Characteristics of Physiological Parameters of Japanese Black Calves Relate to Carcass Weight
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Data Collection
2.2. Blood Sampling and Blood Metabolites Profiling
2.3. Genetic Analyses
2.4. Non-Genetic Analyses
3. Results and Discussion
3.1. Genetic Relationships among Growth Performance, Blood Metabolites, and Carcass Traits in Japanese Black Calves
3.2. Characteristics of Growth Performance and Blood Metabolites According to CW
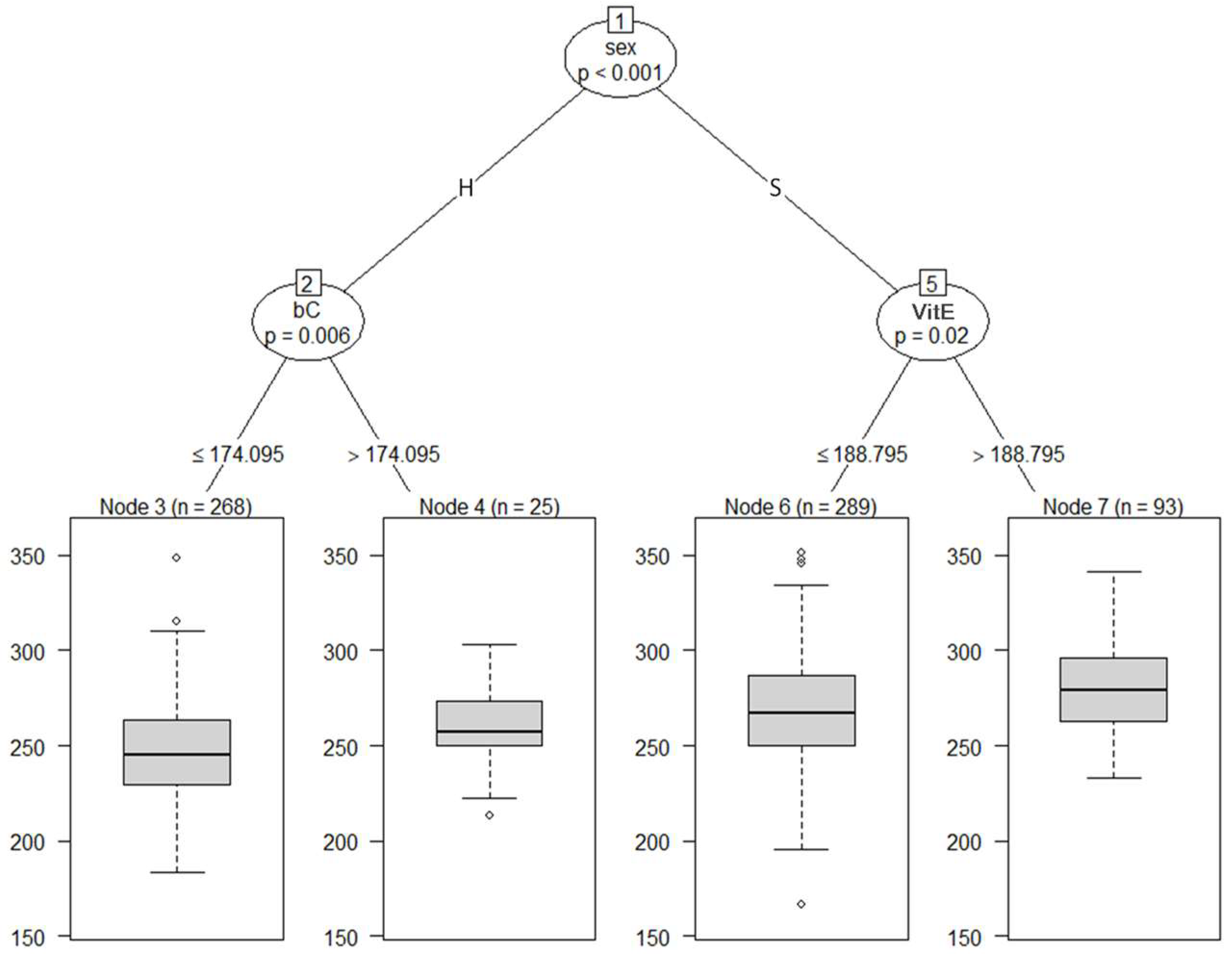
3.3. Regression Tree for CW and BW in Japanese Black Calves
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kida, K. The metabolic profile test: Its practicability in assessing feeding management and periparturient diseases in high yielding commercial dairy herds. J. Vet. Med. Sci. 2002, 64, 557–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kida, K. Relationships of metabolic profiles to milk production and feeding in dairy cows. J. Vet. Med. Sci. 2003, 65, 671–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Payne, J.; Dew, S.M.; Manston, R.; Faulks, M. The use of a metabolic profile test in dairy herds. Vet. Rec. 1970, 87, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, U.; Takagi, M.; Yamato, O.; Otoi, T.; Tshering, C.; Okamoto, K. Metabolic profile of Japanese black breeding cattle herds: Usefulness in selection for nutrient supplementation to enhance reproductive performance and regional differences. J. Vet. Med. Sci. 2013, 75, 481–487. [Google Scholar] [CrossRef] [Green Version]
- Chung, Y.-H.; Rico, D.; Martinez, C.; Cassidy, T.; Noirot, V.; Ames, A.; Varga, G. Effects of feeding dry glycerin to early postpartum Holstein dairy cows on lactational performance and metabolic profiles. J. Dairy Sci. 2007, 90, 5682–5691. [Google Scholar] [CrossRef]
- Hashemzadeh-Cigari, F.; Ghorbani, G.; Khorvash, M.; Riasi, A.; Taghizadeh, A.; Zebeli, Q. Supplementation of herbal plants differently modulated metabolic profile, insulin sensitivity, and oxidative stress in transition dairy cows fed various extruded oil seeds. Prev. Vet. Med. 2015, 118, 45–55. [Google Scholar] [CrossRef]
- Liao, Y.; Hu, R.; Wang, Z.; Peng, Q.; Dong, X.; Zhang, X.; Zou, H.; Pu, Q.; Xue, B.; Wang, L. Metabolomics profiling of serum and urine in three beef cattle breeds revealed different levels of tolerance to heat stress. J. Agric. Food Chem. 2018, 66, 6926–6935. [Google Scholar] [CrossRef]
