Combined Oxygen-Ozone Therapy for Mesh Skin Graft in a Cat with a Hindlimb Extensive Wound
Abstract
:Simple Summary
Abstract
1. Introduction
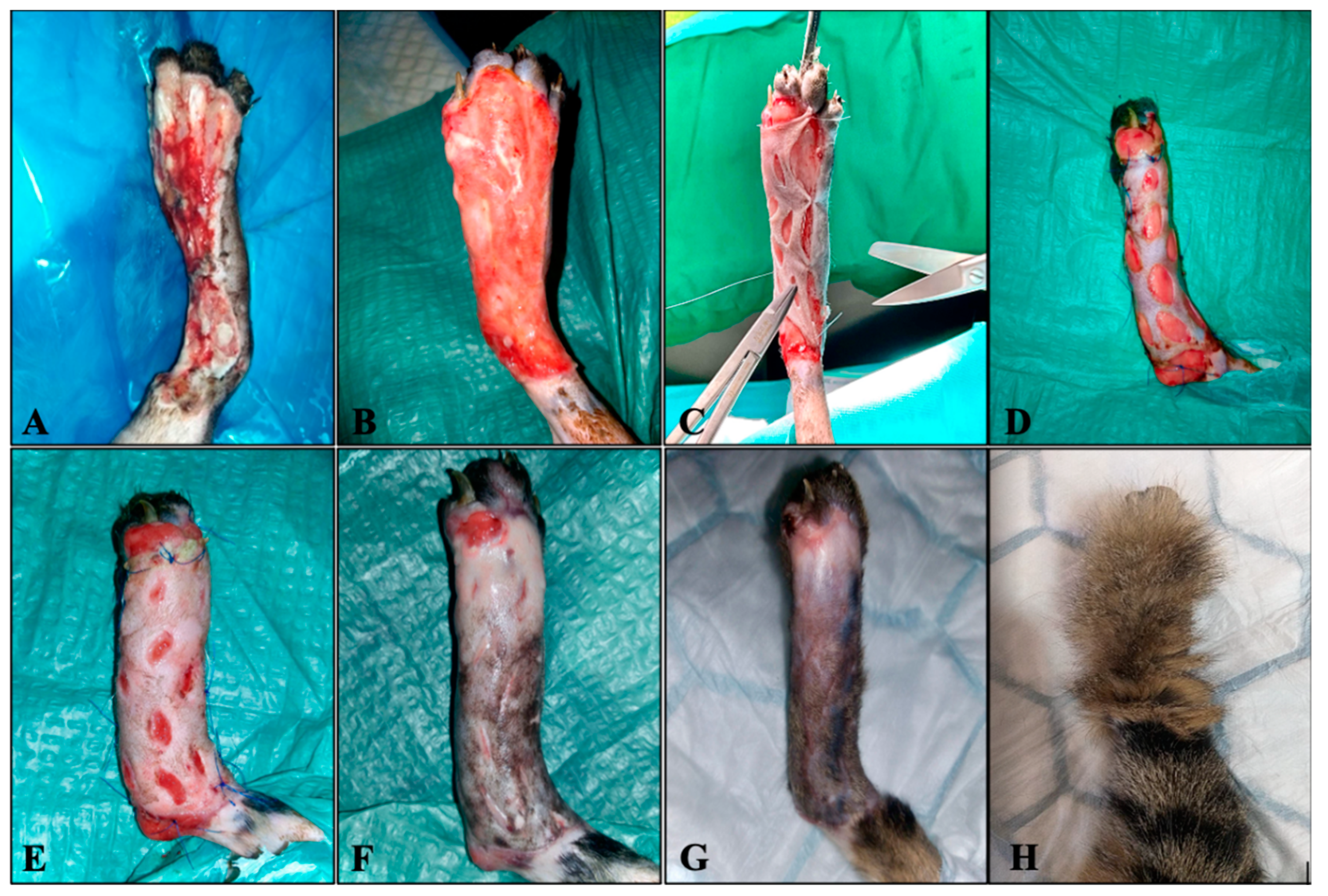
2. Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corr, S. Intensive, Extensive, Expensive: Management of Distal Limb Shearing Injuries in Cats. J. Feline Med. Surg. 2009, 11, 747–757. [Google Scholar] [CrossRef]
- Fossum, T.W.; Duprey, L.P. Small Animal Surgery; Elsevier: Philadelphia, PA, USA, 2019; pp. 179–189. [Google Scholar]
- Shahar, R.; Shamir, M.H.; Brehm, D.M.; Johnston, D.E. Free Skin Grafting for Treatment of Distal Limb Skin Defects in Cats. J. Small Anim. Pract. 1999, 40, 378–382. [Google Scholar] [CrossRef]
- Siegfried, R.; Schmökel, H.; Rytz, U.; Spreng, D.; Schawalder, P. Treatment of Large Distal Extremity Skin Wounds with Autogenous Full-Thickness Mesh Skin Grafts in 5 Cats. Schweiz. Arch. Tierheilkd. 2004, 146, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.A.; Tobias, K.M. Veterinary Surgery: Small Animal; Elsevier: St. Louis, MO, USA, 2018; pp. 3804–3827. [Google Scholar]
- Bocci, V.; Borrelli, E.; Travagli, V.; Zanardi, I. The Ozone Paradox: Ozone Is a Strong Oxidant as well as a Medical Drug. Med. Res. Rev. 2009, 29, 646–682. [Google Scholar] [CrossRef]
- Zhang, J.; Guan, M.; Xie, C.; Luo, X.; Zhang, Q.; Xue, Y. Increased Growth Factors Play a Role in Wound Healing Promoted by Noninvasive Oxygen-Ozone Therapy in Diabetic Patients with Foot Ulcers. Oxidative Med. Cell. Longev. 2014, 2014, 273475. [Google Scholar] [CrossRef] [PubMed]
- ISCO3: Madrid Declaration on Ozone Therapy, 3rd ed.; International Scientific Committee of Ozone Therapy: Madrid, Spain, 2020.
- Bocci, V.A. Scientific and Medical Aspects of Ozone Therapy. State of the Art. Arch. Med. Res. 2006, 37, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Bocci, V. OZONE; Springer: Dordrecht, The Netherlands, 2010; pp. 20–32. [Google Scholar] [CrossRef]
- Silva, R.A.; Garotti, J.E.R.; Silva, R.S.B.; Navarins, A.; Pacheco, A.M., Jr. Analysis of the bactericidal effect of ozone pneumoperitoneum. Acta Cir. Bras. 2009, 24, 124–127. [Google Scholar] [CrossRef]
