Diet of the Insular Lizard, Podarcis lilfordi (Günther, 1874): Complementary Morphological and Molecular Approaches
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling
2.2. Molecular Study of the Diet
2.2.1. DNA Extraction and Library Preparation
2.2.2. Sequence Analyses and Taxonomic Assignment
2.3. Morphological Study of the Diet
2.4. Diet Comparison
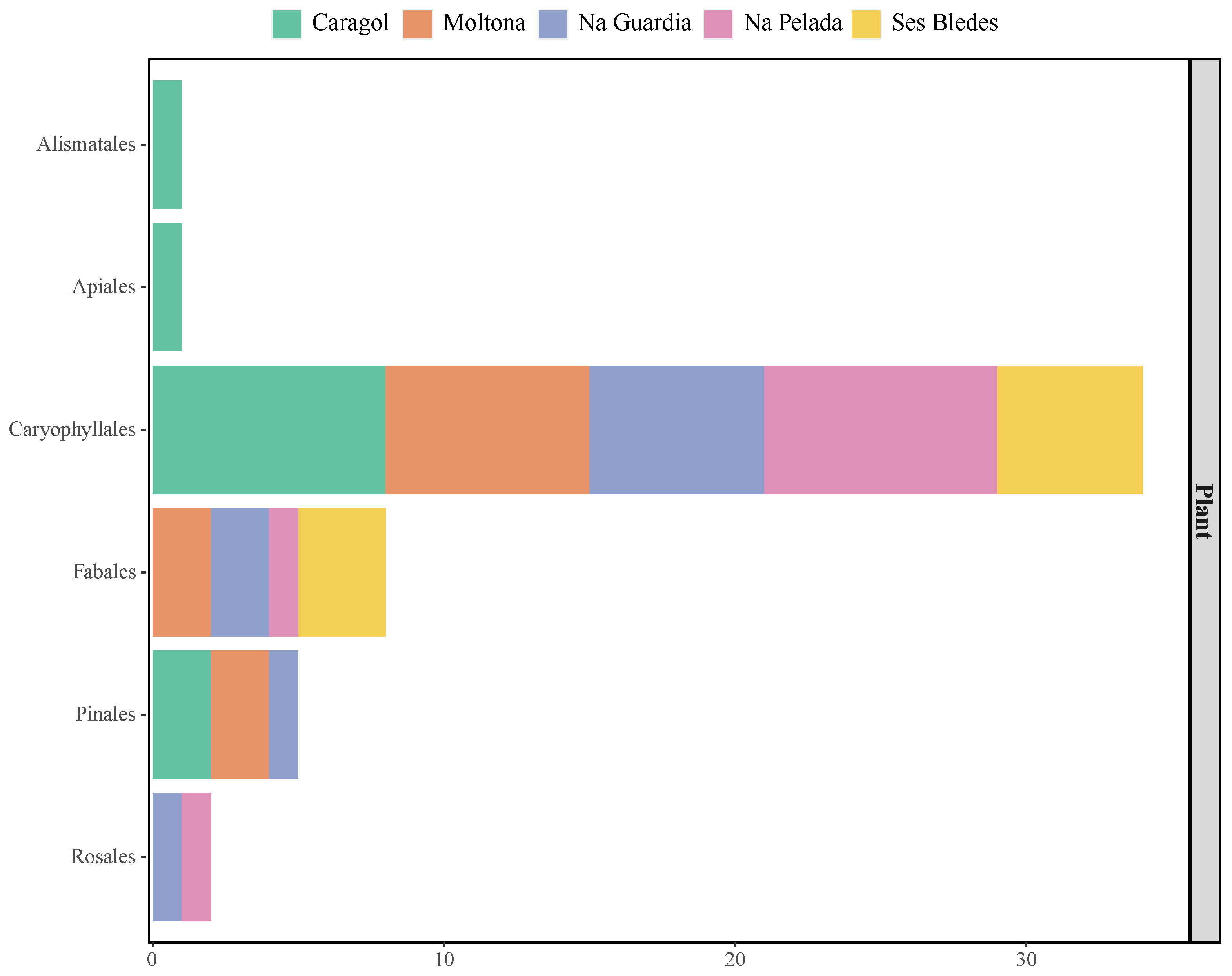
3. Results
3.1. Morphological and Molecular Diet Compositions
3.2. Comparison of Morphological and Molecular Analytical Methods
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
- -
- Alemany, A.; Leza, M.M.; Núñez, L.; Petro, B.; Closa, S.; Miranda, M.A. Estudio del impacto de los tratamientos contra la procesionaria del pino (Thaumetopoea pityocampa, Denn. y Schiff.) en Baleares. XXVI Grupo de Trabajo Fitosanitario, Govern de les Illes Balears, 2009.
- -
- Alonso-Zarazaga, M.A. Elenco sistemático de los Curculionoidea (Coleoptera) de la Península Ibérica e islas Baleares. Boletín de la Sociedad Entomológica Aragonesa (S.E.A.) 2018, 63, 3–44.
- -
- Altaba, C.R. XXVII. Els caragols i llimacs terrestres (Mollusca: Gastropoda). In: Alcover, J.A.; Ballesteros, E.: Fornós, J.J. (Eds.), Història Natural de l’Arxipèlag de Cabrera; CSIC–Editorial Moll, Palma, Spain, 1993, Monografies de la Societat d’Història Natural de les Balears, 2, pp. 409–426.
- -
- Anonymous. Balance fitosanitario–Baleares 2015. Direcció General d’Agricultura I Ramaderia, Conselleria de medi Ambient, Agricultura i Pesca, Govern de les Illes Balears, Palma, Spain, 2015.
- -
- AntWiki. Last updated 01/0/2021. Available at https://www.antwiki.org/ [02/09/2021].
- -
- Arbea, J.I. Los colémbolos de Aragón (Hexapoda: Collembola). Catalogus de la entomofauna aragonesa 2003, 29, 3–23.
- -
- Arbea, J.I.; Jordana, R. Colémbolos de las Islas Baleares (Insecta, Collembola). Redia 1990, 73, 187–200.
- -
- Bach, C.; Molero, R.; Gaju, M. Clase Insecta. Orden Microcoryphia. Revista IDE@-SEA 2015, 38, 1–12.
- -
- Bächli, G.; Báez, M. Drosophilidae. In: Catálogo de los Diptera de España, Portugal y Andorra (Insecta); Carles-Tolrá Hjorth-Andersen, M. (edit.) Monografías S.E.A., Zaragoza, Spain, 2002; 8, 161–162.
- -
- Bahillo de la Puebla, P.; López-Colón, J.I. Citas interesantes de cléridos de la Península Ibérica (Coleoptera, Cleridae). Zoologica baetica 1999, 10, 207–209.
- -
- Bahillo de la Puebla, P.; López-Colón, J.I. El género Opilo Latreille, 1802 en la Península Ibérica (Coleoptera, Cleridae). Boletín de la Asociación Española de Entomología 2000, 24 (1–2), 213–227.
- -
- Baldizzone, G. Contributions à la connaissance des Coleophoridae. XLII. Sur quelques Coleophoridae d’Espagne (Seconde partie: Espèces nouvelles pour la Faune espagnole, ou peu connues). Nota lepidopterrologica 1986, 9(1–2), 2–34.
- -
- Baldock, D. A provisional list of the wasps and bees of Mallorca, Balearic Islands, Spain (Hymenoptera aculeata: Chrysidoidea, Scolioidea, Vespoidea, Apoidea). Entomofauna 2014, 35, 333–404.
- -
- Baquero, E.; Jordana, R. Clase Collembola. Órdenes Poduromorpha, Entomobryomorpha, Neelipleona y Symphypleona. Revista IDE@-SEA 2015, 36, 1–11.
- -
- Baank, R.A. Fauna Europaea: Gastropoda. 2013, Fauna Europaea version 2017.06. https://fauna-eu.org [30/01/2021].
- -
- Barrientos, J.A.; Febrer, B. Arañas (Arachnida, Araneae) de Menorca (Islas Baleares, España). 2: “Adenda et corrigenda”. Descripción de tres especies nuevas. Revista Ibérica de Aracnología 2018, 33, 39–51.
- -
- Blanes-Dalmau, M.; Caballero-López, B.; Pujade-Villar, J. Estudi de les gales de la coŀlecció Vilarrúbia dipositada al Museu de Ciències Naturals de Barcelona. Butlletí de la Institució Catalana d’Història Natural 2017, 81, 137–173.
- -
- Bohn, H. Revision of the Loboptera species of Spain (Blattaria: Blattellidae). Entomologica Scandinavica 1990, 21, 369–403.
- -
- Bouaziz-Yahiatene, H.; Pfarrer, B.; Medjdoub-Bensaad, F.; Neubert, E. Revision of Massylaea Möllendorff, 1898 (Stylommatophora, Helicidae). ZooKeys 2017, 694, 109–133. https://doi.org/10.3897/zookeys.694.15001.
- -
- Boxshall, G. Fauna Europaea: Isopoda, Porcellionidae, Fauna Europaea version 2017.06. https://fauna-eu.org [29/01/2021].
- -
- Branco, V.V.; Morano E.; Cardoso, P. An update to the Iberian spider checklist (Araneae). Zootaxa 2019, 4614 (2), 201–254.
- -
- Cabanillas, D.; Parejo-Pulido, D. Primer registro de Lamyctes (Lamyctes) emarginatus (Newport, 1844) (Chilopoda: Lithobiomorpha: Henicopidae) en la Comunidad Autónoma de Extremadura y otras citas de la provincia de Badajoz (España). Boletín de la Sociedad Entomológica Aragonesa 2019, 64, 307–311.