- Phillips, W.; Juniewicz, P.; Zavy, M.; Tungeln, D.V. The effects of the stress of weaning and transit on performance and metabolic profile of beef calves of different genotypes. Can. J. Anim. Sci. 1987, 67, 991–999. [Google Scholar] [CrossRef]
- Matsuzaki, M.; Takizawa, S.; Ogawa, M. Plasma insulin, metabolite concentrations, and carcass characteristics of Japanese Black, Japanese Brown, and Holstein steers. J. Anim. Sci. 1997, 75, 3287–3293. [Google Scholar] [CrossRef] [Green Version]
- Vázquez-Mosquera, J.M.; de Mercado, E.; Fernández-Novo, A.; Gardón, J.C.; Pesántez-Pacheco, J.L.; Pérez-Solana, M.L.; Revilla-Ruiz, Á.; Martínez, D.; Villagrá, A.; Sebastián, F. Comparison of Pure and Crossbred Japanese Black Steers in Growth Performance and Metabolic Features from Birth to Slaughter at a Spanish Fattening Farm. Animals 2022, 12, 1671. [Google Scholar] [CrossRef]
- Kim, M.; Masaki, T.; Ikuta, K.; Iwamoto, E.; Nishihara, K.; Hirai, M.; Uemoto, Y.; Terada, F.; Roh, S. Physiological responses and adaptations to high methane production in Japanese Black cattle. Sci. Rep. 2022, 12, 11154. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Masaki, T.; Ikuta, K.; Iwamoto, E.; Uemoto, Y.; Terada, F.; Roh, S. Changes in the liver transcriptome and physiological parameters of Japanese Black steers during the fattening period. Sci. Rep. 2022, 12, 4029. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, T.; Nishimura, T.; Kuchida, K.; Mannen, H. The Japanese Wagyu beef industry: Current situation and future prospects—A review. Asian Australas. J. Anim. Sci. 2018, 31, 933–950. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.B.; Gotoh, T.; Greenwood, P.L. Current situation and future prospects for global beef production: Overview of special issue. Asian Australas. J. Anim. Sci. 2018, 31, 927–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fluharty, F.L.; Loerch, S.C.; Turner, T.B.; Moeller, S.J.; Lowe, G.D. Effects of weaning age and diet on growth and carcass characteristics in steers. J. Anim. Sci. 2000, 78, 1759–1767. [Google Scholar] [CrossRef]
