Inclusion of Red Macroalgae (Asparagopsis taxiformis) in Dairy Cow Diets Modulates Feed Intake, Chewing Activity and Estimated Saliva Secretion
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Feeds and Feeding
2.3. Feed Samples, Analysis and Chemical Composition
2.4. Eating, Rumination Activities and Ruminal Fluid pH
2.5. Calculations, Data Summary and Statistical Analyses
3. Results and Discussion
3.1. Feed and Water Intake
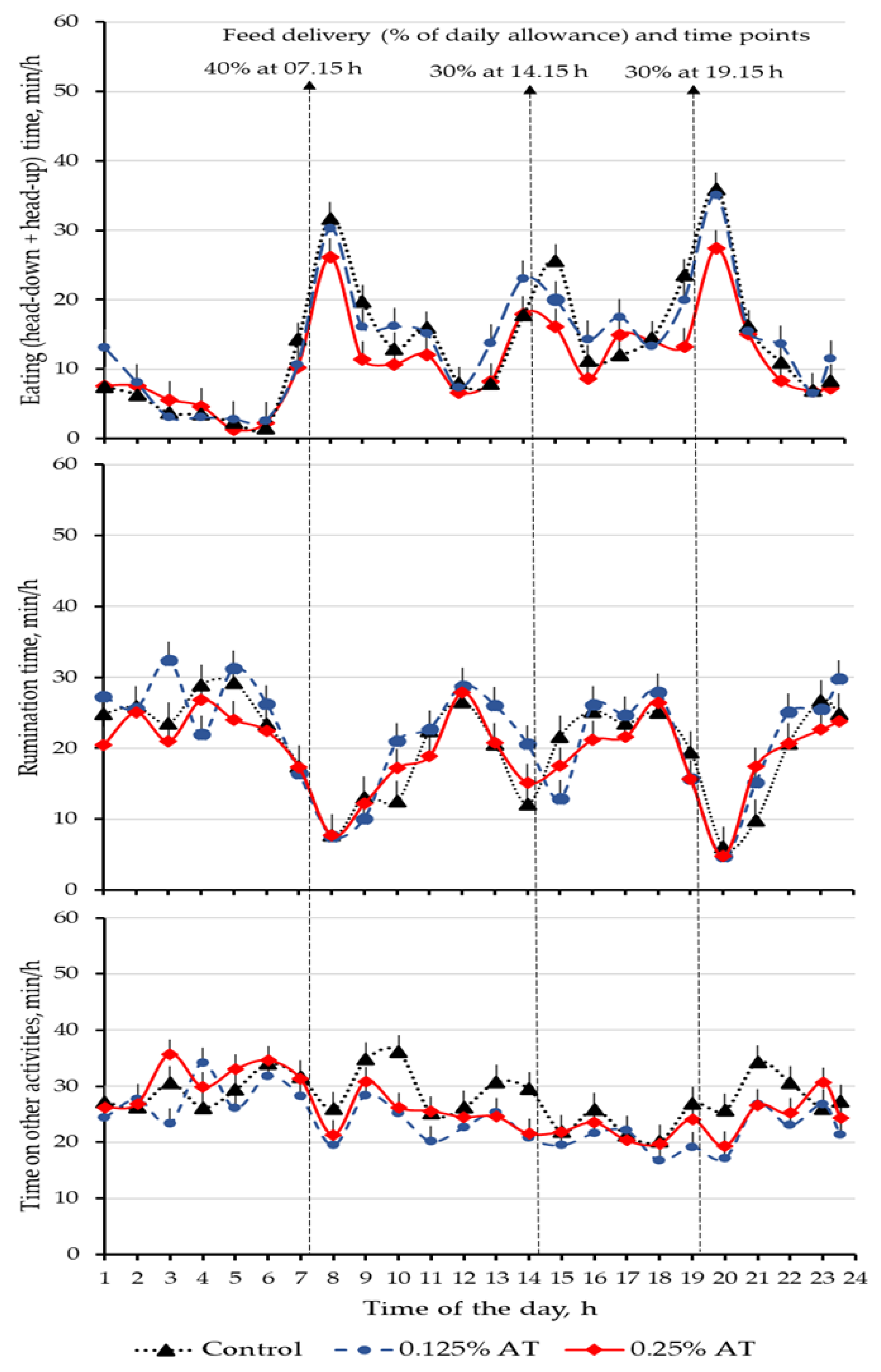
3.2. Time Budget on Eating, Rumination and Other Activities
3.3. Chewing Index and Estimated Saliva Secretion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Green, T.C.; Jago, J.G.; Macdonald, K.A.; Waghorn, G.C. Relationships between residual feed intake, average daily gain, and feeding behavior in growing dairy heifers. J. Dairy Sci. 2013, 96, 3098–3107. [Google Scholar] [CrossRef]
- Richardson, E.C.; Herd, R.M. Biological basis for variation in residual feed intake in beef cattle. 2. Synthesis of results following divergent selection. Aust. J. Exp. Agric. 2004, 44, 431–440. [Google Scholar] [CrossRef]
- Kidane, A.; Prestløkken, E.; Zaralis, K.; Steinshamn, H. Effects of three short-term pasture allocation methods on milk production, methane emission and grazing behaviour by dairy cows. Acta Agric. Scand. A 2018, 68, 87–102. [Google Scholar] [CrossRef]
- Tolkamp, B.J.; Friggens, N.C.; Emmans, G.C.; Kyriazakis, I.; Oldham, J.D. Meal patterns of dairy cows consuming mixed foods with a high or a low ratio of concentrate to grass silage. Anim. Sci. 2002, 74, 369–382. [Google Scholar] [CrossRef]
- Zehner, N.; Umstätter, C.; Niederhauser, J.J.; Schick, M. System specification and validation of a noseband pressure sensor for measurement of ruminating and eating behavior in stable-fed cows. Comput. Electron. Agr. 2017, 136, 31–41. [Google Scholar] [CrossRef]
- Neave, H.W.; Weary, D.M.; von Keyserlingk, M.A.G. Review: Individual variability in feeding behaviour of domesticated ruminants. Animal 2018, 12, s419–s430. [Google Scholar] [CrossRef] [Green Version]
- Roque, B.M.; Salwen, J.K.; Kinley, R.; Kebreab, E. Inclusion of Asparagopsis armata in lactating dairy cows’ diet reduces enteric methane emission by over 50 percent. J. Clean. Prod. 2019, 234, 132–138. [Google Scholar] [CrossRef]
- Li, X.; Hayley, N.; Kinley, R.D.; Laurence, M.; Wilmot, M.; Bender, H.; de Nys, R.; Tomkins, N.W. Asparagopsis taxiformis decreases enteric methane production from sheep. Anim. Prod. Sci. 2016, 58, 681–688. [Google Scholar] [CrossRef]