- -
- Carles-Tolrá, M.; Báez, M. Stratiomyidae. In: Carles-Tolrá Hjorth-Andersen, M. (coord.) Catálogo de los Diptera de España, Portugal y Andorra (Insecta). Monografías S.E.A. 2002, 8, 113–114.
- -
- Carles-Tolrá, M.; Báez, M. Therevidae. In: Carles-Tolrá Hjorth-Andersen, M. (edit.) Catálogo de los Diptera de España, Portugal y Andorra (Insecta). Monografías S.E.A. 2002, 8, 117.
- -
- Cartes, J.E.; Abelló, P.; Torres, P. The occurrence of Hymenopenaeus debilis (Decapoda: Aristeidae: Solenocerinae) in Mediterranean waters: A case of pseudopopulations of Atlantic origin? Journal of the Marine Biological Association of the United Kingdom 2000, 80, 549–550.
- -
- Choi, E.H.; Hwang, U.W. First Record of Maritime Pseudoscorpion Garypus japonicus (Garypidae) from Korea. Animal Systematics, Evolution and Diversity 2009, 25, 261–264. https://doi.org/10.5635/KJSZ.2009.25.3.261.
- -
- Chueca, L.J.; Madeira, M.J.; Gómez-Moliner, B.J. Biogeography of the land snail genus Allognathus (Helicidae): Middle Miocene colonization of the Balearic Islands. Journal of Biogeography 2015, 42, 1845–1857.
- -
- Chueca, L.J.; Forés, M.; Gómez-Moliner, B.J. Actualización taxonómica y nomenclatural de las especies de Xerocrassa (Gastropoda: Geomitridae) endémicas de las islas Baleares. Iberus 2017, 35, 159–184.
- -
- Cifuentes, J. Los isópodos terrestres de Galicia, España (Crustacea: Isopoda, Oniscidea). Graellsia 2019, 75, e098. https://doi.org/10.3989/graellsia.2019.v75.243.
- -
- Cini, A.; Anfora, G.; Escudero-Colomar, L.A.; Grassi, A., Santosuosso, U.; Seljak, G.; Papini, A. Tracking the invasion of the alien fruit pest Drosophila suzukii in Europe. Journal of Pest Science 2014, 87, 559–566.
- -
- Cobo, F.; Soriano, O.; Báez, M. Chironomidae. In: Carles-Tolrá Hjorth-Andersen, M. (coord.) Catálogo de los Diptera de España, Portugal y Andorra (Insecta). Monografías S.E.A. 2002, 8, 35–44.
- -
- Collingwood, C.A.; Yarrow, H.H. A survey of Iberian Formicidae (Hymenoptera). Eos, Revista Española de Entomología 1969, 44, 53–101.
- -
- Cornara, D.; Garzo, E.; Morente, M.; Moreno, A.; Alba-Tercedor, J.; Fereres, A. EPG combined with micro-CT and video recording reveals new insights on the feeding behavior of Philaenus spumarius. PLoS ONE 2018, 13, e0199154. https://doi.org/10.1371/journal.pone.0199154.
- -
- Costas, M.; López, T.; Vázquez, M.A. Checklist de Fauna Ibérica. Superfamilia Lygaeoidea Schilling, 1829 (Insecta: Heteroptera) en la península ibérica, islas Baleares e islas Canarias. In: Ramos, M.A. and Sánchez, M. (eds.) Documentos Fauna Ibérica 2018, 7, 1–29.
- -
- Cruz-Suárez, A. Los Halophilosciidae Verhoeff, 1908 de la Península Ibérica e Islas Baleares (Isopoda: Oniscidea). Boletín de la Asociación Española de Entomología 1992, 16, 113–121.
- -
- Cruz-Suárez, A. El género Armadillidium Brandt, 1833 en la Península Ibérica y Baleares (Isopoda, Oniscidea, Armadillidiidae). Boletín de la Asociación Española de Entomología 1993, 17, 155–181.
- -
- Cruz, A. Redescripción de Agabiformius obtusus (Budde-Lund, 1909) y de Armadillo hirsutus Koch, 1856 (Isopoda: Oniscidea) de la Península Ibérica. Butlletí de la Institució Catalana d’Història Natural 1994, 62, 65–76.
- -
- De Moraes, F.J.; McMurtry, J.A.; Denmark, H.A.; Campos, C.B. A revised catalog of the mite family Phytoseiidae. Zootaxa 2004, 434, 1–494.
- -
- Delgado-Serra, S.; Viader, M.; Ruiz-Arrondo, I.; Miranda, M.A.; Barceló, C.; Bueno-Marí, R.; Hernández-Triana, L.M.; Miquel, M.; Lester, K.; Jurado-Rivera, J.A.; Paredes-Esquivel, C. Molecular Characterization of Mosquito Diversity in the Balearic Islands. Journal of Medical Entomology 2020, 217, 1–8. https://doi.org/10.1093/jme/tjaa217.
- -
- De Lillo, E. Fauna Europaea: Eriophyidae. In Fauna Europaea: Actinotrichida. Magowski, W. 2003, Fauna Europaea version 2017.06. https://fauna-eu.org [29/01/2021].
- -
- Denux, O.; Zagatti, P. Coleoptera families other than Cerambycidae, Curculionidae sensu lato, Chrysomelidae sensu lato and Coccinelidae. Chapter 8.5. In Alien terrestrial arthropods of Europe. Roques, A.; Kenis, M.; Lees, D.; Lopez-Vaamonde, C.; Rabitsch, W.; Rasplus, J.Y.; Roy, D. (Eds.) BioRisk 2010, 4, 315–406. https://doi.org/10.3897/biorisk.4.61.
- -
- Diéguez Fernández, J.M. Nuevas citas y catálogo de los Cantharidae y Dasytidae (Coleoptera) del área iberobalear. Heteropterus Revista de Entomología 2011, 11, 75–85.
- -
- Docavo, A. Contribución al conocimiento de los Braconidae de España. I. Nuevos hallazgos de géneros y especies. Entomophaga 1962, 7, 343–348.
- -
- Ebejer, M.J. Sorne Chloropidae (Diptera) from the Balearic Islands (Spain) with particular reference to Parc Natural de s’Albufera de Mallorca. Bolletí de la Societat d’Història Natural de les Balears 2006, 49, 173–184.
- -
- Eidmann, H. Die Ameisenfauna der Balearen. Zeitschrift für Morphologie und Ökologie der Tiere 1926, 6, 694–742.
- -
- Eidmann, H. Zur Kenntnis der Insektenfauna der balearischen Inseln. Entomologische Mitteilungen 1927, 16: 24–37.
- -
- Eiroa, E.; Báez, M. Tipulidae. In: Carles-Tolrá Hjorth-Andersen, M. (coord.) Catálogo de los Diptera de España, Portugal y Andorra (Insecta). Monografías S.E.A. 2002, 8, 79–81.
- -
- Ellis, W.N. Plant Parasites of Europe. Leafminers, galls and fungi. Available In https://bladmineerders.nl/ [27-01-2021].
- -
- Español, F. De re entomològica Contribució a l’Entomologia de les illes de Cabrera i Foradada (Balears). Butlletí de la Institució Catalana d’Història Natural 1935, 35, 251–253.
- -
- Español, F. Los Cléridos (Cleridae) de Cataluña e Islas Baleares (Col., Cleroidea). Publicaciones del Instituto de Biología Aplicada 1959, 30, 105–146.
- -
- Español, F. Coleoptera, Anobiidae. Fauna Ibérica, vol. 2. Madrid: Museo Nacional de Ciencias Naturales. CSIC; Madrid, Spain 1992, 192 pp.
- -
- FAO FishFinder. Species Fact Sheets. Pleoticus muelleri (Bate, 1888). In FAO Fisheries Division [online]. Rome. [31/01/2021]. 2021, Online at http://www.fao.org/fishery/species/3437/en.
- -
- Faraji, F.; Ueckermann, E.A. A new species of Mediolata Canestrini from Spain (Acari: Stigmaeidae), re-description of M. chanti and a key to the known species of Mediolata. Zootaxa 2006, 1151, 27–39.
- -
- Fet, V.; Soleglad, M.E. Morphology analysis supports presence of more than one species in the “Euscorpius carpathicus” complex (Scorpiones: Euscorpiidae). Euscorpius, Occasional Publications in Scorpiology 2002, 3.
- -
- Fischer S.; Patzner, R.A.; Müller, C.H.G.; Winkler, H.M. Studies on the ichthyofauna of the coastal waters of Ibiza (Balearic Islands, Spain). Rostocker Meeresbiologische Beiträge 2007, 18, 30–62.
- -
- Gabarra, R.; Arnó, J.; Riudavets, J. Drosophila suzukii: Biología y ecología. PHYTOMA España 2015, 269, 12–13.
- -
- Gadea, E. Nematofauna muscícola. In Història Natural de l’Arxipèlag de Cabrera; Alcover, J.A.; Ballesteros, E.; Fornós, J.J. (Eds.); CSIC–Editorial Moll, Palma, Spain, Monografies de la Societat d’Història Natural de les Balears 1993, 2, 269–272.
- -
- Gangwere, S.K.; Llorente, V. Distribution and habits of the Orthoptera (sens. lat.) of the Balearic Islands (Spain). Eos 1992, 68, 51–87.
- -
- Gantenbein, B.; Soleglad, M.E.; Fet, V. Euscorpius balearicus Caporiacco, 1950, stat. nov. (Scorpiones:Euscorpiidae): Molecular (allozymes and mtDNA) and morphological evidence for an endemic Balearic Islands species. Organisms Diversity and Evolution 2001, 1, 301–320.