- Schoonmaker, J.P.; Cecava, M.J.; Fluharty, F.L.; Zerby, H.N.; Loerch, S.C. Effect of source and amount of energy and rate of growth in the growing phase on performance and carcass characteristics of early- and normal-weaned steers. J. Anim. Sci. 2004, 82, 273–282. [Google Scholar] [CrossRef] [Green Version]
- NARO. Japanese Feeding Standard for Beef Cattle; Japan Livestock Industry Association: Tokyo, Japan, 2008. [Google Scholar]
- JMGA. New Beef Carcass Grading Standards; Japan Meat Grading Association: Tokyo, Japan, 1988. [Google Scholar]
- Abe, K. Application of micro particle columns for the high-performance liquid chromatographic determination of vitamin A, E and ubiquinone. Vitamin 1979, 53, 385–390. [Google Scholar]
- Gilmour, A.; Gogel, B.; Cullis, B.; Welham, S.; Thompson, R. ASReml User Guide Release 4.1 Structural Specification; VSN International Ltd.: Hemel Hempstead, UK, 2015. [Google Scholar]
- Hothorn, T.; Hornik, K.; Zeileis, A. Unbiased Recursive Partitioning: A Conditional Inference Framework. J. Comput. Graph. Stat. 2006, 15, 651–674. [Google Scholar] [CrossRef] [Green Version]
- Mukai, F.; Oyama, K.; Kohno, S. Genetic relationships between performance test traits and field carcass traits in Japanese Black cattle. Livest. Prod. Sci. 1995, 44, 199–205. [Google Scholar] [CrossRef]
- Okanishi, T.; Shojo, M.; Katsuta, T.; Oyama, K.; Mukai, F. Genetic analysis of residual feed intakes and other performance test traits of Japanese Black cattle from revised protocol. Anim. Sci. J. 2008, 79, 291–296. [Google Scholar] [CrossRef]
- Shinoda, C.; Yasuda, J.; Yamagata, K.; Suzuki, K.; Satoh, M.; Roh, S.; Uemoto, Y. Genetic relationships of feed efficiency and growth traits with carcass traits in Japanese Shorthorn cattle. Anim. Sci. J. 2022, 93, e13691. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.; Pahnish, O.; Roubicek, C. Hepatic and blood concentrations of carotene and vitamin A in unsupplemented range cattle. J. Anim. Sci. 1968, 27, 1477–1486. [Google Scholar] [CrossRef]
- Kato, Y.; Ito, M.; Hirooka, H. Genetic parameters of serum vitamin A and total cholesterol concentrations and the genetic relationships with carcass traits in an F1 cross between Japanese Black sires and Holstein dams. J. Anim. Sci. 2011, 89, 951–958. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, O.; Yamamoto, N.; Togashi, K.; Minezawa, M. Plasma metabolites concentrations in calves until 90 days of age for estimating genetic ability for milk production traits. Asian Australas. J. Anim. Sci. 2002, 15, 1813–1821. [Google Scholar] [CrossRef]
