Effects of Black Cumin Seed (Nigella sativa) and Coconut Meals (Cocos nucifera) on Broiler Performance and Cecal Microbiota
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Performance Traits
2.3. Cecal Microbiota DNA Extraction and Relative Quantitative RT-qPCR
2.4. Relative Quantification Protocol
2.5. Statistical Analysis
3. Results
3.1. Performance Traits
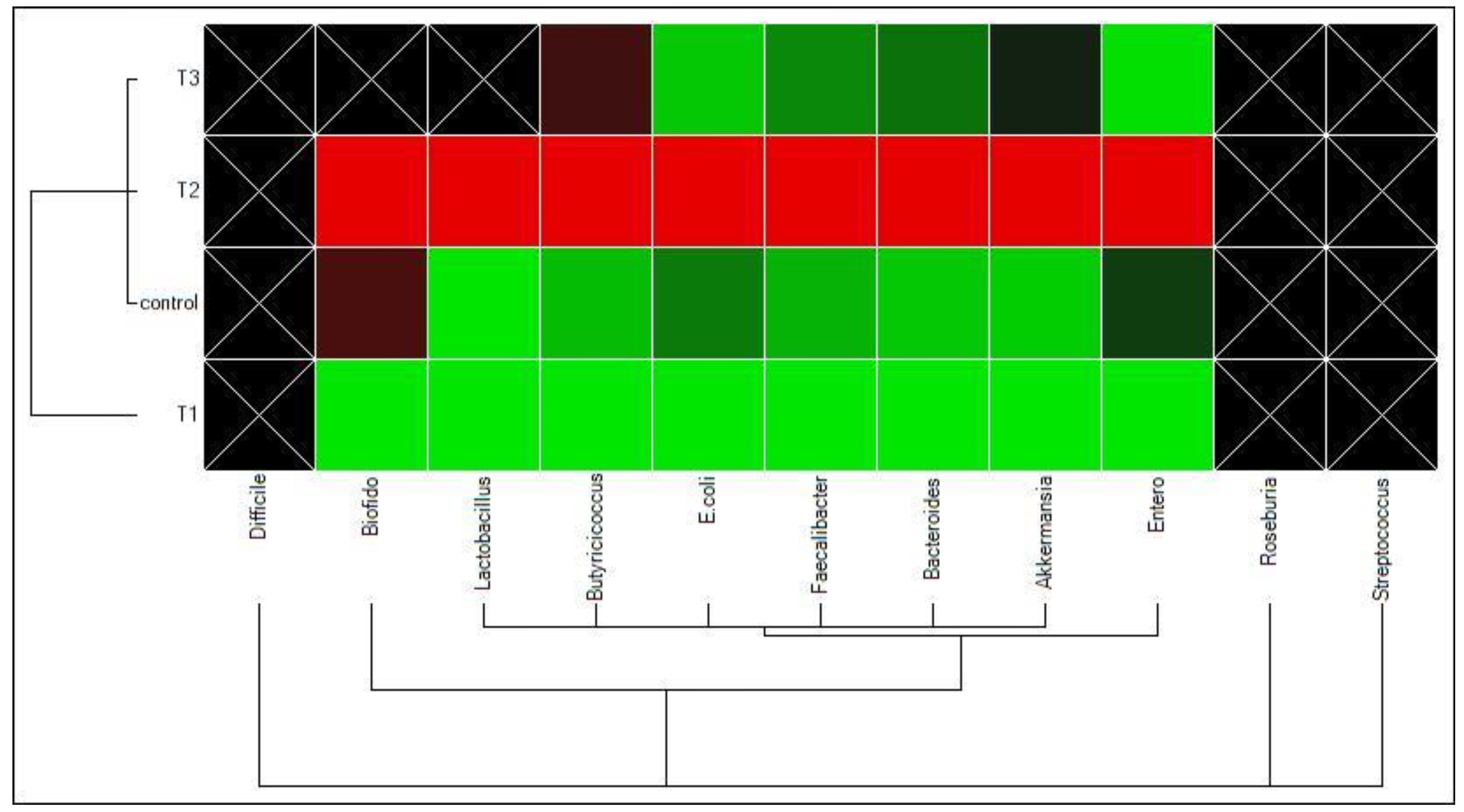
3.2. Cecal Microbiota
4. Discussion
4.1. Performance Traits
4.2. Cecal Microbiota
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akhtar, M.S.; Nasir, Z.; Abid, A. Effect of feeding powdered Nigella Sativa L. seeds on poultry egg production and their suitability for human consumption. Vet. Arhiv. 2003, 73, 181–190. [Google Scholar]
- Guler, T.; Dalkılıç, B.; Ertas, O.N.; Çiftçi, M. The effect of dietary black cumin seeds (Nigella Sativa L.) on the performance of broilers. Asian-Australas. J. Anim. Sci. 2006, 19, 425–430. [Google Scholar] [CrossRef]
- Erener, G.; Altop, A.; Ocak, N.; Aksoy, H.M.; Cankaya, S.; Ozturk, E. Influence of black cumin seed (Nigella sativa L.) and seed extract on broilers performance and total coliform bacteria count. Asian J. Anim. Vet. Adv. 2010, 5, 128–135. [Google Scholar] [CrossRef]
- Hassan, S.S.A.; Mandour, M.A. Effect of Nigella sativa seeds on growth performance, carcass traits and economic efficiency of broiler chicks under Egyptian condition. Egypt. Poult. Sci. J. 2018, 38, 331–344. [Google Scholar]
- Hermes, I.H.; Attia, F.A.M.; Ibrahim, K.A.; El-Nesr, S.S. Effect of dietary Nigella sativa L. on productive performance and nutrients utilization of broiler chicks raised under summer conditions of Egypt. Egypt. Poult. Sci. J. 2009, 29, 145–172. [Google Scholar]
- Khan, S.H.; Ansari, J.; Haq, A.U.; Abbas, G. Black cumin seeds as phytogenic product in broiler diets and its effects on performance, blood constituents, immunity and caecal microbial population. Ital. J. Anim. Sci. 2012, 11, e77. [Google Scholar] [CrossRef]