- Onal, O.; Yetisir, F.; Sarer, A.E.S.; Zeybek, N.D.; Onal, C.O.; Yurekli, B.; Celik, H.T.; Wire, A.; Kılıc, M. Prophylactic Ozone Administration Reduces Intestinal Mucosa Injury Induced by Intestinal Ischemia-Reperfusion in the Rat. Mediat. Inflamm. 2015, 2015, 792016. [Google Scholar] [CrossRef]
- Bocci, V.; Zanardi, I.; Huijberts, M.S.P.; Travagli, V. Diabetes and Chronic Oxidative Stress. A perspective based on the possible use of ozone therapy. Diabetes Metab. Syndr. Clin. Res. Rev. 2011, 5, 45–49. [Google Scholar] [CrossRef]
- Bocci, V.; Di Paolo, N. Oxygen-Ozone Therapy in Medicine: An Update. Blood Purif. 2009, 28, 373–376. [Google Scholar] [CrossRef]
- Valacchi, G.; Lim, Y.; Belmonte, G.; Miracco, C.; Zanardi, I.; Bocci, V.; Travagli, V. Ozonated Sesame Oil Enhances Cutaneous Wound Healing in SKH1 Mice. Wound Repair Regen. 2010, 19, 107–115. [Google Scholar] [CrossRef]
- Kim, H.S.; Noh, U.S.; Inn, Y.W.; Kim, K.M.; Kang, H.; Kim, H.O.; Park, Y.M. Therapeutic Effects of Topical Application of Ozone on Acute Cutaneous Wound Healing. J. Korean Med. Sci. 2009, 24, 368. [Google Scholar] [CrossRef] [PubMed]
- Travagli, V.; Zanardi, I.; Valacchi, G.; Bocci, V. Ozone and Ozonated Oils in Skin Diseases: A Review. Mediat. Inflamm. 2010, 2010, 610418. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.D.; Morais, T.M.L.; Araújo, R.M.F.G.; Meyer, P.F.; Oliveira, E.A.F.; Silva, R.M.V.; Carreiro, E.M.; Carreiro, E.P.; Belloco, V.G.; Mariz, B.A.L.A.; et al. Effects of Subcutaneous Injection of Ozone during Wound Healing in Rats. Growth Factors 2019, 37, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Repciuc, C.C.; Toma, C.G.; Ober, C.A.; Oana, L.I. Management of Surgical Wound Dehiscence by Oxygen-Ozone Therapy in a FIV-Positive Cat—A Case Report. Acta Vet. Brno 2020, 89, 189–194. [Google Scholar] [CrossRef]
- Viebahn-Haensler, R.; León Fernández, O.S. Ozone in Medicine. The Low-Dose Ozone Concept and Its Basic Biochemical Mechanisms of Action in Chronic Inflammatory Diseases. Int. J. Mol. Sci. 2021, 22, 7890. [Google Scholar] [CrossRef] [PubMed]
- Moger, G.; Khatri, I.; Kumar, N. Evaluation of Effect of Topical Ozone Therapy on Salivary Candidal Carriage in Oral Candidiasis. Indian J. Dent. Res. 2015, 26, 158. [Google Scholar] [CrossRef]
- Al-Saadi, H.; Potapova, I.; Rochford, E.T.; Moriarty, T.F.; Messmer, P. Ozonated Saline Shows Activity against Planktonic and Biofilm Growing Staphylococcus Aureusin Vitro: A Potential Irrigant for Infected Wounds. Int. Wound J. 2015, 13, 936–942. [Google Scholar] [CrossRef]