- Baco, S.; Harada, H.; Fukuhara, R. Genetic relationships of body measurements at registration to a couple of reproductive traits in Japanese Black cows. Anim. Sci. Technol. 1998, 69, 1–7. [Google Scholar]
- Bene, S.; Nagy, B.; Nagy, L.; Kiss, B.; Polgár, J.P.; Szabo, F. Comparison of body measurements of beef cows of different breeds. Arch. Anim. Breed. 2007, 50, 363–373. [Google Scholar] [CrossRef]
- Puppel, K.; Kuczyńska, B. Metabolic profiles of cow’s blood; a review. J. Sci. Food Agric. 2016, 96, 4321–4328. [Google Scholar] [CrossRef]
- Kohn, R.; Dinneen, M.; Russek-Cohen, E. Using blood urea nitrogen to predict nitrogen excretion and efficiency of nitrogen utilization in cattle, sheep, goats, horses, pigs, and rats. J. Anim. Sci. 2005, 83, 879–889. [Google Scholar] [CrossRef] [Green Version]
- Jovaisiene, J.; Bakutis, B.; Baliukoniene, V.; Gerulis, G. Fusarium and Aspergillus mycotoxins effects on dairy cow health, performance and the efficacy of Anti-Mycotoxin Additive. Pol. J. Vet. Sci. 2016, 19, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Otomaru, K.; Shiga, H.; Kanome, J.; Yanagita, K. Serum biochemical values in Japanese Black fattening steers in Kagoshima Prefecture. Jpn. J. Vet. Res. 2015, 5, 185–190. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, D.; Ando, T.; Asai, S.; Ohtsuka, H.; Takagishi, K.; Ohhashi, S.; Kumada, S.; Shiba, F.; Oikawa, M. Association of plasma gamma-glutamyl transferase (GGT) with ageand other blood chemistry findings in Japanese Black beef cattle. Jpn. J. Vet. Res. 2010, 1, 177–183. [Google Scholar] [CrossRef] [Green Version]
- Chew, B.P.; Park, J.S. Carotenoid action on the immune response. J. Nutr. 2004, 134, 257S–261S. [Google Scholar] [CrossRef] [PubMed]
- De Ondarza, M.; Wilson, J.; Engstrom, M. Case study: Effect of supplemental β-carotene on yield of milk and milk components and on reproduction of dairy cows. Prof. Anim. Sci. 2009, 25, 510–516. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, R.C.; Guerreiro, B.M.; Morais Junior, N.N.; Araujo, R.L.; Pereira, R.A.N.; Pereira, M.N. Supplementation of prepartum dairy cows with β-carotene. J. Dairy Sci. 2015, 98, 6304–6314. [Google Scholar] [CrossRef] [PubMed]