- -
- García, L. Halophiloscia ischiana Verhoeff, 1933, un isòpode terrestre nou per a la fauna de Mallorca. Aubaïna 2000, 2, 18.
- -
- García, L. Armadillidium cruzi. In Bioatles. Palma: Servei de Protecció d’Especies, Conselleria de Medi Ambient, Palma, Spain, 2006.
- -
- García, L.; Cruz, A. XIX. Els isopòdes terrestres (Crustacea: Isopoda: Oniscidea). In, Història Natural de l’Arxipèlag de Cabrera; Alcover, J.A.; Ballesteros, E.; Fornós, J.J. (Eds.); CSIC–Editorial Moll, Palma, Spain, Monografies de la Societat d’Història Natural de les Balears 1993, 2, 323–332.
- -
- García, L.; Cruz, A. Els isòpodes terrestres (Crustacea: Isopoda: Oniscidea) de les iIles Balears: Catàleg d’especies. Bolletí de la Societat d’Història Natural de les Baleares 1996, 39, 77–99.
- -
- García, L.; Gross, A.; Riddiford, N. Armadillidium album, un isopode terrestre nou per a la fauna balear (Isopoda, Crinocheta, Armadillidiidae). Bolletí de la Societat d’Història Natural de les Baleares 2004, 46, 91–94.
- -
- García-Barros, E.; Romo, H.; Sarto i Monteys, V.; Munguira, M.L.; Baixeras, J.; Vives Moreno, A.; Yela García, J.L. Clase Insecta, Orden Lepidoptera. Revista IDE@-SEA 2006, 65: 1–21.
- -
- García-Romera, C.; Báez, M. Phoridae. In Catálogo de los Diptera de España, Portugal y Andorra (Insecta); Carles-Tolrá Hjorth-Andersen, M. (coord.). Monografías S.E.A. 2002, 8, 125–129.
- -
- García-Romera, C.; Barrientos, J.A. La fauna de Phoridae (Diptera) en el parque natural del Montseny (Cataluña, España). Citas nuevas para la Península Ibérica. Boletín de la Sociedad Entomológica Aragonesa, 2014, 54, 237–261.
- -
- Gasull, L. Las Helicella (Xeroplexa) de Baleares, Gasteropoda Pulmonata. Boletín de la Sociedad de Historia Natural de Baleares 1964, 10, 3–70.
- -
- Gasull, L. Algunos moluscos terrestres y de agua dulce de Baleares. Boletín de la Sociedad de Historia Natural de Baleares 1965, 11, 7–161.
- -
- GBIF.org. GBIF: The Global Biodiversity Information Facility. Available in https://www.gbif.org [22-01-2021].
- -
- Germann, C.; Torres, J.L.; Borovec, R. Confirmative records of Trachyphloeus nodipennis Chevrolat, 1860 for the Iberian Peninsula (Coleoptera: Curculionidae: Entiminae) with a key to the Spanish species of the nominal subgenus. Boletín de la SAE 2017, 27, 23–28.
- -
- Gnezdilov, V.M.; Holzinger, W.E.; Wilson, M.R. The Western Palaearctic Issidae (Hemiptera, Fulgoroidea): An Illustrated Checklist and Key to Genera and Subgenera. Proceedings of the Zoological Institute RAS 2014, 318 (1), 5–118.
- -
- Goldarazena, A. Clase Insecta, Orden Thysanoptera. Revista IDE@-SEA 2015, 52, 1–20.
- -
- Gómez, K. Citas nuevas o interesantes de hormigas (Hymenoptera: Formicidae) para la isla de Mallorca (Baleares, España). Boletín de la Sociedad Entomológica Aragonesa 2004, 34, 107–108.
- -
- Gómez, K.; Espadaler, X. La hormiga argentina (Linepithema humile) en las Islas Baleares. Listado preliminar de las Hormigas de las Islas Baleares. Documentos Técnicos de Conservación. Conselleria de Medi Ambient. Govern de les Illes Balears 2005, 13, 68 pp.
- -
- Gómez, K.; Espadaler, X. Exotic ants (Hymenoptera: Formicidae) in the Balearic Islands. Myrmecologische Nachrichten 2006, 8, 225–233.
- -
- González, M.A.; Terra, L.S.W.; García de Jalón, D.; Cobo, F. Lista faunística y bibliográfica de los Tricópteros (Trichoptera) de la Península Ibérica e Islas Baleares. Listas de la Flora y Fauna de las aguas continentales de la Península Ibérica, 1992, 11. Madrid: Asociación Española de Limnología. 200pp.
- -
- González Peña, C.F.; Vives i Noguera, E.; de Sousa Zuzarte, A.J. Nuevo catálogo de los Cerambycidae (Coleoptera) de la Península Ibérica, islas Baleares e islas atlánticas: Canarias, Açores y Madeira. Monografías S.E.A. 2007, 12, 1–211.
- -
- Govern Balear. La procesionaria del pino. Conselleria d’Agricultura i Pesca, Direcció General de Producció i Industries Agràries, Palma, Spain, 2011.
- -
- Gravestein, W.H. Twaalf nieuwe Hemiptera Heteroptera voor de fauna van Mallorca. Entomologische Berichten 1969, 29, 156–158.
- -
- Gravestein, W.H. Hemiptera Heteroptera new to the Baleares, in particular to the Island of Mallorca. Entomologische Berichten 1978, 38, 37–39.
- -
- Harbach, R.E. Genus Acartomyia Theobald, 1903. Mosquito Taxonomic Inventory. Available in http://mosquito-taxonomic-inventory.info/genus-acartomyia-theobald-1903-0#overlay-context=genus-acartomyia-theobald-1903-0 [05/02/2021].
- -
- Harvey, M.S.; Hillyer, M.J.; Carvajal, J.I.; Huey, J.A. Supralittoral pseudoscorpions of the genus Garypus (Pseudoscorpiones: Garypidae) from the Indo-West Pacific region, with a review of the subfamily classification of Garypidae. Invertebrate Systematics 2020, 34, 34–87. https://doi.org/10.1071/IS19029.
- -
- Heckman, C.W. Neuroptera (Including Megaloptera)., Springer, Switzerland, 2017 621pp.
- -
- Henry, T.J.; Dellapé, P.M.; Scudder, G.G.E. Resurrection of the Genera Crophius Stål and Mayana Distant from Synonymy Under Anomaloptera Amyot and Serville, Description of a New Genus, and a Key to the New World Oxycarenid Genera (Hemiptera: Heteroptera: Oxycarenidae). Proceedings- Entomological Society of Washington 2015, 117, 367–380.
- -
- Hodkinson, I.D.; Hollis, D. The psyllids (Homoptera: Psylloidea) of Mallorca. Entomologica Scandinavica 1981, 12, 65–77.
- -
- Hurtado L.A.; Lee E.J.; Mateos M.; Taiti S. Global Diversification at the Harsh Sea-Land Interface: Mitochondrial Phylogeny of the Supralittoral Isopod Genus Tylos (Tylidae, Oniscidea). PLoS ONE 2014, 9, e94081. https://doi.org/10.1371/journal.pone.0094081.
- -
- Iaciofano, D.; Lo Brutto, S. Re-description of Orchestia stephenseni Cecchini, 1928: Designation of neotype and senior synonym to Orchestia constricta A. Costa, 1853 (Crustacea: Amphipoda: Talitridae) by Reversal of Precedence. Zootaxa 2016, 4150 (1), 40–60.
- -
- Iberfauna. IBERFAUNA. El Banco de Datos de la Fauna Ibérica. Museo Nacional de Ciencias Naturales (CSIC). Available in http://iberfauna.mncn.csic.es/showficha.aspx?rank=T&idtax=9657 [27/01/2021].
- -
- Jaramillo, E.; Cifuentes, S.; Duarte, C.; Contreras, H. Relationships between bioturbation by Tylos spinulosus (Crustacea, Isopoda) and its distribution on sandy beaches of north-central Chile. Marine Ecology 2008, 29, 37–42.
- -
- Jordana, R.; Arbea, J.I. Clave de identificación de los géneros de colémbolos de España (Insecta: Collembola). Publicaciones de Biología de la Universidad de Navarra, Serie Zoológica 1989, 19, 1–16.
- -
- Jurado-Rivera, J.A.; Álvarez, G.; Caro, J.A.; Juan, C.; Pons, J.; Jaume, D. Molecular systematics of Haploginglymus, a genus of subterranean amphipods endemic to the Iberian Peninsula (Amphipoda: Niphargidae). Contributions to Zoology 2017, 86 (3), 239–260.
- -
- Karsholt, O.; Nieukerken, E.J. van. Lepidoptera. Fauna Europaea version 2017.06, https://fauna-eu.org [29/01/2021].
- -
- Kawachino, Y. Spawning record of Pythia cecillei (Philippi, 1847). Transactions of the Nagasaki Biological Society 2015, 76, 62–66.
- -
- Kazantsev, S. Fauna Europaea: Malthodes. In: Alonso-Zarazaga, M.A. 2013. Fauna Europaea: Coleoptera, Cantharidae. Fauna Europaea version 2017.06. https://fauna-eu.org [29/01/2021].
- -
- Kennedy, M.; Spencer, H.G. Classification of the cormorants of the world. Molecular Phylogenetics and Evolution 2014, 79, 249–257.