- Stefenoni, H.A.; Räisänen, S.E.; Cueva, S.F.; Wasson, D.E.; Lage, C.F.A.; Melgar, A.; Fetter, M.E.; Smith, P.; Hennessy, M.; Vecchiarelli, B.; et al. Effects of the macroalga Asparagopsis taxiformis and oregano leaves on methane emission, rumen fermentation, and lactational performance of dairy cows. J. Dairy Sci. 2021, 104, 4157–4173. [Google Scholar] [CrossRef]
- Garcia, F.; Muñoz, C.; Martínez-Ferrer, J.; Urrutia, N.L.; Martínez, E.D.; Saldivia, M.; Immig, I.; Kindermann, M.; Walker, N.; Ungerfeld, E.M. 3-Nitrooxypropanol substantially decreased enteric methane emissions of dairy cows fed true protein- or urea-containing diets. Heliyon 2022, 8, e09738. [Google Scholar] [CrossRef]
- Roque, B.M.; Venegas, M.; Kinley, R.D.; de Nys, R.; Duarte, T.L.; Yang, X.; Kebreab, E. Red seaweed (Asparagopsis taxiformis) supplementation reduces enteric methane by over 80 percent in beef steers. PLoS ONE 2021, 16, e0247820. [Google Scholar] [CrossRef]
- Reynolds, C.K.; Humphries, D.J.; Kirton, P.; Kindermann, M.; Duval, S.; Steinberg, W. Effects of 3-nitrooxypropanol on methane emission, digestion, and energy and nitrogen balance of lactating dairy cows. J. Dairy Sci. 2014, 97, 3777–3789. [Google Scholar] [CrossRef] [Green Version]
- Haisan, J.; Sun, Y.; Guan, L.L.; Beauchemin, K.A.; Iwaasa, A.D.; Duval, S.M.; Kindermann, M.; Barreda, D.R.; Oba, M. The effects of feeding 3-nitrooxypropanol at two doses on milk production, rumen fermentation, plasma metabolites, nutrient digestibility, and methane emissions in lactating Holstein cows. Anim. Prod. Sci. 2016, 57, 282–289. [Google Scholar] [CrossRef]
- Muizelaar, W.; Groot, M.; van Duinkerken, G.; Peters, R.; Dijkstra, J. Safety and transfer study: Transfer of bromoform present in Asparagopsis taxiformis to milk and urine of lactating dairy cows. Foods 2021, 10, 584. [Google Scholar] [CrossRef]
- Beauchemin, K.A. Invited review: Current perspectives on eating and rumination activity in dairy cows. J. Dairy Sci. 2018, 101, 4762–4784. [Google Scholar] [CrossRef] [Green Version]
- Volden, H. (Ed.) NorFor: NorFor-The Nordic Feed Evaluation System; Wageningen Academic Publishers: Wageningen, The Netherlands, 2011. [Google Scholar]
- Kidane, A.; Gregersen, V.S.; Ferneborg, S.; Skeie, S.; Olsen, M.A.; Mydland, L.T.; Øverland, M.; Prestløkken, E. Cyberlindnera jadinii yeast as a protein source in early- to mid-lactation dairy cow diets: Effects on feed intake, ruminal fermentation, and milk production. J. Dairy Sci. 2022, 105, 2343–2353. [Google Scholar] [CrossRef]