- Aragona, K.M.; Rice, E.M.; Engstrom, M.; Erickson, P.S. Effect of β-carotene supplementation to prepartum Holstein cows on colostrum quality and calf performance. J. Dairy Sci. 2021, 104, 8814–8825. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Sun, L.; Zhao, G. Effect of β-carotene on selected indices of in vitro rumen fermentation in goats. J. Anim. Feed Sci 2007, 16, 581–585. [Google Scholar] [CrossRef]
- Borel, P.; Troadec, R.; Damiani, M.; Halimi, C.; Nowicki, M.; Guichard, P.; Margier, M.; Astier, J.; Grino, M.; Reboul, E. β-Carotene Bioavailability and Conversion Efficiency Are Significantly Affected by Sex in Rats: First Observation Suggesting a Possible Hormetic Regulation of Vitamin A Metabolism in Female Rats. Mol. Nutr. Food Res. 2021, 65, 2100650. [Google Scholar] [CrossRef]
- Galan, P.; Viteri, F.; Bertrais, S.; Czernichow, S.; Faure, H.; Arnaud, J.; Ruffieux, D.; Chenal, S.; Arnault, N.; Favier, A. Serum concentrations of β-carotene, vitamins C and E, zinc and selenium are influenced by sex, age, diet, smoking status, alcohol consumption and corpulence in a general French adult population. Eur. J. Clin. Nutr. 2005, 59, 1181–1190. [Google Scholar] [CrossRef] [Green Version]
- Ozkaya, S.; Bozkurt, Y. The accuracy of prediction of body weight from body measurements in beef cattle. Arch. Anim. Breed. 2009, 52, 371–377. [Google Scholar] [CrossRef]
- Allison, R.; Laven, R. Effect of vitamin E supplementation on the health and fertility of dairy cows: A review. Vet. Rec. 2000, 147, 703–708. [Google Scholar]
- Haga, S.; Ishizaki, H.; Roh, S. The Physiological Roles of Vitamin E and Hypovitaminosis E in the Transition Period of High-Yielding Dairy Cows. Animals 2021, 11, 1088. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Panjono, P.; Kang, S.M.; Kim, T.S.; Park, Y.S. The effects of dietary sulfur and vitamin E supplementation on the quality of beef from the longissimus muscle of Hanwoo bulls. Asian Australas. J. Anim. Sci. 2008, 21, 1059–1066. [Google Scholar] [CrossRef]
- Marques, R.; Cooke, R.F.; Francisco, C.; Bohnert, D. Effects of twenty-four hour transport or twenty-four hour feed and water deprivation on physiologic and performance responses of feeder cattle. J. Anim. Sci. 2012, 90, 5040–5046. [Google Scholar] [CrossRef] [PubMed]