- -
- Klimov, P.B.; OConnor, B.; Ochoa, R.; Bauchan, G.R.; Redford, A.J.; Scher, J. Bee Mite ID: Bee-Associated Mite Genera of the World. USDA APHIS Identification Technology Program (ITP), Fort Collins, CO. [24-01-2021] Available in http://idtools.org/id/mites/beemites/index.php.
- -
- Kuschel, G. Curculionoidea (weevils) of New Caledonia and Vanuatu: Ancestral families and some Curculionidae. In:, Zoologia Neocaledonica 6. Biodiversity studies in New Caledonia; Grandcolas, P. (ed.); Mémoires du Muséum National d’Histoire Naturelle 2006, 197, 99–249.
- -
- Lomnicki, J. Une contribution à la connaissance de la faune des fourmis des îles Baléares. Polskie Pismo Entomologiczne-Bulletin Entomologique de la Pologne 1925, 4, 1–3.
- -
- Lowry, J.K.; Myers, A.A. New genera of Talitridae in the revised Superfamily Talitroidea Bulycheva 1957 (Crustacea, Amphipoda, Senticaudata). Zootaxa 2019, 4553 (1), 1–100.
- -
- Lucena-Moya, P.; Abraín, R.; Pardo, I.; Hermida, B.; Domínguez, M. Invertebrate species list of coastal lagoons in the Balearic Islands. Transitional Waters Bulletin 2010, 4 (1), 1–11.
- -
- Lundqvist, L. Fauna Europaea: Acari, Mesostigmata. 2013, Fauna Europaea version 2017.06. https://fauna-eu.org [29/01/2021].
- -
- Mahnert, V. XXII. Els pseudoscorpins (Arachnida, Pseudoscorpiones). In, Història Natural de l’Arxipèlag de Cabrera, Alcover, J.A., Ballesteros, E. and Fornós, J.J. (Eds.); CSIC–Editorial Moll, Palma, Spain; Monografies de la Societat d’Història Natural de les Balears 1993, 2, 355–360.
- -
- Malicky, L.H. Beschreibungen von neuen mediterranen Köcherfliegen und Bemerkungen zu bekannten (Trichoptera). Zeitschrift der Arbeitsgemeinschaft Österreichischer Entomologen 1980, 32, 1–17.
- -
- Malo, J.; García-Avilés, J. Contribución al conocimiento de los quironómidos (Diptera, Chironomidae) de las Islas Baleares. Zoologica Baetica 1999, 10, 211–214.
- -
- Marcos-García, M.A.; Rojo, S.; Pérez-Bañón, C. Syrphidae. In: Catálogo de los Diptera de España, Portugal y Andorra (Insecta). Carles-Tolrá Hjorth-Andersen, M. (coord.); Monografías S.E.A. 2002, 8, 132–136.
- -
- Martín, J.L.; Izquierdo, I.; Oromí, P. The genus Loboptera in the Canary Islands; description of five new hypogean species. Vieraea 1999, 27, 255–286.
- -
- Martínez-Fernández, J.C. Un nuevo representante del género Blaps Fabricius, 1775 de la Península Ibérica: Blaps tichyi n. sp. (Coleoptera, Tenebrionidae). Boletín de la Sociedad Entomológica Aragonesa 2010, 47, 181–185.
- -
- Mastrantonio, V.; Porretta, D.; Bellini, R.; Nascetti, G.; Urbanelli, S. Molecular Systematics and Origin of the Mediterranean Sea Rock-Pool Mosquitoes of the Aedes mariae (Diptera: Culicidae) Complex. Annals of the Entomological Society of America 2015, 108: 593–599. https://doi.org/10.1093/aesa/sav031.
- -
- Mauriés, J.P.; Vicente, M.C. Miriápodos de Baleares. Boletín de la Sociedad de Historia Natural de Baleares 1976, 21, 33–46.
- -
- Meliá, A. Contribución al conocimiento de los pulgones (Homoptera, aphidoidea) sobre plantas agrícolas y forestales en España. Boletín de sanidad vegetal. Plagas 1986, 12, 335–342.
- -
- Mendoza-Roldan, J.A.; Colella, V.; Lia, R.P.; Nguyen, V.L.; Barros-Battesti, D.M.; Iatta, R.; Dantas-Torres, F.; Otranto, D. Borrelia burgdorferi (sensu lato) in ectoparasites and reptiles in southern Italy. Parasites & Vectors 2019, 12, 35 https://doi.org/10.1186/s13071-019-3286-1.
- -
- Menozzi, C.C. Zur Kenntnis der Ameisenfauna der Balearen. Zoologische Anzeiger 1926, 66(7–8), 180–182.
- -
- Michalska, K.; Skoracka, A.; Navia, D.; Amrine, J.W. Behavioural studies on eriophyoid mites: An overview. Experimental and Applied Acarology 2009, 51, 31–59. https://doi.org/10.1007/s10493-009-9319-2.
- -
- Miranda, M.A.; López-Mercadal, J.; Tugores, M.A.; Delgado, S.; Seguí, G.; Lalucat, J.; Gomila, M.; Ruíz, M.; Lester, K.; Kenyon, D.M.; Paredes-Esquivel, C. Recogida de datos e información en las Islas Baleares sobre la biología de vectores de Xylella fastidiosa. XLIX Foro INIA. 2019, Xylella fastidiosa en el contexto del cambio climático.
- -
- Monserrat, V.J. Catálogo de los Neurópteros de Baleares con nuevos datos sobre su fauna (Insecta, Neuroptera). Bolletí de la Societat d’Història Natural de les Baleares 2005, 48, 71–85.
- -
- Monserrat, V.J. Los crisópidos de la Península Ibérica y Baleares (Insecta, Neuropterida, Neuroptera: Chrysopidae). Graellsia 2016a, 72, e037. https://doi.org/10.3989/graellsia.2016.v72.143.
- -
- Monserrat, V.J. Los coniopterígidos de la Península Ibérica e Islas Baleares (Insecta: Neuropterida, Neuroptera: Coniopterygidae). Graellsia 2916b, 72, e047. http://dx.doi.org/10.3989/graellsia.2016.v72.157.
- -
- Monserrat, V.J.; Acevedo, F.; Pantaleoni, R.A. Nuevos datos sobre algunas especies de crisópidos de la Península Ibérica, Islas Baleares e Islas Canarias (Insecta, Neuroptera, Chrysopidae). Graellsia, 2014, 70, e002;. https://doi.org/10.3989/graellsia.2014.v70.100.
- -
- Montesanto, G.; Deidun, A.; Sciberras, A.; Sciberra, J.; Lombardo, B.M. Current distribution of two species of Tylos (Isopoda: Oniscidea) in the central Mediterranean and the influence of beach sand grain-size parameters. Journal of Crustacean Biology 2014, 34, 47–53. https://doi.org/10.1163/1937240X-00002206.
- -
- Moraza, M.L.; Irwin, N.R.; Godinho, R.; Baird, S.J.E.; Bellocq, J.G. A new species of Ophionyssus Mégnin (Acari: Mesostigmata: Macronyssidae) parasitic on Lacerta schreiberi Bedriaga (Reptilia: Lacertidae) from the Iberian Peninsula, and a world key to species. Zootaxa 2009, 2007, 58–68.
- -
- Moreno, A.G. Orden Astigmata. Revista IDE@-SEA 2015, 15, 1–19.
- -
- Myers, A.A.; Lowry, J.K. A revision of the genus Orchestia Leach, 1814 with the reinstatement of O. inaequalipes (K.H. Barnard, 1951), the designation of a neotype for Orchestia gammarellus (Pallas, 1776) and the description of three new species (Crustacea: Amphipoda: Talitridae: Talitrinae). Zootaxa 2020, 4808 (2): 201–250.
- -
- Nentwig, W.; Blick, T.; Bosmans, R.; Gloor, D.; Hänggi, A.; Kropf, C. Spiders of Europe. Version 01.2021. Online at https://www.araneae.nmbe.ch, [22-01-2021]. https://doi.org/10.24436/1.
- -
- Núñez, L. Plagas de frondosas en las Illes Balears. Servicio de Sanidad Forestal, Govern de les Illes Balears. Palma, Spain.
- -
- OConnor; B.M. Evolutionary ecology of Astigmatid mites. Annual Review of Entomology 1982, 27, 385–409.
- -
- Oosterbroek, P. Notes on western Palaearctic species of the Tipula (Yamatotipula) lateralis group, with the description of a new species from Turkey (Diptera: Tipulidae). European Journal of Entomology 1994, 91, 429–435.
- -
- Ouvrard, D. Psyl’list-The World Psylloidea Database; Available in http://www.hemiptera-databases.com/psyllist [08/12/2020]. https://doi.org/10.5519/0029634.
- -
- Palau, J.M. Algunas consideraciones sobre los embiópteros de Mallorca y, en especial, sobre el género Haploembia Verh. Bolletí de la Societat d’Història Natural de les Balears 1956, 2, 23–25.
- -
- Palau, J.M. Pequeño catálogo de hemípteros heterópteros de Mallorca. Boletín de la Sociedad de Historia Natural de Baleares 1959, 5, 7–11.
- -
- Palmer, M.; Petitpierre, E. XXVI. Els coleòpters de Cabrera: Llista faunística i perspectives d’estudi. In Història Natural de l’Arxipèlag de Cabrera; Alcover, J.A.; Ballesteros, E.; Fornós, J.J. (Eds.); CSIC–Editorial Moll, Monografies de la Societat d’Història Natural de les Balears 1993, 2, 383–407.
- -
- Pape, T.; González-Mora, D.; Peris, S.V.; Báez, M. Sarcophagidae. In Catálogo de los Diptera de España, Portugal y Andorra (Insecta); Carles-Tolrá Hjorth-Andersen, M. (coord.); Monografías S.E.A. 2002, 8, 218–221.