- Zehner, N.; Niederhauser, J.J.; Nydegger, F.; Grothmann, A.; Keller, M.; Hoch, M.; Haeussermann, A.; Schick, M. Validation of a New health monitoring system (RumiWatch) for combined automatic measurement of rumination, feed intake, water intake and locomotion in dairy cows. Infomation Technology, Automation and Precision Farming. In Proceedings of the International Conference of Agricultural Engineering—CIGR-AgEng 2012: Agriculture and Engineering for a Healthier Life, Valencia, Spain, 8–12 July 2012; Volume 5. Available online: https://www.rumiwatch.com/files/Zehner-et-al-2012_Validation-of-RumiWatch_CIGR-AGENG-2012.pdf (accessed on 21 December 2022).
- Norbu, N.; Alvarez-Hess, P.S.; Leury, B.J.; Wright, M.M.; Douglas, M.L.; Moate, P.J.; Williams, S.R.O.; Marett, L.C.; Garner, J.B.; Wales, W.J.; et al. Assessment of RumiWatch noseband sensors for the quantification of ingestive behaviors of dairy cows at grazing or fed in stalls. Anim. Feed Sci. Technol. 2021, 280, 115076. [Google Scholar] [CrossRef]
- Maekawa, M.; Beauchemin, K.A.; Christensen, D.A. Effect of concentrate level and feeding management on chewing activities, saliva production, and ruminal pH of lactating dairy cows. J. Dairy Sci. 2002, 85, 1165–1175. [Google Scholar] [CrossRef]
- Bailey, C.B. Saliva secretion and its relation to feeding in cattle: 3. The rate of secretion of mixed saliva in the cow during eating, with an estimate of the magnitude of the total daily secretion of mixed saliva. Br. J. Nutr. 1961, 15, 443–451. [Google Scholar] [CrossRef]
- Sclafani, A. Learned controls of ingestive behaviour. Appetite 1997, 29, 153–158. [Google Scholar] [CrossRef]
- Janssen, P.H. Influence of hydrogen on rumen methane formation and fermentation balances through microbial growth kinetics and fermentation thermodynamics. Anim. Feed Sci. Technol. 2010, 160, 1–22. [Google Scholar] [CrossRef]
- Castle, M.E.; Thomas, T.P. The water intake of British Friesian cows on rations containing various forages. Anim. Sci. 1975, 20, 181–189. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Dairy Cattle, 7th ed.; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- Dado, R.G.; Allen, M.S. Variation in and relationships among feeding, chewing, and drinking variables for lactating dairy cows. J. Dairy Sci. 1994, 77, 132–144. [Google Scholar] [CrossRef]
- Holter, J.B.; Urban, W.E. Water partitioning and intake prediction in dry and lactating Holstein cows. J. Dairy Sci. 1992, 75, 1472–1479. [Google Scholar] [CrossRef]
- Beauchemin, K.A. Ingestion and mastication of feed by dairy cattle. Vet. Clin. N. Am. Food Anim. Pract. 1991, 7, 439–463. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; Farr, B.I.; Rode, L.M.; Schaalje, G.B. Optimal neutral detergent fiber concentration of barley-based diets for lactating dairy cows. J. Dairy Sci. 1994, 77, 1013–1029. [Google Scholar] [CrossRef]