- Chirase, N.K.; Greene, L.W.; Purdy, C.W.; Loan, R.W.; Auvermann, B.W.; Parker, D.B.; Walborg, E.F.; Stevenson, D.E.; Xu, Y.; Klaunig, J.E. Effect of transport stress on respiratory disease, serum antioxidant status, and serum concentrations of lipid peroxidation biomarkers in beef cattle. Am. J. Vet. Res. 2004, 65, 860–864. [Google Scholar] [CrossRef] [Green Version]
- Cooke, R.F. Invited Paper: Nutritional and management considerations for beef cattle experiencing stress-induced inflammation. Prof. Anim. Sci. 2017, 33, 1–11. [Google Scholar] [CrossRef]
- Agenäs, S.; Heath, M.; Nixon, R.; Wilkinson, J.; Phillips, C. Indicators of undernutrition in cattle. Anim. Welf. 2006, 15, 149–160. [Google Scholar] [CrossRef]
- Bertoni, G.; Trevisi, E.; Han, X.; Bionaz, M. Effects of inflammatory conditions on liver activity in puerperium period and consequences for performance in dairy cows. J. Dairy Sci. 2008, 91, 3300–3310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otomaru, K.; Wataya, K.; Uto, T.; Kasai, K. Blood biochemical values in Japanese black calves in Kagoshima prefecture, Japan. J. Vet. Med. Sci. 2015, 78, 15–0381. [Google Scholar] [CrossRef]
- Kojima, T.; Aoki, N.; Ookawa, T. Effect of Feeding Cut Sudangrass Hay as a Replacement for Timothy Hay in the Early Fattening Period on Growth, Carcass Characteristics and Profitability in Japanese Black Steers. Res.Bull.Aichi Agric.Res.Ctr. 2020, 52, 87–91. [Google Scholar]
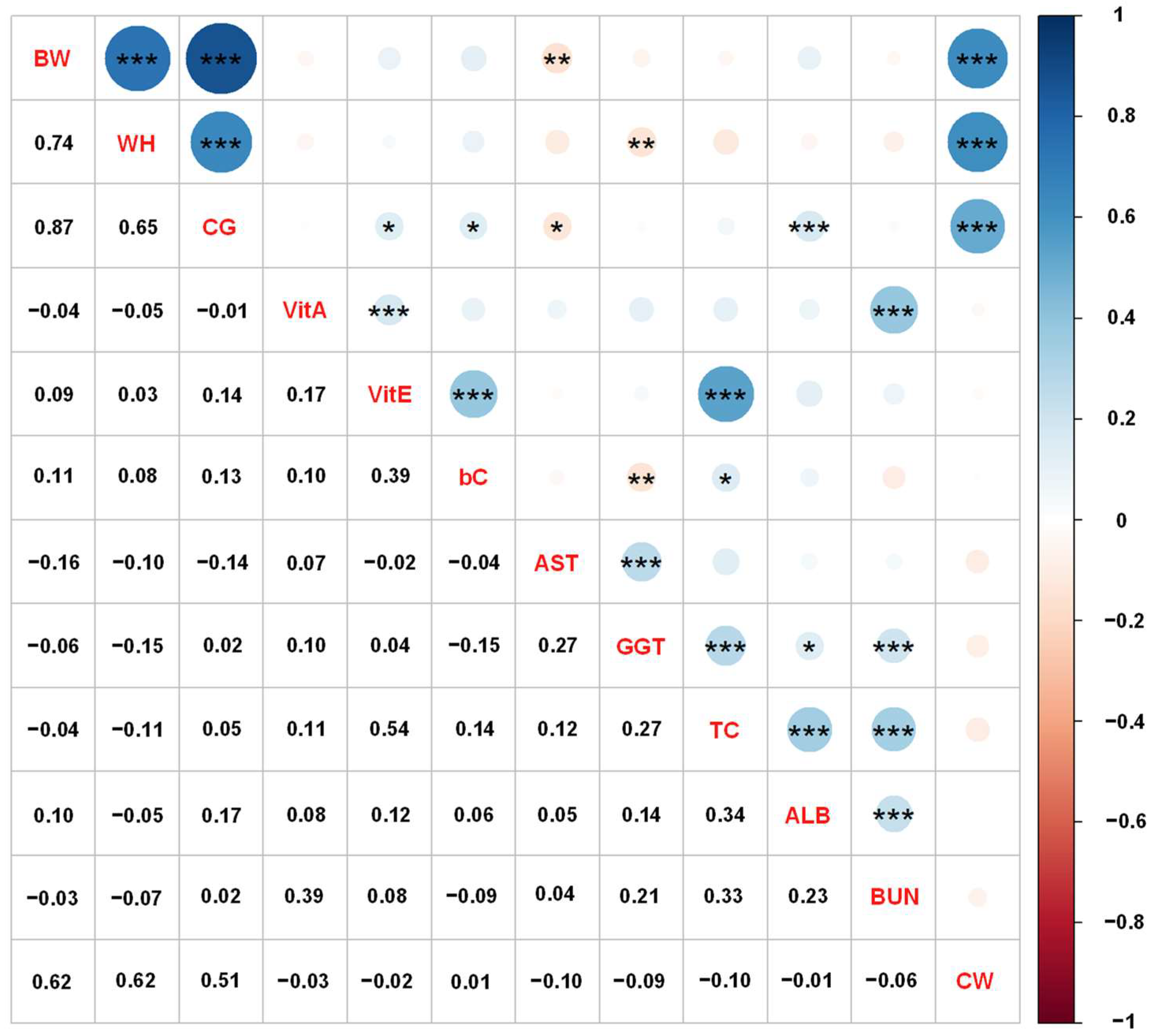

| Traits | Abbreviations | Min | Max | Mean | SD |
|---|---|---|---|---|---|
| Growth performance | |||||
| Body weight (Kg) | BW | 164 | 397 | 280.95 | 34.12 |
| Wither height (cm) | WH | 103 | 127 | 115.07 | 3.72 |
| Chest girth (cm) | CG | 130 | 172 | 151.91 | 6.58 |
| Blood metabolites | |||||
| Vitamin A (IU/dL) | VitA | 39.07 | 213.79 | 105.04 | 28.78 |
| Vitamin E (μg/dL) | VitE | 27.98 | 518.02 | 172.3 | 78.75 |
| β-carotene (μg/dL) | bC | 2.98 | 282.62 | 69.2 | 52.49 |
| Asparate aminotransferase (U/L) | AST | 46 | 170 | 71.23 | 16.68 |
| γ-glutamyl transferase (U/L) | GGT | 12 | 56 | 22.69 | 5.22 |
| Total cholesterol (mg/dL) | TC | 52 | 279 | 132.12 | 39.64 |
| Albumin (g/dL) | ALB | 2.9 | 4.8 | 3.77 | 0.26 |
| Blood urea nitrogen (mg/dL) | BUN | 4.4 | 26.8 | 13.92 | 3.46 |