- Hassan, M.M.; Ashmawy, E.S.; Abd-Elsamee, M.O.; Ibrahim, M.R. Effect of phytochemicals and active components of some natural feed additives on growth performance, antioxidative properties and economic efficiency of Muscovy ducklings. Egypt. Poult. Sci. J. 2019, 39, 81–97. [Google Scholar] [CrossRef]
- Osman, M. Beneficial effects of black seed oil inclusion in broiler diet on performance and carcass characteristics. Egypt. Poult. Sci. J. 2002, 22, 839–853. [Google Scholar]
- Attia, Y.A.; Al-Harthi, M.A. Nigella seed oil as an alternative to antibiotic growth promoters for broiler chickens. Europ. Poult. Sci. 2015, 79. [Google Scholar] [CrossRef]
- Demirci, M.; Karsli, M.A.; Aluc, Y. Determining the effects of black cumin seed oil on performance and meat fatty acid profile of broiler chickens. S. Afr. J. Anim. Sci. 2019, 49, 890–897. [Google Scholar] [CrossRef]
- El-Deek, A.A.; Hamdy, S.M.; Attia, Y.A.; Khalifah, M.M. Nigella Sativa seed oil meal as a source of plant protein in broiler diets. Egypt. Poult. Sci. J. 2009, 29, 39–52. [Google Scholar]
- Jahan, M.S.; Khairunnesa, M.; Afrin, S.; Ali, M.S. Dietary black cumin (Nizella sativa) seed meal on growth and meat yield performance of broilers. SAARC J. Agric. 2015, 13, 151–160. [Google Scholar] [CrossRef]
- Asghar, M.U.; Doğan, S.C.; Wilk, M.; Korczyński, M. Effect of Dietary Supplementation of Black Cumin Seeds (Nigella sativa) on Performance, Carcass Traits, and Meat Quality of Japanese Quails (Coturnix coturnix japonica). Animals 2022, 12, 1298. [Google Scholar] [CrossRef] [PubMed]
- FAO/IAEA-Food and Agriculture Organization of the United Nations. Manual on the Application of the HACCP System in Mycotoxin Prevention and Control; FAO, Food and Nutrition: Rome, Italy, 2001. [Google Scholar]
- Daghir, N.J. Poultry Production in Hot Climates, 2nd ed.Cabi SeriesCABI: Oxfordshire, UK, 2008.