- Woodford, J.A.; Jorgensen, N.A.; Barrington, G.P. Impact of dietary fiber and physical form on performance of lactating dairy cows. J. Dairy Sci. 1986, 69, 1035–1047. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; Farr, B.I.; Rode, L.M. Enhancement of the effective fiber content of barley-based concentrates fed to dairy cows. J. Dairy Sci. 1991, 74, 3128–3139. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; Eriksen, L.; Nørgaard, P.; Rode, L.M. Short communication: Salivary secretion during meals in lactating dairy cattle. J. Dairy Sci. 2008, 91, 2077–2081. [Google Scholar] [CrossRef] [Green Version]
- DeVries, T.J.; Gill, R.M. Adding liquid feed to a total mixed ration reduces feed sorting behavior and improves productivity of lactating dairy cows. J. Dairy Sci. 2012, 95, 2648–2655. [Google Scholar] [CrossRef]
- Coppock, C.E.; Bath, D.L.; Harris, B. From feeding to feeding systems. J. Dairy Sci. 1981, 64, 1230–1249. [Google Scholar] [CrossRef]
- Miller-Cushon, E.K.; DeVries, T.J. Effect of dietary dry matter concentration on the sorting behavior of lactating dairy cows fed a total mixed ration. J. Dairy Sci. 2009, 92, 3292–3298. [Google Scholar] [CrossRef] [PubMed]
- Miller-Cushon, E.K.; DeVries, T.J. Feed sorting in dairy cattle: Causes, consequences, and management. J. Dairy Sci. 2017, 100, 4172–4183. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, C.; Armentano, L.E. Short communication: Feed selection by dairy cows fed individually in a tie-stall or as a group in a free-stall barn. J. Dairy Sci. 2007, 90, 2386–2389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krause, K.M.; Oetzel, G.R. Understanding and preventing subacute ruminal acidosis in dairy herds: A review. Anim. Feed Sci. Technol. 2006, 126, 215–236. [Google Scholar] [CrossRef]
- Nørgaard, P.; Nadeau, E.; Randby, Å.; Volden, H. Chewing index system for predicting physical structure of the diet. In NorFor-The Nordic Feed Evaluation System; Volden, H., Ed.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2011; pp. 127–132. [Google Scholar]
- Van Soest, P.J. Nutritional Ecology of the Ruminant, 2nd ed.; Cornell University Press: Ithaca, NY, USA, 1994. [Google Scholar]
| Item | Total Mixed Ration 1 | Asparagopsis taxiformis |
|---|---|---|
| DM content (g/kg feed) | 360.0 | 950.0 |
| Organic matter | 922.9 | 483.0 |
| Ash | 77.1 | 517.0 |
| Crude protein | 178.1 | na |
| NDFom 2 | 371.7 | na |
| ADFom 3 | 196.8 | na |
| Starch | 134.5 | na |