| Carcass weight (Kg) | CW | 317 | 670.5 | 482.24 | 65.01 |
| Traits | Genetic Variance | Heritability | CW | BW | ||||
|---|---|---|---|---|---|---|---|---|
| Estimate | SE | Estimate | SE | rG | SE | rG | SE | |
| Growth performance | ||||||||
| BW | 388.25 | 155.92 | 0.48 | 0.17 | 0.55 | 0.20 | - | - |
| WH | 5.29 | 2.08 | 0.51 | 0.17 | 0.61 | 0.19 | 0.71 | 0.15 |
| CG | 21.92 | 7.14 | 0.74 | 0.19 | 0.42 | 0.22 | 0.80 | 0.09 |
| Blood metabolites | ||||||||
| VitA | 185.31 | 94.54 | 0.30 | 0.14 | −0.38 | 0.27 | −0.66 | 0.19 |
| VitE | 2115.56 | 983.98 | 0.36 | 0.15 | 0.00 | 0.31 | −0.30 | 0.29 |
| bC | 447.86 | 290.82 | 0.19 | 0.12 | −0.39 | 0.38 | 0.01 | 0.37 |
| AST | 106.55 | 50.74 | 0.39 | 0.16 | −0.08 | 0.31 | −0.41 | 0.27 |
| GGT | 10.08 | 4.54 | 0.38 | 0.15 | −0.08 | 0.30 | −0.57 | 0.25 |
| TC | 659.16 | 282.90 | 0.47 | 0.17 | −0.15 | 0.29 | −0.69 | 0.23 |
| ALB | 0.01 | 0.01 | 0.25 | 0.14 | 0.04 | 0.35 | −0.37 | 0.33 |
| BUN | 6.39 | 2.53 | 0.51 | 0.17 | −0.40 | 0.24 | −0.64 | 0.18 |
| CW | 1287.57 | 523.40 | 0.46 | 0.16 | - | - | - | - |
| Traits | Steer | Heifer | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| High (N = 64) | Low (N = 60) | p-Value | High (N = 48) | Low (N = 48) | p-Value | |||||
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | |||
| Growth performance | ||||||||||
| BW | 293.66 | 3.15 | 250.92 | 3.23 | <0.001 | 272.33 | 3.02 | 230.26 | 2.92 | <0.001 |
| WH | 117.18 | 0.37 | 112.74 | 0.37 | <0.001 | 113.84 | 0.32 | 109.31 | 0.39 | <0.001 |
| CG | 153.52 | 0.62 | 147.54 | 0.65 | <0.001 | 150.41 | 0.59 | 144.80 | 0.55 | <0.001 |
| Blood metabolites | ||||||||||
| VitA | 88.11 | 3.10 | 90.77 | 3.14 | n.s. | 89.13 | 3.82 | 89.72 | 3.81 | n.s. |
| VitE | 147.70 | 8.33 | 144.50 | 8.61 | n.s. | 172.46 | 11.12 | 165.07 | 10.05 | n.s. |
| bC | 93.51 | 6.20 | 95.39 | 6.17 | n.s. | 108.50 | 8.22 | 88.46 | 5.44 | <0.05 |
| AST | 68.32 | 1.86 | 71.76 | 2.11 | n.s. | 72.72 | 2.46 | 71.22 | 1.47 | n.s. |
| GGT | 19.07 | 0.41 | 21.35 | 0.79 | <0.05 | 22.18 | 0.72 | 21.26 | 0.73 | n.s. |
| TC | 106.31 | 4.01 | 111.88 | 4.05 | n.s. | 122.63 | 5.44 | 121.26 | 4.75 | n.s. |
| ALB | 3.63 | 0.03 | 3.61 | 0.02 | n.s. | 3.66 | 0.03 | 3.65 | 0.02 | n.s. |
| BUN | 11.15 | 0.34 | 12.35 | 0.47 | <0.05 | 12.98 | 0.50 | 12.14 | 0.48 | n.s. |