- Sundu, B.; Kumar, A.; Dingle, J. Feeding value of copra meal for broilers. World Poult. Sci. J. 2009, 65, 481–491. [Google Scholar] [CrossRef]
- Sundu, B.; Kumar, A.; Dingle, J. The effect of levels of copra meal and enzymes on bird performance. In Proceedings of the 16th Australian Poultry Science Symposium, Sydney, NSW, Australia, 9–11 February 2004; pp. 52–54. [Google Scholar]
- Sundu, B.; Kumar, A.; Dingle, J. Response of broiler chicks fed increasing levels of copra meal and enzymes. Int. J. Poult. Sci. 2006, 5, 13–18. [Google Scholar]
- Panigrahi, S.; Machin, D.H.; Parr, W.H.; Bainton, J. Responses of broiler chicks to dietary copra cake of high lipid content. Br. Poult. Sci. 1987, 28, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Sundu, B.; Kumar, A.; Dingle, J. Growth pattern of broilers fed a physically or enzymatically treated copra meal diet. In Proceedings of the 17th Australian Poultry Science Symposium, Sydney, NSW, Australia, 7–9 February 2005; pp. 291–294. [Google Scholar]
- Jácome, I.M.T.D.; da Silva Gomes, L.P.; Guim, A.; Lima, D.Q.; Almeida, M.M.; de Araújo, M.J.; Oliveira, V.P.; Silva, J.D.B.; Martins, T.D.D. Effect of different levels of coconut meal in broiler chicken's diets upon the carcass yield. Acta. Sci. Anim. Sci. 2002, 24, 1015–1019. [Google Scholar] [CrossRef]
- Bastos, S.C.; Fuentes, M.F.F.; Freitas, E.R.; Espíndola, G.B.; Braga, C.V.P. Efeito da inclusão do farelo de coco emrações para frangos de corte. Rev. Ciên. Agron. 2007, 38, 297–303. [Google Scholar]
- Sundu, B.; Kumar, A.; Dingle, J. The Effect of proportion of crumbled copra meal and enzyme supplementation on broiler growth and gastrointestinal development. Int. J. Poult. Sci. 2008, 7, 511–515. [Google Scholar] [CrossRef]
- Engberg, R.M.; Hedemann, M.S.; Steenfeldt, S.; Jensen, B.B. Influence of whole wheat and xylanase on broiler performance and microbial composition and activity in the digestive tract. Poult. Sci. 2004, 83, 925–938. [Google Scholar] [CrossRef]
- Alwafa, R.A.; Mudalal, S.; Mauriello, G. Origanum syriacum L. (Za’atar), from Raw to Go: A Review. Plants 2021, 10, 1001. [Google Scholar] [CrossRef] [PubMed]
- Mudalal, S.; Zaazaa, A.; Omar, J.A. Effects of medicinal plants extract with antibiotic free diets on broilers growth performance and incidence of muscles abnormalities. Braz. J. Poult. Sci. 2021, 23, 1–8. [Google Scholar] [CrossRef]
- Zaazaa, A.; Mudalal, S.; Alzuheir, I.; Samara, M.; Jalboush, N.; Fayyad, A.; Petracci, M. The Impact of Thyme and Oregano Essential Oils Dietary Supplementation on Broiler Health, Growth Performance, and Prevalence of Growth-Related Breast Muscle Abnormalities. Animals 2022, 12, 3065. [Google Scholar] [CrossRef] [PubMed]
