Feeding Management and Albendazole Pharmacokinetics in Pigs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
2.3. Experimental Design, Treatments, and Sampling
2.4. Analytical Procedures
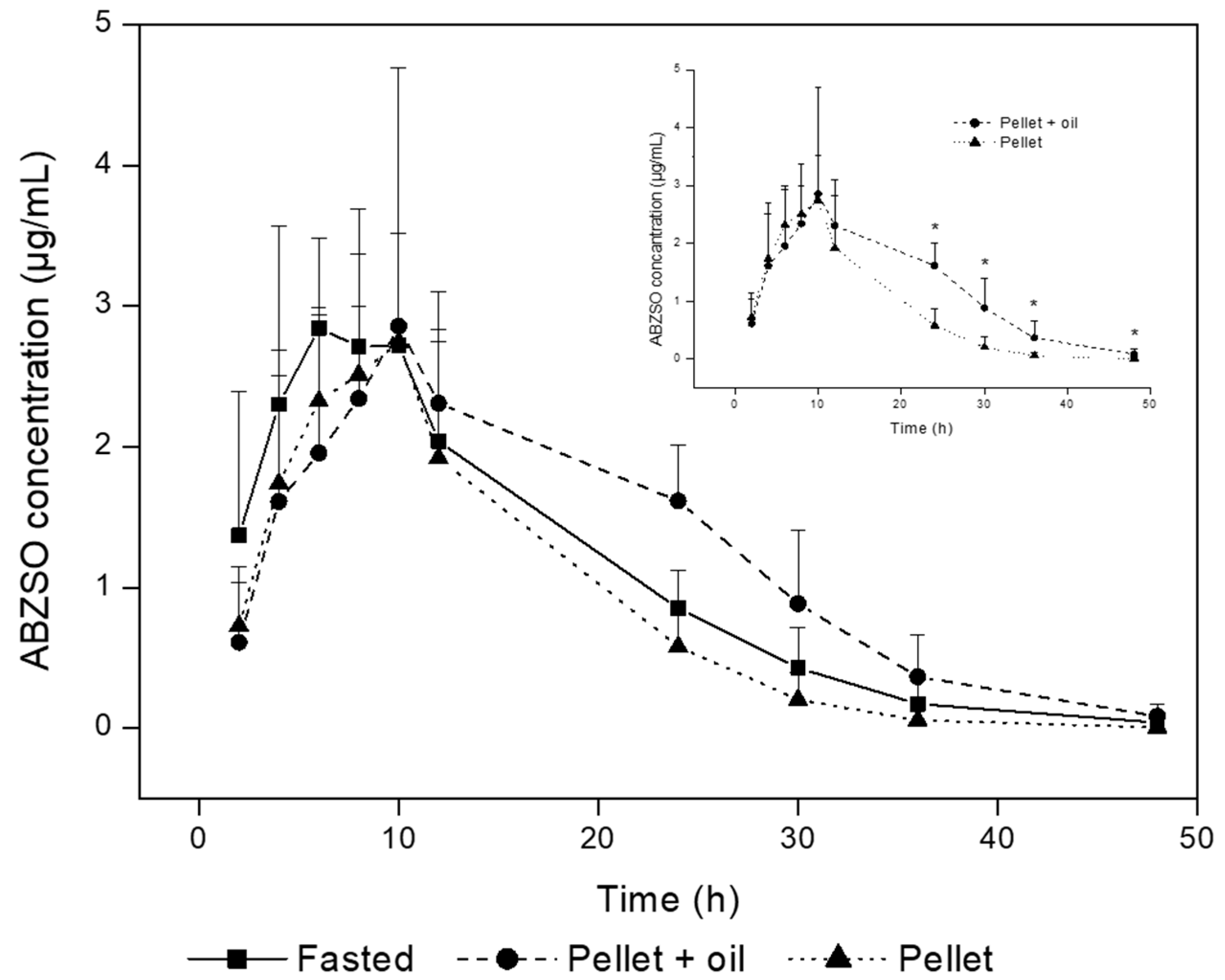
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Carstensen, L.; Vaarst, M.; Roepstorff, A. Endoparasite infections in Danish organic swine herds. Vet. Parasitol. 2002, 106, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Roepstorff, A.; Mejer, H.; Nejsum, P.; Thamsborg, S.M. Helminth parasites in pigs: New challenges in pig production and current research highlights. Vet. Parasitol. 2011, 180, 72–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pettersson, E.; Sjölund, M.; Dórea, F.C.; Lind, E.O.; Grandi, G.; Jacobson, M.; Höglund, J.; Wallgren, P. Gastrointestinal parasites in Swedish pigs: Prevalence and associated risk factors for infection in herds where animal welfare standards are improved. Vet. Parasitol. 2021, 295, 109459. [Google Scholar] [CrossRef]
- Kaplan, R.M.; Vidyashankar, A.N. An inconvenient truth: Global worming and anthelmintic resistance. Vet. Parasitol. 2012, 186, 70–78. [Google Scholar] [CrossRef]
- Charlier, J.; Thamsborg, S.M.; Bartley, D.J.; Skuce, P.J.; Kenyon, F.; Geurden, T.; Hoste, H.; Williams, A.R.; Sotiraki, S.; Höglund, J.; et al. Mind the gaps in research on the control of gastrointestinal nematodes of farmed ruminants and pigs. Transbound Emerg. Dis. 2018, 1, 217–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, W.C. Benzimidazoles: Veterinary uses. Parasitol. Today 1990, 6, 130–133. [Google Scholar] [CrossRef]
- McKellar, Q.A.; Scott, E.W. The benzimidazole anthelmintic agents—A review. J. Vet. Pharmacol. Ther. 1990, 13, 223–247. [Google Scholar] [CrossRef]
- Prichard, R.K. Interaction of host physiology and efficacy of antiparasitic drugs. Vet. Parasitol. 1985, 18, 103–110. [Google Scholar] [CrossRef]
- Thompson, D.; Geary, T. Biochemistry and Molecular Biology of Parasites; Harr, J., Muller, M., Eds.; Academic: London, UK, 1995; pp. 203–232. [Google Scholar]
- Lanusse, C.E.; Prichard, R.K. Clinical pharmacokinetics and metabolism of benzimidazole anthelmintics in ruminants. Drug Metab. Rev. 1993, 25, 235–279. [Google Scholar] [CrossRef]