- -
- Peña, L.E. Nuevas especies del género Psammetichus Latr., (Coleoptera-Tenebrionidae) para Chile y Perú. Revista Chilena de Entomología 1973, 7, 137–144.
- -
- Perera, A.; Maia, J.P.M.C.; Jorge, F.; Harris, D.J. Molecular screening of nematodes in lacertid lizards from the Iberian Peninsula and Balearic Islands using 18S rRNA sequences. Journal of Helminthology 2012, 87, 189–194. https://doi.org/10.1017/S0022149x12000181.
- -
- Pérez-Íñigo, C. jr. Acari Oribatei, Poronota. Fauna Ibérica, vol. 3, Museo Nacional de Ciencias Naturales. CSIC, Madrid, Spain, 1993.
- -
- Petitpierre, E.; Sacarés, A.; Jurado-Rivera, J.A. Updated checklist of Balearic leaf beetles (Coleoptera: Chrysomelidae). Zootaxa 2017, 4272 (2), 151–177.
- -
- Pons, G.X. XX. Estudi preliminar sobre la fauna d’aranèids (Arachnida, Araneae). In, Història Natural de l’Arxipèlag de Cabrera; Alcover, J.A.; Ballesteros, E.; Fornós, J.J. (Eds); CSIC–Editorial Moll, Monografies de la Societat d’Història Natural de les Balears 1993, 2, 333–350.
- -
- Pons, G.X. Noves dades biogeografiques i taxonomiques sobre els escorpins (Arachnida; Scorpiones: Euscorpiidae) de les Illes Balears. Bolletí de la Societat d’Història Natural de les Baleares 2001, 44, 103–109.
- -
- Pons, G.X.; Palmer, M. Fauna endèmica de les illes Balears. Palma (Spain): Institut d’Estudis Baleàrics, Conselleria d’Obres Públiques, Ordenació del Territori i Medi Ambient (Dir. Gen. Medi Ambient). Societat d’Història Natural de les Balears. Palma, Spain, 1996, 307pp.
- -
- Pons, G.X; Palmer, M. Invertebrats endemics i Illes: (Tenebrionidae i Araneae) introduccions i extincions aIs illots de Cabrera (Illes BaIears). In: Alcover, J.A. (coord.), Ecologia de les illes. Mon. Soc. Hist. Nat. Balears 6/Mon. Inst. Est. Bal. 1999, 66, 105–122. Palma de Mallorca, Spain.
- -
- Pons, G.X.; Vadell, M. Biospeleologia de les cavitats de les Illes Balears: Invertebrats terrestres. Endins 2011, 35, 241–256.
- -
- Pons, G.X.; Jaume, D.; Damians, J. Fauna cavernícola de Mallorca/Cavernicolous Fauna of Mallorca. Endins 1995, 20, 125–144.
- -
- Pons, G.X.; Palmer, M.; García, Ll. Isópodos terrestres (Isopoda, Oniscidea) de las Islas Chafarinas (N África, Mediterráneo Occidental). Bolletí de la Societat d’Història Natural de les Baleares 1999, 42, 139–146.
- -
- Pont, A.C.; Báez, M. Muscidae. In: Carles-Tolrá Hjorth-Andersen, M. (coord.) Catálogo de los Diptera de España, Portugal y Andorra (Insecta). Monografías S.E.A. 2002, 8, 210–214.
- -
- Redondo, V.M.; Gastón, F.J.; Gimeno, R. Geometridae Ibericae; Apollo Books; Stenstrup; Denmark, 2009, 360p.
- -
- Remaudiére, G.; Nieto, J.M.; Mier, M.P. Nuevas aportaciones al conocimiento de la fauna española de pulgones (Hom. Aphidoidea). Boletín de la Asociación Española de Entomología 1986, 10, 313–333.
- -
- Requena, E. Noves dades sobre la distribució del genere Agdistis Hübner, [1825], a Catalunya (Lepidoptera: Pterophoridae). Butlleti-Societat Catalana de Lepidopterologia 1999, 84, 9–16.
- -
- Requena, E. Aproximació a la fauna dels gelèquids de Catalunya i Balears (Lepidoptera: Gelechiidae). Treballs de la Societat Catalana de Lepidopterologia 2009, 16, 5–77.
- -
- Ribera, I.; Melic, A. Clase Insecta. Orden Neuroptera s.s. (Planipennia). Revista IDE@-SEA, 2015, 58, 1–12.
- -
- Ribes, J. Hemípteros de Mallorca. Publicaciones del Instituto de Biología Aplicada 1965, 39, 71–95.
- -
- Ribes, J. XXIII. Els heteròpters. In Història Natural de l’Arxipèlag de Cabrera. Alcover, J.A.; Ballesteros, E.; Fornós, J.J. (Eds.); CSIC–Editorial Moll, Monografies de la Societat d’Història Natural de les Balears 1993, 2, 361–364.
- -
- Robertson, P.L. A revision of the genus Tyrophagus, with a discussion on its taxonomic position in the Acarina. Australian Journal of Zoology 1959, 7, 146–181.
- -
- Roca, V.; Hornero, M.J. Strongyloides ophiusensis sp. n. (Nematoda: Strongyloididae), parasite of an insular lizard, Podarcis pityusensis (Sauria: Lacertidae). Folia Parasitologica 1992, 39, 369–373.
- -
- Roca-Cusachs, M.; Goula, M.; Prieto, F.; Pérez, J. Checklist de Fauna Ibérica. Superfamilias Aradoidea, Coreoidea y Pyrrhocoroidea (Insecta: Heteroptera) en la península ibérica, islas Baleares e islas Canarias. In: Ramos, M.A. and Sánchez, M. (eds.). Documentos Fauna Ibérica 2018, 6. Museo Nacional de Ciencias Naturales, CSIC. Madrid. 14 pp.
- -
- Rozkosny, R. Fauna Europaea: Stratiomyidae. In Fauna Europaea: Diptera, Brachycera; Pape, T.; Beuk, P. Fauna Europaea version 2017.06. https://fauna-eu.org [29/01/2021].
- -
- Schmalfuss, H. World catalog of terrestrial isopods (Isopoda: Oniscidea). Stuttgarter Beiträge zur Naturkunde 2003, Serie A, 654: 1–341.
- -
- Schubart, C.D.; Cuesta, J.A.; Felder, D.L. Phylogeography of Pachygrapsus transversus (Gibbes, 1850): The effect of the American continent and the Atlantic Ocean as gene flow barriers and recognition of Pachygrapsus socius Stimpson 1871 as a valid species. Nauplius 2005, 13, 99–113.
- -
- Seco, M.V.; Mier, M.P. Contribuciones al conocimiento de los pulgones (Hom., Aphidoidea) de las Islas Baleares. 1. Introducción y afidofauna de Mallorca. Bolletí de la Societat d’Història Natural de les Balears 1986, 30, 5–17.
- -
- Seifert, B.; d’Eustacchio, D.; Kaufmann, B.; Centorame, M.; Lorite, P.; Modica, M.V. Four species within the supercolonial ants of the Tapinoma nigerrimum complex revealed by integrative taxonomy (Hymenoptera: Formicidae). Myrmecological News 2017, 24, 123–144.
- -
- Siddiqi M.R. Tylenchida. Parasites of plants and insects., 2nd ed. CABI Publishing. 2000, 848 pp.
- -
- Skuhravá, M. and Skuhravý, V. Gall midges (Cecidomyiidae, Diptera) of Mallorca (Balearic Islands, Spain). Boletín de la Asociación Española de Entomología 2004, 28 (1–2), 105–119.
- -
- Skuhravá, M.; Skuhravý, V. Species richness of gall midges (Diptera: Cecidomyiidae) in Europe (West Palaearctic): Biogeography and coevolution with host plants. Acta Societatis Zoologicae Bohemicae 2009, 73, 87–156.
- -
- Skuhravá, M.; Blasco-Zumeta, J.; Pujade-Villar, J. Cecidomyiidae. In Catálogo de los Diptera de España, Portugal y Andorra (Insecta); Carles-Tolrá Hjorth-Andersen, M. (coord.); Monografías S.E.A. 2002, 8, 21–25.
- -
- Sláma, P.M.; Berger, P. Contribution to the knowledge of the genus Nathrius Brèthes, 1916, with the description of N. cypericus n. sp. from Cyprus (Coleoptera: Cerambycidae). Biocosme Mésogéen 2006, 23, 55–65.
- -
- Soghigian, J.; Andreadis, T.G.; Livdahl, T.P. From ground pools to treeholes: Convergent evolution of habitat and phenotype in Aedes mosquitoes. BMC Evolutionary Biology 2017, 17: 262. https://doi.org/10.1186/s12862-017-1092-y.
- -
- Soler-Membrives, A.; Munilla, T. PYCNOIB: Biodiversity and Biogeography of Iberian Pycnogonids. PLoS ONE 2015, 10, e0120818. https://doi.org/10.1371/journal.pone.0120818.
- -
- Spelda, J. Clase Diplopoda, Orden Julida. Revista IDE@-SEA 2015, 27, 1–18.
- -
- Stehlík, J.L.; Kment, P. Antilochus (Neaeretus) pterobrachys sp. nov. and the correct name of the subgenus Afroantilochus (Hemiptera: Heteroptera: Pyrrhocoridae). Acta Entomologica Musei Nationalis Pragae 2011, 51, 49–53.
- -
- Stock, J.H. On the identity of Porrassia mallorquensis Marcus, 1912, an amphipod supposedly endemic in Mallorca. Crustaceana 1976, 30 (1), 110–111.