- Indira, M.; Venkateswarulu, T.C.; Abraham Peele, K.; Nazneen Bobby, M.; Krupanidhi, S. Bioactive molecules of probiotic bacteria and their mechanism of action: A review. 3 Biotech 2019, 9, 306. [Google Scholar] [CrossRef] [PubMed]
- Oakley, B.B.; Lillehoj, H.S.; Kogut, M.H.; Kim, W.K.; Maurer, J.J.; Pedroso, A.; Lee, M.D.; Collett, S.R.; Johnson, T.J.; Cox, N.A. The chicken gastrointestinal microbiome. FEMS Microbiol. Lett. 2014, 360, 100–112. [Google Scholar] [CrossRef]
- NRC—National Research Council. Nutrient Requirements of Poultry, 9th ed.; National Academy Press: Washington, DC, USA, 1994. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Lee, K.W.; Everts, H.; Kappert, H.J.; Yeom, K.H.; Beynen, A.C. Dietary carvacrol lowers body weight gain but improves feed conversion in female broiler chickens. J. Appl. Poult. Res. 2003, 12, 394–399. [Google Scholar] [CrossRef]
- Kaur, G.; Sankrityayan, H.; Dixit, D.; Jadhav, P. Cocos nucifera and metformin combination for modulation of diabetic symptoms in streptozotocin induced diabetic rats. J. Ayurveda Integr. Med. 2020, 11, 3–9. [Google Scholar] [CrossRef]
- Mohd Nor, N.N.; Abbasiliasi, S.; Marikkar, M.N.; Ariff, A.; Amid, M.; Lamasudin, D.U.; Abdul Manap, M.Y.; Mustafa, S. Defatted coconut residue crude polysaccharides as potential prebiotics: Study of their effects on proliferation and acidifying activity of probiotics in vitro. J. Food Sci. Technol. 2017, 54, 164–173. [Google Scholar]
- Guo, Q.; Goldenberg, J.Z.; Humphrey, C.; El Dib, R.; Johnston, B.C. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst. Rev. 2019, 4, CD004827. [Google Scholar] [CrossRef]
- Singh, V.P.; Sharma, J.; Babu, S.; Singla, A. Role of probiotics in health and disease: A review. J. Pak. Med. Assoc. 2013, 63, 253–257. [Google Scholar] [PubMed]
- Ahmad, A.; Husain, A.; Mujeeb, M.; Khan, S.A.; Najmi, A.K.; Siddique, N.A.; Damanhouri, Z.A.; Anwar, F.A. Review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac. J. Trop. Biomed. 2013, 3, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Kooti, W.; Hasanzadeh-Noohi, Z.; Sharafi-Ahvazi, N.; Asadi-Samani, M.; Ashtary-Larky, D. Phytochemistry, pharmacology, and therapeutic uses of black seed (Nigella sativa). Chin. J. Nat. Med. 2016, 14, 732–745. [Google Scholar] [CrossRef] [PubMed]
| Ingredient | C | T1 | T2 | T3 |
|---|---|---|---|---|
| Yellow corn | 317.0 | 311.5 | 322.0 | 236.0 |
| Soybean meal | 295.0 | 292.5 | 282.0 | 268.0 |
| Wheat | 250 | 139 | 139 | 139 |
| Sunflower | 50 | 50 | 50 | 50 |
| Black cumin seed meal | 0 | 100 | 0 | 50 |