- Hennessy, D.R. The disposition of antiparasitic drugs in relation to the development of resistance by parasites of livestock. Acta Trop. 1994, 56, 125–141. [Google Scholar] [CrossRef]
- Alvarez, L.I.; Saumell, C.A.; Sanchez, S.F.; Lanusse, C.E. Plasma disposition kinetics of albendazole metabolites in pigs fed different diets. Res. Vet. Sci. 1996, 60, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Welling, P. Effects of food on drug absorption. Pharmacol. Ther. 1989, 43, 425–441. [Google Scholar] [CrossRef] [PubMed]
- Lifschitz, A.; Virkel, G.; Mastromarino, M.; Lanusse, C. Enhanced plasma availability of the metabolites of albendazole in fasted adult sheep. Vet. Res. Commun. 1997, 21, 201–211. [Google Scholar] [CrossRef]
- Sánchez, S.; Alvarez, L.; Sallovitz, J.; Lanusse, C. Enhanced plasma and target tissue availabilities of albendazole and albendazole sulphoxide in fasted calves: Evaluation of different fasting intervals. J. Vet. Pharmacol. Ther. 2000, 23, 193–201. [Google Scholar] [CrossRef]
- Ceballos, L.; Nieves, E.; Juárez, M.; Aveldaño, R.; Travacio, M.; Martos, J.; Cimino, R.; Walson, J.L.; Krolewiecki, A.; Lanusse, C.; et al. Assessment of Diet-Related Changes on Albendazole Absorption; Systemic Exposure; and Pattern of Urinary Excretion in Treated Human Volunteers. Antimicrob. Agents. Chemother. 2021, 65, e0043221. [Google Scholar] [CrossRef]
- Singh, D.; Sanyal, P.K.; Swarnkar, C.P.; Khan, F.A.; Bhagwan, P.S. Influence of diet type and pretreatment fasting on the disposition kinetics of albendazole in sheep. Vet. Res. Commun. 1999, 23, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Marriner, S.E.; Bogan, J.A. Pharmacokinetics of albendazole in sheep. Am. J. Vet. Res. 1980, 41, 1126–1129. [Google Scholar] [PubMed]
- Ngomuo, A.J.; Marriner, S.E.; Bogan, J.A. The pharmacokinetics of fenbendazole and oxfendazole in cattle. Vet. Res. Commun. 1984, 8, 187–193. [Google Scholar] [CrossRef]
- Sánchez, S.F.; Alvarez, L.I.; Lanusse, C.E. Fasting-induced changes to the pharmacokinetic behaviour of albendazole and its metabolites in calves. J. Vet. Pharmacol. Ther. 1997, 20, 38–47. [Google Scholar] [CrossRef]
- Bogan, J.; Benoit, E.; Delatour, P. Pharmacokinetics of oxfendazole in goats: A comparison with sheep. J. Vet. Pharmacol. Ther. 1987, 10, 305–309. [Google Scholar] [CrossRef]