| Crude fat | 39.1 | na |
| RestCHO + FFP 4 | 199.5 | na |
| NEL20 (MJ/kg DM) 5 | 6.63 | na |
| Parameters | AT Inclusion (%, OM Basis) | Statistics | Contrasts 1 | ||||
|---|---|---|---|---|---|---|---|
| Intake | 0 | 0.125 | 0.25 | SE | p-Value | L | Q |
| Dry matter intake, kg/d | 22.1 b | 22.4 b | 19.9 a | 0.53 | 0.009 | 0.017 | 0.043 |
| Water intake, kg/d | 70.1 | 77.5 | 65.9 | 4.67 | 0.190 | 0.466 | 0.121 |
| Time Budget, min/d | |||||||
| Eating, head-up | 139.1 | 138.0 | 121.5 | 24.1 | 0.873 | 0.636 | 0.78 |
| Eating, head-down | 90.5 a | 162.0 b | 246.2 c | 26.8 | 0.021 | 0.009 | 0.836 |
| Sum eating (head-up + head-down) | 229.5 | 301.4 | 367.4 | 36.4 | 0.092 | 0.042 | 0.944 |
| Rumination | 474.1 | 517.4 | 488.6 | 32.1 | 0.516 | 0.770 | 0.332 |
| Other activities | 688.8 | 596.2 | 567.9 | 59.3 | 0.317 | 0.221 | 0.623 |
| Parameters | AT Inclusion (%, OM Basis) | Statistics | Contrasts 1 | ||||
|---|---|---|---|---|---|---|---|
| 0 | 0.125 | 0.25 | SE | p-Value | L | Q | |
| DMI intake rate, g/min | 103.3 b | 67.0 a | 62.4 a | 5.13 | <0.001 | <0.001 | 0.06 |
| EI, min/kg DMI | 10.7 a | 14.2 ab | 17.3 c | 1.38 | 0.031 | 0.011 | 0.90 |
| RI, min/kg DMI | 21.7 a | 22.8 ab | 24.0 b | 0.53 | 0.018 | 0.006 | 0.99 |
| CI, min/kg DMI | 32.1 a | 37.6 b | 41.3 c | 0.99 | <0.001 | <0.001 | 0.42 |
| Chews, counts/kg DMI | 1922 a | 2322 b | 2608 b | 177 | 0.0016 | 0.0005 | 0.22 |
| Saliva secretion, L/d | 236 | 255 | 254 | 7.10 | 0.058 | 0.033 | 0.11 |
| Saliva per kg DMI, L | 10.7 a | 11.4 a | 13.0 b | 0.38 | 0.0001 | <0.001 | 0.14 |
| Ruminal fluid pH | 6.09 a | 6.14 a | 6.37 b | 0.064 | 0.028 | 0.012 | 0.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nyløy, E.; Prestløkken, E.; Eknæs, M.; Eikanger, K.S.; Heldal Hagen, L.; Kidane, A. Inclusion of Red Macroalgae (Asparagopsis taxiformis) in Dairy Cow Diets Modulates Feed Intake, Chewing Activity and Estimated Saliva Secretion. Animals 2023, 13, 489. https://doi.org/10.3390/ani13030489
Nyløy E, Prestløkken E, Eknæs M, Eikanger KS, Heldal Hagen L, Kidane A. Inclusion of Red Macroalgae (Asparagopsis taxiformis) in Dairy Cow Diets Modulates Feed Intake, Chewing Activity and Estimated Saliva Secretion. Animals. 2023; 13(3):489. https://doi.org/10.3390/ani13030489
Chicago/Turabian StyleNyløy, Emma, Egil Prestløkken, Margrete Eknæs, Katrine Sømliøy Eikanger, Live Heldal Hagen, and Alemayehu Kidane. 2023. "Inclusion of Red Macroalgae (Asparagopsis taxiformis) in Dairy Cow Diets Modulates Feed Intake, Chewing Activity and Estimated Saliva Secretion" Animals 13, no. 3: 489. https://doi.org/10.3390/ani13030489