- Thanomsub, B.; Anupunpisit, V.; Chanphetch, S.; Watcharachaipong, T.; Poonkhum, R.; Srisukonth, C. Effects of Ozone Treatment on Cell Growth and Ultrastructural Changes in Bacteria. J. Gen. Appl. Microbiol. 2002, 48, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Zanardi, I.; Burgassi, S.; Paccagnini, E.; Gentile, M.; Bocci, V.; Travagli, V. What Is the Best Strategy for Enhancing the Effects of Topically Applied Ozonated Oils in Cutaneous Infections? BioMed Res. Int. 2013, 2013, 702949. [Google Scholar] [CrossRef]
- Riggs, J.; Jennings, J.L.F.; Friend, E.J.; Halfacree, Z.; Nelissen, P.; Holmes, M.A.; Demetriou, J.L. Outcome of Full-Thickness Skin Grafts Used to Close Skin Defects Involving the Distal Aspects of the Limbs in Cats and Dogs: 52 Cases (2005–2012). J. Am. Vet. Med. Assoc. 2015, 247, 1042–1047. [Google Scholar] [CrossRef]
- Nolff, M.C. Filling the Vacuum: Role of Negative Pressure Wound Therapy in Open Wound Management in Cats. J. Feline Med. Surg. 2021, 23, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Nolff, M.C.; Meyer-Lindenberg, A. Negative Pressure Wound Therapy Augmented Full-Thickness Free Skin Grafting in the Cat: Outcome in 10 Grafts Transferred to Six Cats. J. Feline Med. Surg. 2015, 17, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Re, L.; Mawsouf, M.N.; Menéndez, S.; León, O.S.; Sánchez, G.M.; Hernández, F. Ozone Therapy: Clinical and Basic Evidence of Its Therapeutic Potential. Arch. Med. Res. 2008, 39, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Rilling, S.; Viebahn, R. The Use of Ozone in Medicine; Haug Publishers: Heidelberg, Germany, 1987; p. 17. [Google Scholar]
- Pavletic, M.M. Skin Flap and Skin Grafting Technique in Small Animal Surgery. Vet. Q. 1997, 19 (Suppl. 1), 24–25. [Google Scholar] [CrossRef] [PubMed]
- Swaim, S. The full thickness mesh graft. Vet. Med. 1986, 81, 524–531. [Google Scholar]
- Elvis, A.M.; Ekta, J.S. Ozone Therapy: A Clinical Review. J. Nat. Sci. Biol. Med. 2011, 2, 66–70. [Google Scholar] [CrossRef]
- Orakdogen, M.; Uslu, S.; Emon, S.T.; Somay, H.; Meric, Z.C.; Hakan, T. The Effect of Ozone Therapy on Experimental Vasospasm in the Rat Femoral Artery. Turk. Neurosurg. 2015, 26, 860–865. [Google Scholar] [CrossRef]