- -
- Subías, L.S.; Shtanchaeva, U.Y.; Arillo, A. Oribátidos (Acari, Oribatida) de España peninsular e Islas Baleares. Distribución. Monografías electrónicas S.E.A. 2013, 5. Sociedad Entomológica Aragonesa.
- -
- Teruel, R.; Melic, A. Orden Scorpiones. Revista IDE@-SEA 2015, 18, 1–17.
- -
- Thanou, E.; Sponza, S.; Nelson, E.J.; Perry, A.; Wanless, S.; Daunt, F.; Cavers, S. Genetic structure in the European endemic seabird, Phalacrocorax aristotelis, shaped by a complex interaction of historical and contemporary, physical and nonphysical drivers. Molecular Ecology 2017, 26, 2796–2811.
- -
- Torres-Vila, L.M.; McMinn, M.; Rodríguez-Molina, A.; Rodríguez-Molina, M.C. Primera cita de Lobesia botrana Den. et Schiff. (Lepidoptera: Tortricidae) en la isla de Cabrera, Islas Baleares. Bolletí de la Societat d’Història Natural de les Balears 2006, 49, 45–49.
- -
- Vadell, M.; Pons, G.X. Aportaciones al conocimiento de los quilópodos (Chilopoda; Geophilomorpha) de la Serra de na Burguesa (Mallorca, Islas Baleares). Bolletí de la Societat d’Història Natural de les Balears 2009, 52, 169–182.
- -
- Vadell, M.; Zaragoza, J.A. Estudio preliminar de la fauna invertebrada terreste de la Cova des Coll (Felanitx, Mallorca). Endins 2005, 27, 187–204.
- -
- Vallhonrat, F. Aportació a la fauna de geomètrids de les illes Balears (Lepidoptera: Geometridae). Butlletí-Societat Catalana de Lepidopterologia 2004, 93, 43–51.
- -
- Vallhonrat, F.; Pérez, J.J.; Requena, E. Heteròcers nous o interessants de les illes Balears (Lepidoptera). Butlletí-Societat Catalana de Lepidopterologia 2011, 102, 67–72.
- -
- Vicens, P. Primer cens hivernal de corb marí gros Phalacrocorax carbo a les zones de colgada a Balears. Anuari Ornitològic de les Balears 2012, 27, 15–21.
- -
- Viejo, J.L.; González, J.; Gómez, C. Biodiversidad de lepidópteros en relación con sus hábitats, formaciones vegetales y flora de Las Marismillas (Parque Nacional de Doñana, Huelva, Sur de España). Resultados preliminares. Boletín de la Real Sociedad Española de Historia Natural, Sección biológica 2014, 108, 79–101.
- -
- Wang, M.; Zhang, Y.; Bourgoin, T. Planthopper family Issidae (Insecta: Hemiptera: Fulgoromorpha): Linking molecular phylogeny with classification. Molecular Phylogenetics and Evolution 2016, 105, 224–234.
- -
- Wheeler, W.M. Ants of the Balearic Islands. Folia Myrmecologica et Termitologica 1926, 1, 1–6.
- -
- Wirth, S. Description of a new species, Bonomoia opuntiae n. sp. (Histiostomatidae, Astigmata), with observations on the function of its eyes. Acarologia 2005, XLV, 4, 303–319.
- -
- World Spider Catalog. Version 22.0. Natural History Museum Bern, online at http://wsc.nmbe.ch [22-01-2021]. https://doi.org/10.24436/2.
- -
- Wulcan, J.M.; Dennis, M.M.; Ketzis, J.K.; Bevelock, T.J.; Verocai, G.G. Strongyloides spp. in cats: A review of the literature and the first report of zoonotic Strongyloides stercoralis in colonic epithelial nodular hyperplasia in cats. Parasites Vectors 2019, 12, 349.
- -
- WWF España. Informe del Inventario: MAL028-S’Albufera de Mallorca. EsIsWet-Base de datos de los humedales insulares españoles. Updated: 05.2020. Online at: https://www.humedalesdebaleares.es/general/report.php?id=58&lang=es_ES [22.10.2020].
- -
- Yunker, C.E. Studies on the Snake Mite, Ophionyssus natricis, in Nature. Science 1956, 124, 979–980.
- -
- Zahradnik, P. Fauna Europaea: Gastrallus. In Fauna Europaea: Coleoptera, Anobiidae. Audisio, P. (edit.); Fauna Europaea version 2017.06. https://fauna-eu.org [29/01/2021].
- -
- Zaragoza, J.A. Catálogo de los Pseudoescorpiones de la Península Ibérica e Islas Baleares (Arachnida: Pseudoscorpiones). Revista Ibérica de Aracnología 2006, 13, 3–91.
- -
- Balaguer, P.; Gómez-Pujol, L.; Fornós, J.J. 1240 Acantilados con vegetación de las costas mediterráneas con Limonium spp. endémicos. In: Fornós, J.J. (coord.), Bases ecológicas preliminares para la conservación de los tipos de hábitat de interés comunitario en España. Madrid: Ministerio de Medio Ambiente y Medio Rural y Marino, 2009, 1–66.
- -
- Bibiloni, G.; Alomar, G.; Rita, J. XII. Flora vascular dels illost I addicions a la flora de Cabrera Gran. In, Història Natural de l’Arxipèlag de Cabrera; Alcover, J.A.; Ballesteros, E.; Fornós, J.J. (Eds.) CSIC–Editorial Moll, Monografies de la Societat d’Història Natural de les Balears 1993, 2, 179–206.
- -
- Cano, M.J.; Gallego, M.T.; Garilleti, R.; Juaristi, R.; Lara, F., Martínez, J.; Mazimpaka, V.; Rosselló, J.A.; Sánchez-Moya, M.C.; Urdíroz, A. Aportaciones al conocimiento de la flora briológica española. Nótula XIII: Hepáticas y musgos de Mallorca (Islas Baleares). Boletín de la Sociedad Española de Briología 2001, 18/19, 103–110.
- -
- Casas, C.; Cros, R.M.; Muñoz, J. Triquetrella arapilensis y especies afines: Su morfología y distribución geográfica. The Bryologist 1993, 96(1), 122–131.
- -
- Cirujano, S. Tamaricaceae. In. Flora Ibérica, Castroviejo, S. (coord.), 2005, III, 437–445.
- -
- Cros, R.M. Algunos briofitos interesantes para la flora balear. Acta Botánica Malacitana 1982, 7: 141–150.
- -
- Cros, R.M.; Brugués, M.; Sérgio, C.; Infante, M.; Heras, P. Ephemerum recurvifolium. In: Brugués, M., Cros, R.M. and Sérgio, C. (coord.). Cartografia de Briòfits. Península Ibèrica i Illes Balears, Available at http://briofits.iec.cat [11-03-2021].
- -
- Herbari Virtual del Mediterrani Occidental. Available at http://herbarivirtual.uib.es/ [30-08-2021].
- -
- Infante, M.; Sérgio, C.; Heras, P.; Cros, R.M.; Brugués, M. Ephemerum sessile. In Cartografia de Briòfits. Península Ibèrica i Illes Balears. Brugués, M.; Cros, R.M.; Sérgio, C. (coord.); 2019, Available at http://briofits.iec.cat [11-03-2021].
- -
- Puche, F.; Rosselló, J.A. XI. Flora Briològica i Pteridològica. In, Història Natural de l’Arxipèlag de Cabrera; Alcover, J.A., Ballesteros, E. and Fornós, J.J. (Eds.) CSIC–Editorial Moll, Monografies de la Societat d’Història Natural de les Balears 1993, 2, 175–178.
- -
- Pujadas, A.J. Daucus. In: Castroviejo, S. (coord.) Flora Ibérica, 2003, X, 97–125.
- -
- Rita, J.; Bibiloni, G. XIII. La vegetació (Memòria del mapa de les comunitats vegetals). In, Història Natural de l’Arxipèlag de Cabrera; Alcover, J.A.; Ballesteros, E.; Fornós, J.J. (Eds.) CSIC–Editorial Moll, Monografies de la Societat d’Història Natural de les Balears 1993, 2, 207–255.
- -
- Röser, M. Stipellula, a new genus, and new combination in feather grasses (Poaceae tribe Stipeae). Schlechtendalia 2012, 24, 91–93.
- -
- Sáez, L.; Brugués, M.; Casas, C.; Cros, R.M; Balaguer, P. Briófitos nuevos o interesantes para las Islas Baleares. Boletín de la Sociedad Española de Briología 2006, 28, 11–23.
- -
- SITIBSA-GOIB. Projecte BioAtles. Last updated 17/08/2021. Conselleria de Medi Ambient i Territori, Govern de les Illes Balears. 2018, Available at: http://bioatles.caib.es/serproesfront/VisorServlet [31/08/2020].
- -
- Stevens, P.F. Angiosperm Phylogeny Website. Version 14. Last updated 06/02/2021. 2017, Available at: http://www.mobot.org/MOBOT/research/APweb/ [12/03/2021].
- -
- Talavera, S. Cullen. In Flora Ibérica; Castroviejo, S. (coord.); 2000, VII: 357–360.
- -
- Talavera, S. Cymodocea. Flora Ibérica, Talavera, S., Gallego, M.J.; Romero, C.; Herrero, A. (eds.) 2010, XVII: 104–107.