- Aksit, D.; Yalinkilinc, H.S.; Sekkin, S.; Boyacioğlu, M.; Cirak, V.Y.; Ayaz, E.; Gokbulut, C. Comparative pharmacokinetics and bioavailability of albendazole sulfoxide in sheep and goats; and dose-dependent plasma disposition in goats. BMC Vet. Res. 2015, 11, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gokbulut, C.; Akar, F.; McKellar, Q.A. Plasma disposition and faecal excretion of oxfendazole; fenbendazole and albendazole following oral administration to donkeys. Vet. J. 2006, 172, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Gokbulut, C.; Bilgili, A.; Hanedan, B.; McKellar, Q.A. Comparative plasma disposition of fenbendazole; oxfendazole and albendazole in dogs. Vet. Parasitol. 2007, 148, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Edwards, G.; Breckenridge, A. Clinical pharmacokinetics of anthelmintic drugs. Clin. Pharm. 1988, 15, 67–93. [Google Scholar] [CrossRef] [PubMed]
- Ceballos, L.; Krolewiecki, A.; Juarez, M.; Moreno, L.; Schaer, F.; Alvarez, L.; Cimino, R.; Walson, J.; Lanusse, C. Assessment of serum pharmacokinetics and urinary excretion of albendazole and its metabolites in human volunteers. PLoS Negl. Trop. Dis. 2018, 12, e0005945. [Google Scholar] [CrossRef] [Green Version]
- Gyurik, R.J.; Chow, A.W.; Zaber, B.; Brunner, E.L.; Miller, J.A.; Villani, A.J.; Petka, L.A.; Parish, R.C. Metabolism of albendazole in cattle; sheep; rats and mice. Drug Metab. Dispos. 1981, 9, 503–508. [Google Scholar]
- Villaverde, C.; Alvarez, A.I.; Redondo, P.; Voces, J.; Del Estal, J.L.; Prieto, J.G. Small intestinal sulphoxidation of albendazole. Xenobiotica 1995, 25, 5–433. [Google Scholar] [CrossRef]
- Virkel, G.; Lifschitz, A.; Sallovitz, J.; Pis, A.; Lanusse, C. Comparative hepatic and extrahepatic enantioselective sulfoxidation of albendazole and fenbendazole in sheep and cattle. Drug Metab. Dispos. 2004, 32, 536–544. [Google Scholar] [CrossRef] [Green Version]
- AVMA Panel on Euthanasia. American Veterinary Medical Association. 2000 Report of the AVMA Panel on Euthanasia. J. Am. Vet. Med. Assoc. 2001, 218, 669–696. [Google Scholar] [CrossRef]
- ICH Topic Q2 (R1) Harmonised tripartite guideline validation of analytical procedures: Text and methodology. In International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use; Somatek Inc.: San Diego, CA, USA, 2014.