- Bohling, M.W.; Henderson, R.A.; Swaim, S.F.; Kincaid, S.A.; Wright, J.C. Comparison of the Role of the Subcutaneous Tissues in Cutaneous Wound Healing in the Dog and Cat. Vet. Surg. 2006, 35, 3–14. [Google Scholar] [CrossRef] [PubMed]

| Day | Session | Method | Ozone Concentration-Time (Minutes) | Observations |
|---|---|---|---|---|
| 0 | 1 | bagging | 60 μg/Nml-5 | atonic wound, infected with devitalized edges and necrotic tissue |
| subcutaneous infiltration | 15 μg/Nml | |||
| 1 | 2 | bagging | 60 μg/Nml-5 | exudative wound |
| subcutaneous infiltration | 15 μg/Nml | |||
| 2 | 3 | bagging | 60 μg/Nml-5 | exudative wound with delimited edges |
| subcutaneous infiltration | 15 μg/Nml | |||
| 5 | 4 | bagging | 30 μg/Nml-10 | exudative wound with adherent edges to the underlying tissue, without clinical signs of infection |
| 8 | 5 | bagging | 20 μg/Nml-10 | exudate in small quantities, with fine-grained buds |
| 11 | 6 | bagging | 10 μg/Nml-20 | homogeneous grain tissue of pale pink color |
| 14 | 7 | bagging | 10 μg/Nml-20 | uniform granulation tissue with the beginning of epithelialization at the periphery |
| 17 | 8 | bagging | 10 μg/Nml-20 | application of ozone before surgery |
| 17 | 9 | bagging | 10 μg/Nml-20 | immediate post-grafting ozone application |
| 20 | 10 | bagging | 10 μg/Nml-20 | graft of pink skin without pathological exudate |
| 23 | 11 | bagging | 10 μg/Nml-20 | graft of skin adhering to the underlying tissue and the beginning of epithelization is visible on the periphery of the graft incisions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oros, N.-V.; Repciuc, C.; Ober, C.; Mihai, M.; Oana, L.-I. Combined Oxygen-Ozone Therapy for Mesh Skin Graft in a Cat with a Hindlimb Extensive Wound. Animals 2023, 13, 513. https://doi.org/10.3390/ani13030513
Oros N-V, Repciuc C, Ober C, Mihai M, Oana L-I. Combined Oxygen-Ozone Therapy for Mesh Skin Graft in a Cat with a Hindlimb Extensive Wound. Animals. 2023; 13(3):513. https://doi.org/10.3390/ani13030513
Chicago/Turabian StyleOros, Nicuşor-Valentin, Călin Repciuc, Ciprian Ober, Mihaela Mihai, and Liviu-Ioan Oana. 2023. "Combined Oxygen-Ozone Therapy for Mesh Skin Graft in a Cat with a Hindlimb Extensive Wound" Animals 13, no. 3: 513. https://doi.org/10.3390/ani13030513