- -
- Traveset, A.; Rita, J. Els illots de l’Arxipèlag de Cabrera: Refugis de biodiversitat. In Arxipèlag de Cabrera: Historia Natural. Mallorca; Grau, A.M; Fornós, J.J.; Mateu, G.; Oliver, P.A.; Terrasa, B. (eds.); Monografies de la Societat d’Historia Natural de les Balears 2020, 30, 513–533.
- -
- Vives, J. Vegetació briofítica. Impressions sobre la vegetació de l’illa de Cabrera. Treballs de la Institució Catalana d’Historia Natural 1976, 7, 119–121.
- -
- World Flora Online. Beta L. 2021, Available at www.worldfloraonline.org/taxon/wfo-4000004539. [26-07-2021].
References
- Pianka, E.; Vitt, L.J. Lizards, Windows to the Evolution of Diversity; University of California Press: Berkeley, CA, USA, 2003. [Google Scholar]
- Pough, F.H. Lizard energetics and diet. Ecology 1973, 54, 837–844. [Google Scholar] [CrossRef]
- Pianka, E.R. Ecology and Natural History of Desert Lizards; Princeton University Press: Princeton, NJ, USA, 1986. [Google Scholar]
- Pérez-Mellado, V.; Corti, C. Dietary adaptations and herbivory in lacertid lizards of the genus Podarcis from western Mediterranean islands (Reptilia: Sauria). Bonner Zool. Beiträge 1993, 44, 193–220. [Google Scholar]
- Legler, J.M.; Sullivan, L.J. The application of stomach-flushing to lizards and anurans. Herpetol. J. 1979, 35, 107–110. [Google Scholar]
- Herrel, A.; Joachim, R.; Vanhooydonck, B.; Irschick, D.J. Ecological consequences of ontogenetic changes in head shape and bite performance in the Jamaican lizard Anolis lineatopus. Biol. J. Linn. Soc. 2006, 86, 443–454. [Google Scholar] [CrossRef]
- Sáez, E.; Traveset, A. Fruit and nectar feeding by Podarcis lilfordi (Lacertidae) on Cabrera Archipelago (Balearic Islands). Herpetol. Rev. 1995, 26, 121–123. [Google Scholar]
- Luiselli, L.; Akani, G.C.; Nwabueze, E.; Pérez-Mellado, V. Stomach flushing affects survival/emigration in wild lizards: A study case with rainbow lizards (Agama agama) in Nigeria. Amphibia. -Reptil. 2011, 32, 253–260. [Google Scholar]
- Pérez-Cembranos, A.; León, A.; Pérez-Mellado, V. Omnivory of an insular lizard: Sources of variation in the diet of Podarcis lilfordi (Squamata, Lacertidae). PLoS ONE 2016, 11, e0148947. [Google Scholar] [CrossRef]
- Pérez-Mellado, V.; Pérez-Cembranos, A.; Garrido, M.; Corti, C.; Luiselli, L. Using faecal samples in lizard dietary studies. Amphibia. -Reptil. 2011, 32, 1–7. [Google Scholar] [CrossRef]
- Casper, R.M.; Jarman, S.N.; Deagle, B.E.; Gales, N.J.; Hindell, M.A. Detecting prey from DNA in predator scats: A comparison with morphological analysis, using Arctocephalus seals fed a known diet. J. Exp. Mar. Bio. Ecol. 2007, 347, 144–154. [Google Scholar] [CrossRef]
- Alemany, I.; Pérez-Cembranos, A.; Pérez-Mellado, V.; Castro, J.A.; Picornell, A.; Ramon, C.; Jurado-Rivera, J.A. DNA metabarcoding the diet of Podarcis lizards endemic to the Balearic Islands. Curr. Zool. 2022, 20, zoac073. [Google Scholar] [CrossRef]
- Jurado-Rivera, J.A.; Vogler, A.P.; Reid, C.A.M.; Petitpierre, E.; Gómez-Zurita, J. DNA barcoding insect-host plant associations. Proc. R. Soc. B Biol. Sci. 2009, 276, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Navarro, S.P.; Jurado-Rivera, J.A.; Gómez-Zurita, J.; Lyal, C.H.C.; Vogler, A.P. DNA profiling of host-herbivore interactions in tropical forests. Ecol. Entomol. 2010, 35, 18–32. [Google Scholar] [CrossRef]
- García-Robledo, C.; Erickson, D.L.; Staines, C.L.; Erwin, T.L.; Kress, W.J. Tropical plant-herbivore networks: Reconstructing species interactions using DNA barcodes. PLoS ONE 2013, 8, e52967. [Google Scholar] [CrossRef]
- Admassu, B.; Juen, A.; Traugott, M. Earthworm primers for DNA-based gut content analysis and their cross-reactivity in a multi-species system. Soil Biol. Biochem. 2006, 38, 1308–1315. [Google Scholar] [CrossRef]
- Dasmahapatra, K.K.; Mallet, J. Taxonomy: DNA barcodes: Recent successes and future prospects. Heredity 2006, 97, 254–255. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cembranos, A.; Pérez-Mellado, V.; Alemany, I.; Bassitta, M.; Terrasa, B.; Picornell, A.; Castro, J.A.; Brown, R.P.; Ramon, C. Morphological and genetic diversity of the Balearic lizard, Podarcis lilfordi (Günther, 1874): Is it relevant to its conservation? Divers. Distrib. 2020, 26, 1122–1141. [Google Scholar] [CrossRef]
- Pérez-Mellado, V. Estudio ecológico de la Lagartija Balear Podarcis lilfordi (Günther, 1874) en Menorca. Rev. Menorca 1989, 80, 455–511. [Google Scholar]
- Leray, M.; Yang, J.Y.; Meyer, C.P.; Mills, S.C.; Agudelo, N.; Ranwez, V.; Boehm, J.T.; Machida, R.J. A new versatile primer set targeting a short fragment of the mitochondrial COI region for metabarcoding metazoan diversity: Application for characterizing coral reef fish gut contents. Front. Zool. 2013, 10, 34. [Google Scholar] [CrossRef]
- Geller, J.B.; Meyer, C.P.; Parker, M.; Hawk, H. Redesign of PCR primers for mitochondrial Cytochrome c oxidase subunit I for marine invertebrates and application in all-taxa biotic surveys. Mol. Ecol. Resour. 2013, 13, 851–861. [Google Scholar] [CrossRef]
- Mallott, E.K.; Malhi, R.S.; Garber, P.A. Brief communication: High-Throughput sequencing of fecal DNA to Identify Insects Consumed by Wild Weddell’s Saddleback Tamarins (Saguinus weddelli, Cebidae, Primates) in Bolivia. Am. J. Phys. Anthropol. 2015, 156, 474–481. [Google Scholar] [CrossRef]
- Sang, T.; Crawford, D.J.; Stuessy, T.F. Chloroplast DNA phylogeny, reticulate evolution, and biogeography of Paeonia (Paeoniaceae). Am. J. Bot. 1997, 84, 1120–1136. [Google Scholar] [CrossRef] [PubMed]
- Tate, J.A.; Simpson, B.B. Paraphyly of Tarasa (Malvaceae) and Diverse Origins of the Polyploid Species. Syst. Bot. 2003, 28, 723–737. [Google Scholar]
- Kress, W.; Erickson, D.L.; Jones, F.A.; Swenson, N.G.; Perez, R.; Sanjur, O.; Bermingham, E. Plant DNA barcodes and a community phylogeny of a tropical forest dynamics plot in Panama. Proc. Natl. Acad. Sci. USA 2009, 106, 18621–18626. [Google Scholar] [CrossRef]
- Levin, R.A.; Wagner, W.L.; Hoch, P.C.; Nepokroeff, M.; Pires, J.C.; Zimmer, E.A.; Sytsma, K.J. Family-level relationships of Onagraceae based on chloroplast rbcL and ndhF data. Am. J. Bot. 2003, 90, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Albanese, D.; Fontana, P.; De Filippo, C.; Cavalieri, D.; Donati, C. MICCA: A complete and accurate software for taxonomic profiling of metagenomic data. Sci. Rep. 2015, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [Green Version]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree v. 1.4.4; Institute of Evolutionary Biology, University of Edinburgh: Edinburgh, Scotland, 2014; Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 3 February 2021).
- Hillis, D.M.; Bull, J.J. An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Syst. Biol. 1993, 42, 182–192. [Google Scholar] [CrossRef]
- Da Silva, L.P.; Mata, V.A.; Lopes, P.B.; Pereira, P.; Jarman, S.N.; Lopes, R.J.; Beja, P. Advancing the integration of multi-marker metabarcoding data in dietary analysis of trophic generalists. Mol. Ecol. Resour. 2019, 19, 1420–1432. [Google Scholar] [CrossRef]
- Cronquist, A. The Evolution and Classification of Flowering Plants, 2nd ed.; New York Botanical Garden: New York, NY, USA, 1988. [Google Scholar]
- Bremer, B.; Bremer, K.; Chase, M.W.; Fay, M.F.; Reveal, J.L.; Soltis, D.E. THE ANGIOSPERM PHYLOGENY GROUP, An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Bot. J. Linn. Soc. 2009, 161, 105–121. [Google Scholar]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2008, 26, 32–46. [Google Scholar] [CrossRef]
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B.; Stevens, M.H.H.; Oksanen, M.J.; Suggests, M. The vegan package. Community Ecol. Packag. 2007, 10, 631–637. [Google Scholar]
- Pallmann, P.; Schaarschmidt, F.; Hothorn, L.A.; Fischer, C.; Nacke, H.; Priesnitz, K.U. Assessing group differences in biodiversity by simultaneously testing a user-defined selection of diversity indices. Mol. Ecol. Resour. 2012, 12, 1068–1078. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.O. Diversity and evenness: A unifying notation and its consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef]
- Magurran, A.E. Measuring Biological Diversity; Blackwell Publishing: Oxford, UK, 2004. [Google Scholar]
- Westfall, P.H.; Young, S.S. Resampling-Based Multiple Testing: Examples and Methods for p-Value Adjustment; John Wiley & Sons, Inc.: New York, NY, USA, 1993. [Google Scholar]
- Scherer, R.; Pallmann, P. simboot: Simultaneous inference for diversity indices. R package, version 0.2-5; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 22 January 2020).
