | 30.2, 12.5 | 11.9 | 16.7, 6.9 | |
| Long-nosed potoroo | 19.3 | 23.8, 15.0 | 10.7 | 13.1, 8.3 | |
| Red fox | 1.8 | 4.5, 1.4 | - | - | |
| Silver gull | >118 | - | >64.9 | - | |
| Southern brown-bandicoot | 0.7 | 0.8, 0.7 | 0.4 | 0.4, 0.4 | |
| Spotted-tailed quoll | 3.2 | 3.4, 2.4 | 1.8 | 1.9, 1.3 | |
| Swamp rat | 3.1 | 3.5, 2.6 | 1.7 | 1.9, 1.5 | |
| Tasmanian devil | 14.2 | 18.4, 8.4 | 7.8 | 10.1, 4.6 | |
| Tasmanian pademelon | 39.3 | 46.6, 12.1 | 21.7 | 25.7, 6.7 | |
| Wild dog | - | - | 0.6 | 1.3, 0.3 | |
| Mean | 34.1 | 20.1 | |||
| p < 0.05 | 21.5 | 11.9 | |||
| Species | PAPP | Placebo | ||||
|---|---|---|---|---|---|---|
| Monitored | KTBA | Died | Monitored | KTBA | Died | |
| Antechinus | 5 | 5 | 0 | - | - | - |
| Brushtail possum | 10 | 10 | 0 | 6 | 6 | 0 |
| Eastern quoll | 9 | 9 | 0 | 9 | 9 | 0 |
| Little Australian raven | 6 | 4 | 2 | 6 | 6 | 0 |
| Long-nosed potoroo | 6 | 6 | 0 | 6 | 6 | 0 |
| Tasmanian pademelon | 8 | 8 | 0 | 8 | 8 | 0 |
| Silver gull | 5 | 5 | 0 | 7 | 6 | 1 |
| Southern brown bandicoot | 4 | 4 | 0 | 6 | 6 | 0 |
| Spotted-tailed quoll | 5 | 5 | 0 | 1 | 1 | 0 |
| Swamp rat | 10 | 10 | 0 | 5 | 5 | 0 |
| Tasmanian devil | 9 | 9 | 0 | 5 | 5 | 0 |
| Total | 77 | 75 | 2 | 59 | 58 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marks, C.A.; Allen, L.; Lindeberg, H. Non-Lethal Dose-Response Models Replace Lethal Bioassays for Predicting the Hazard of Para-Aminopropiophenone to Australian Wildlife. Animals 2023, 13, 472. https://doi.org/10.3390/ani13030472
Marks CA, Allen L, Lindeberg H. Non-Lethal Dose-Response Models Replace Lethal Bioassays for Predicting the Hazard of Para-Aminopropiophenone to Australian Wildlife. Animals. 2023; 13(3):472. https://doi.org/10.3390/ani13030472
Chicago/Turabian StyleMarks, Clive A., Lee Allen, and Heli Lindeberg. 2023. "Non-Lethal Dose-Response Models Replace Lethal Bioassays for Predicting the Hazard of Para-Aminopropiophenone to Australian Wildlife" Animals 13, no. 3: 472. https://doi.org/10.3390/ani13030472