- Hennessy, D.R.; Ali, D.N.; Sillince, J. The effect of a short-term reduction in feed on the pharmacokinetics and efficacy of albendazole in sheep. Aust. Vet. J. 1995, 72, 29–30. [Google Scholar] [CrossRef]
- McKellar, Q.A.; Galbraith, E.A.; Baxter, P. Oral absorption and bioavailability of fenbendazole in the dog and the effect of concurrent ingestion of food. J. Vet. Pharmacol. Ther. 1993, 16, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Bistoletti, M.; Alvarez, L.; Lanusse, C.; Moreno, L. Effects of feeding on the plasma disposition kinetics of the anthelmintic albendazole in laying hens. Br. Poult. Sci. 2014, 55, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.J. Gastrointestinal Physiology and Nutrition; Xu and Cranwell: Nottingham, UK, 2003; pp. 117–155. [Google Scholar]
- Whittaker, C.; Chesnais, C.B.; Pion, S.D.S.; Kamgno, J.; Walker, M.; Basáñez, M.G.; Boussinesq, M. Factors associated with variation in single-dose albendazole pharmacokinetics: A systematic review and modelling analysis. PLoS Negl. Trop Dis. 2022, 16, e0010497. [Google Scholar] [CrossRef] [PubMed]
- Low, A.; Pittman, R.; Elliott, R. Gastric emptying of barley-soya-bean diets in the pig: Effects of feeding level; supplementary maize oil; sucrose or cellulose; and water intake. Br. J. Nutr. 1985, 54, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Gregory, P.C.; McFadyen, M.; Rayner, D.V. Relation between gastric emptying and short-term regulation of food intake in the pig. Physiol. Behav. 1989, 45, 677–683. [Google Scholar] [CrossRef]
- Toutain, P.L.; Ferran, A.; Bousquet-Mélou, A. Species differences in pharmacokinetics and pharmacodynamics. Handb Exp Pharmacol. 2010, 199, 19–48. [Google Scholar] [CrossRef]
| PK Parameters | Fasting | Pellet + Oil | Pellet |
|---|---|---|---|
| Cmax (µg/mL) | 3.2 ± 0.8 a | 3.3 ± 1.5 a | 2.9 ± 0.6 a |
| Tmax (h) | 7.0 ± 2.1 a | 12.0 ± 6.0 a | 9.0 ± 2.1 a |
| AUC0–LOQ (µg.h/mL) | 50.2 ± 11.1 a | 58.6 ± 14.2 a | 40.5 ±11.0 a |
| AUC12–48h (µg.h/mL) | 24.3 ± 7.2 a | 37.7 ± 8.5 b | 17.5 ± 9.1 a |
| AUC0–∞ (µg.h/mL) | 50.7 ± 13.5 a | 59.7 ± 14.7 a | 40.6 ± 11.0 a |
| MRT (h) | 13.5 ± 3.4 a | 17.8 ± 4.0 a | 12.0 ± 1.9 a |
| T½el (h) | 6.4 ± 2.3 a | 6.7 ± 3.4 a | 5.3 ± 2.1 a |
| PK Parameters | Fasting | Pellet + Oil | Pellet |
|---|---|---|---|
| Cmax (µg/mL) | 0.8 ± 0.3 | 0.9 ± 0.2 | 0.8 ± 0.2 |
| Tmax (h) | 19.6 ± 6.7 | 26.0 ± 3.1 | 16.8 ± 6.5 |
| AUC0–LOQ (µg.h/mL) | 15.5 ± 4.4 | 18.2 ± 4.1 | 13.1 ± 4.3 |
| MRT (h) | 18.5 ± 4.0 | 24.6 ± 4.9 | 16.9 ± 3.5 |
| T½el (h) | 4.2 ± 2.0 | 4.4 ± 2.3 | 4.5 ± 2.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ignacio, A.L.; Valentina, C.; Lucila, M.; Paula, D.; Candela, C.; Carlos, L.; Laura, C. Feeding Management and Albendazole Pharmacokinetics in Pigs. Animals 2023, 13, 474. https://doi.org/10.3390/ani13030474
Ignacio AL, Valentina C, Lucila M, Paula D, Candela C, Carlos L, Laura C. Feeding Management and Albendazole Pharmacokinetics in Pigs. Animals. 2023; 13(3):474. https://doi.org/10.3390/ani13030474
Chicago/Turabian StyleIgnacio, Alvarez Luis, Chiappetta Valentina, Moriones Lucila, Dominguez Paula, Cantón Candela, Lanusse Carlos, and Ceballos Laura. 2023. "Feeding Management and Albendazole Pharmacokinetics in Pigs" Animals 13, no. 3: 474. https://doi.org/10.3390/ani13030474
APA StyleIgnacio, A. L., Valentina, C., Lucila, M., Paula, D., Candela, C., Carlos, L., & Laura, C. (2023). Feeding Management and Albendazole Pharmacokinetics in Pigs. Animals, 13(3), 474. https://doi.org/10.3390/ani13030474