- Gil, V.; Pinho, C.J.; Aguiar, C.A.S.; Jardim, C.; Rebelo, R.; Vasconcelos, R. Questioning the proverb ‘more haste, less speed’: Classic versus metabarcoding approaches for the diet study of a remote island endemic gecko. PeerJ 2020, 8, e8084. [Google Scholar] [CrossRef]
- Calvo, M. Reflexiones en torno a los esquemas de racionalidad espacial reflejados en el paisaje durante la Prehistoria de Mallorca. Pyrenae 2009, 40, 37–78. [Google Scholar]
- Crocetta, F.; Mifsud, S.; Paolini, P.; Piscopo, J.; Schembri, P. New records of the genus Pachygrapsus (Crustacea: Decapoda) from the central Mediterranean Sea with a review of its Mediterranean zoogeography. Mediterr. Mar. Sci. 2011, 12, 75–94. [Google Scholar] [CrossRef]
- Álvarez, E.; Grau, A.M.; Marbà, N.; Carreras, D. Praderas de angiospermas marinas de las Islas Baleares. In Atlas de las Praderas Marinas de España; Ruiz, J.M., Guillén, J.E., Ramos Segura, A., Otero, M.M., Eds.; IEO/IEL/UICN, Murcia-Alicante-Málaga: Murcia, Spain, 2015; pp. 179–219. [Google Scholar]
- Hofreiter, M.; Kreuz, E.; Eriksson, J.; Schubert, G.; Hohmann, G. Vertebrate DNA in fecal samples from Bonobos and Gorillas: Evidence for meat consumption or artefact? PLoS ONE 2010, 5, e9419. [Google Scholar] [CrossRef] [Green Version]



| Phylum | Class | Order | Family | Genus | Species | Ses Bledes | Caragol | Na Guardia | Na Moltona | Na Pelada |
|---|---|---|---|---|---|---|---|---|---|---|
| Arthropoda | ||||||||||
| Arachnida | Araneae | Gnaphosidae | 1 | |||||||
| Pseudoscorpiones | 1 | |||||||||
| Trombidiformes | 1 | 2 | ||||||||
| Eriophyidae | 1 | |||||||||
| Tydeidae | 1 | 3 | ||||||||
| Malacostraca | Decapoda | Grapsidae | Pachygrapsus | marmoratus | 1 | |||||
| Isopoda | 1 | 5 | 2 | 7 | 6 | |||||
| Armadillidae | Armadillo | officinalis | 1 | |||||||
| Halophilosciidae | Halophiloscia | 2 | 1 | 4 | ||||||
| Halophiloscia | couchii | 2 | ||||||||
| Ligiidae | Ligia | italica | 3 | 2 | ||||||
| Insecta | 5 | |||||||||
| Archaeognatha | Machilidae | 1 | ||||||||
| Coleoptera | 1 | 3 | 1 | 1 | ||||||
| Anobiidae | Gastrallus | 1 | 1 | |||||||
| Curculionidae | Tychius | 1 | ||||||||
| Diptera | 1 | 1 | ||||||||
| Sarcophagidae | Sarcophaga | 1 | ||||||||
| Syrphidae | 1 | |||||||||
| Embioptera | 2 | |||||||||
| Oligotomidae | Haploembia | 1 | ||||||||
| Hemiptera | Lygaeidae | Nysius | 3 | |||||||
| Miridae | 1 | |||||||||
| Scutelleridae | Odontoscelis | 1 | ||||||||
| Hymenoptera | Formicidae | 1 | 1 | |||||||
| Messor | bouvieri | 2 | 1 | 1 | ||||||
| Pheidole | 6 | 4 | 5 | |||||||
| Plagiolepis | 1 | 1 | ||||||||
| Tetramorium | semilaeve | 1 | ||||||||
| Lepidoptera | Crambidae | Pyrausta | sanguinalis | 1 | ||||||
| Psychidae | 1 | |||||||||
| Pterophoridae | Agdistis | meridionalis | 4 | |||||||
| Tortricidae | 1 | |||||||||
| Lobesia | 6 | |||||||||
| Neuroptera | Coniopterydidae | 1 | ||||||||
| Psocoptera | Peripsocidae | Peropsocus | 1 | |||||||
| Trogiidae | 1 | 2 | ||||||||
| Cerobasis | 3 | |||||||||
| Diplopoda | Julida | Julidae | 2 | 3 | 1 | |||||
| Chordata | ||||||||||
| Mammalia | Rodentia | Muridae | Mus | 2 | ||||||
| Mollusca | ||||||||||
| Gastropoda | Stylommatophora | Helicidae | Theba | 1 | 1 | |||||
| Theba | pisana | 2 | ||||||||
| Streptophyta | ||||||||||
| Magnoliopsida | Alismatales | 1 | ||||||||
| Apiales | Apiaceae | 1 | ||||||||
| Caryophyllales | 2 | 1 | 2 | 2 | ||||||
| Amaranthaceae | 2 | 2 | 2 | 4 | 5 | |||||
| Beta | vulgaris | 2 | 1 | |||||||
| Suaeda | 2 | 7 | 6 | 6 | 8 | |||||
| Plumbaginaceae | Limonium | 1 | 3 | 2 | 2 | 8 | ||||
| Polygonaceae | 1 | |||||||||
| Fabales | Fabaceae | 1 | 2 | 2 | 1 | |||||
| Medicago | 2 | |||||||||
| Prunus | 1 | 1 | ||||||||
| Pinopsida | Pinales | Pinaceae | Pinus | 2 | 1 | 2 | ||||
| Ses Bledes | Caragol | Na Guardia | Na Moltona | Na Pelada | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Taxon | Morpho | DNA | Morpho | DNA | Morpho | DNA | Morpho | DNA | Morpho | DNA |
| Gastropoda | 1 | 0 | 1 | 0 | 1 | 5 | 1 | 1 | 0 | |
| Pseudoscorpionida | 1 | 0 | 0 | 1 | ||||||
| Araneae | 0 | 1 | 1 | 0 | 0 | 2 | 2 | 0 | ||
| Acarina | 0 | 3 | 0 | 3 | ||||||
| Isopoda | 1 | 2 | 6 | 4 | 0 | 1 | 0 | 7 | 2 | 5 |
| Crustacea | 0 | 3 | 0 | 3 | 0 | 1 | ||||
| Diplopoda | 1 | 2 | 1 | 3 | 0 | 1 | ||||
| Blattodea | 1 | 0 | ||||||||
| Isoptera | 1 | 0 | ||||||||
| Orthoptera | 1 | |||||||||
| Dermaptera | 0 | 1 | 0 | |||||||
| Embioptera | 0 | 2 | 0 | 1 | ||||||
| Homoptera | 0 | 3 | 0 | |||||||
| Heteroptera | 3 | 0 | 0 | 1 | 0 | 3 | ||||
| Diptera | 0 | 1 | 1 | 1 | 1 | 1 | ||||
| Lepidoptera | 0 | 2 | 0 | 1 | 0 | 6 | ||||
| Neuroptera | 0 | 5 | 0 | 1 | ||||||
| Coleoptera | 1 | 2 | 8 | 4 | 4 | 1 | 4 | 1 | 3 | 1 |
| Hymenoptera | 1 | 0 | 2 | 0 | 1 | 0 | 3 | 0 | ||
| Formicidae | 7 | 6 | 8 | 5 | 3 | 1 | 6 | 4 | 6 | 0 |
| Psocoptera | 0 | 1 | 0 | 3 | 0 | 2 | ||||
| Archaeognatha | 0 | 1 | ||||||||
| Mammalia | 0 | 1 | ||||||||
| Aves | 2 | 0 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alemany, I.; Pérez-Cembranos, A.; Castro, J.A.; Picornell, A.; Pérez-Mellado, V.; Ramon, C. Diet of the Insular Lizard, Podarcis lilfordi (Günther, 1874): Complementary Morphological and Molecular Approaches. Animals 2023, 13, 507. https://doi.org/10.3390/ani13030507
Alemany I, Pérez-Cembranos A, Castro JA, Picornell A, Pérez-Mellado V, Ramon C. Diet of the Insular Lizard, Podarcis lilfordi (Günther, 1874): Complementary Morphological and Molecular Approaches. Animals. 2023; 13(3):507. https://doi.org/10.3390/ani13030507
Chicago/Turabian StyleAlemany, Iris, Ana Pérez-Cembranos, José A. Castro, Antònia Picornell, Valentín Pérez-Mellado, and Cori Ramon. 2023. "Diet of the Insular Lizard, Podarcis lilfordi (Günther, 1874): Complementary Morphological and Molecular Approaches" Animals 13, no. 3: 507. https://doi.org/10.3390/ani13030507
APA StyleAlemany, I., Pérez-Cembranos, A., Castro, J. A., Picornell, A., Pérez-Mellado, V., & Ramon, C. (2023). Diet of the Insular Lizard, Podarcis lilfordi (Günther, 1874): Complementary Morphological and Molecular Approaches. Animals, 13(3), 507. https://doi.org/10.3390/ani13030507