| CW | 577.91 | 2.88 | 425.67 | 2.00 | <0.001 | 515.19 | 3.63 | 365.97 | 2.70 | <0.001 |
| Traits | Steer | Heifer | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| High (N = 57) | Low (N = 55) | p-Value | High (N = 43) | Low (N = 46) | p-Value | |||||
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | |||
| Growth performance | ||||||||||
| BW | 317.20 | 1.77 | 228.38 | 2.02 | <0.001 | 286.01 | 2.15 | 209.61 | 1.46 | <0.001 |
| WH | 118.11 | 0.37 | 111.12 | 0.35 | <0.001 | 114.67 | 0.32 | 108.70 | 0.29 | <0.001 |
| CG | 157.60 | 0.37 | 143.72 | 0.42 | <0.001 | 152.84 | 0.48 | 140.62 | 0.55 | <0.001 |
| Blood metabolites | ||||||||||
| VitA | 85.35 | 2.97 | 91.06 | 3.40 | n.s. | 82.57 | 3.18 | 91.92 | 4.44 | n.s. |
| VitE | 163.88 | 9.98 | 126.64 | 5.58 | <0.01 | 167.46 | 10.81 | 144.55 | 8.49 | n.s. |
| bC | 98.69 | 7.05 | 80.58 | 4.37 | <0.05 | 116.85 | 9.63 | 85.59 | 6.46 | <0.01 |
| AST | 67.45 | 2.15 | 74.37 | 2.74 | <0.05 | 72.46 | 2.58 | 76.01 | 1.97 | n.s. |
| GGT | 19.25 | 0.50 | 20.41 | 0.87 | n.s. | 21.86 | 0.84 | 21.30 | 0.80 | n.s. |
| TC | 108.95 | 4.32 | 111.61 | 4.29 | n.s. | 125.45 | 6.06 | 112.64 | 6.12 | n.s. |
| ALB | 3.64 | 0.03 | 3.60 | 0.03 | n.s. | 3.74 | 0.04 | 3.65 | 0.03 | <0.05 |
| BUN | 11.90 | 0.44 | 12.28 | 0.48 | n.s. | 11.87 | 0.50 | 12.49 | 0.57 | n.s. |
| CW | 538.77 | 6.88 | 458.85 | 5.03 | <0.001 | 487.03 | 6.91 | 402.43 | 5.36 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arakawa, S.; Kim, M.; Aonuma, T.; Takagi, M.; Watanabe, S.; Lee, H.; Nishihara, K.; Haga, S.; Uemoto, Y.; Roh, S. Characteristics of Physiological Parameters of Japanese Black Calves Relate to Carcass Weight. Animals 2023, 13, 487. https://doi.org/10.3390/ani13030487
Arakawa S, Kim M, Aonuma T, Takagi M, Watanabe S, Lee H, Nishihara K, Haga S, Uemoto Y, Roh S. Characteristics of Physiological Parameters of Japanese Black Calves Relate to Carcass Weight. Animals. 2023; 13(3):487. https://doi.org/10.3390/ani13030487
Chicago/Turabian StyleArakawa, Shotaro, Minji Kim, Tatsuya Aonuma, Michihiro Takagi, Satoshi Watanabe, Huseong Lee, Koki Nishihara, Satoshi Haga, Yoshinobu Uemoto, and Sanggun Roh. 2023. "Characteristics of Physiological Parameters of Japanese Black Calves Relate to Carcass Weight" Animals 13, no. 3: 487. https://doi.org/10.3390/ani13030487