| Coconut meal | 0 | 0 | 100 | 50 |
| Premix 1 | 4 | 4 | 4 | 4 |
| Oil | 41 | 60 | 60 | 60 |
| Limestone calcium | 13 | 13 | 13 | 13 |
| Dicalcium phosphate | 16.5 | 16.5 | 16.5 | 16.5 |
| Sodium bicarbonate | 1 | 1 | 1 | 1 |
| Salt | 4.5 | 4.5 | 4.5 | 4.5 |
| L-lysine | 4.5 | 4.5 | 4.5 | 4.5 |
| DL-methionine | 2.5 | 2.5 | 2.5 | 2.5 |
| Threonine | 1 | 1 | 1 | 1 |
| Calculated analysis (%) | ||||
| Crude protein | 22 | 22 | 22 | 22 |
| Crude fat | 5.80 | 6.20 | 6.40 | 6.30 |
| Fiber | 3.80 | 3.90 | 3.90 | 3.90 |
| Calcium | 0.92 | 0.92 | 0.92 | 0.93 |
| Available P | 0.47 | 0.47 | 0.47 | 0.47 |
| ME, Kcal/kg ration | 3041 | 3036 | 3040 | 3020 |
| Ingredient | C | T1 | T2 | T3 |
|---|---|---|---|---|
| Yellow corn | 479.0 | 387.0 | 397.0 | 329.5 |
| Soybean meal | 242.0 | 229.0 | 219.0 | 213.5 |
| Wheat | 150 | 150 | 150 | 100 |
| Sunflower | 60 | 60 | 60 | 60 |
| Black cumin seed meal | 0 | 100 | 0 | 50 |
| Coconut meal | 0 | 0 | 100 | 50 |
| Premix 1 | 4 | 4 | 4 | 4 |
| Oil | 30 | 35 | 35 | 58 |
| Limestone Calcium | 11.2 | 11.2 | 11.2 | 11.2 |
| Dicalcium phosphate | 14 | 14 | 14 | 14 |
| Sodium bicarbonate | 1 | 1 | 1 | 1 |
| Salt | 2.5 | 2.5 | 2.5 | 2.5 |
| L-lysine | 4 | 4 | 4 | 4 |
| DL-methionine | 1.9 | 1.9 | 1.9 | 1.9 |
| Threonine | 0.4 | 0.4 | 0.4 | 0.4 |
| Calculated analysis (%) | ||||
| Crude protein | 20 | 20 | 20 | 20 |
| Crude fat | 4.20 | 4.40 | 4.40 | 6.40 |
| Fiber | 3.90 | 4.10 | 4.20 | 4.20 |
| Calcium | 0.90 | 0.90 | 0.90 | 0.90 |
| Available P | 0.46 | 0.46 | 0.46 | 0.46 |
| ME, Kcal/kg ration | 3062 | 3010 | 3010 | 3040 |
| qPCR (Target Gene) | Pos/Neg (%) | Sequence | Length (bp) |
|---|---|---|---|
| Total bacteria 16S rRNA | 96/0 | F: ACTCCTACGGGAGGCAGCAGTR: GTAATTCCGCGGCTGCTGGCAC | 194–200 |
| Akkermansia muciniphila | 89/11 | F: CAGCACGTGAAGGTGGGGACR: CCTTGCGGTTGGCTTCAGAT | 329 |
| Bacteroides spp. | 100/0 | F: GGGTTTAAAGGGAGCGTAGGR: CTACACCACGAATTCCGCCT | 116 |
| Bifidobacterium spp. | 100/0 | F: GAATAGCTCCTGGAAACGR: ATAGGACGCGACCCCA | 99 |
| Butyricicoccus spp. | 100/0 | F: ACCTGAAGAGAATAAGCTCCR: GATAACGCTTGCTCCCTACGT | 69 |
| Clostridioides difficile | 9/91 | F: GCAAGTTGAGCGATTTTACTTCGGTR: GTACTGGCTCACCTTTGATATTYAAGAG | 155 |
| Enterobacteriaceae spp. | 97/0 | F: CATTGACGTTACCCGCAGAAGAAGCR: CTCTACGAGACTCAAGCTTGC | 190 |
| Escherichia coli | 97/3 | F: CAACGAACTGAACTGGCAGAR: CATTACGCTGCGATGGAT | 121 |
| Faecalibacterium prausnitzii | 100/0 | F: GGAGGAAGAAGGTCTTCGGR: AATTCCGCCTACCTCTGCACT | 248 |
| Lactobacillus spp. | 90/9 | F: AGCAGTAGGGAATCTTCCAR: CACCGCTACACATGGAG | 340–346 |
| Roseburia spp. | 99/0 | F: TACTGCATTGGAAACTGR: CGGCACCGAAGAGCAAT | 230 |
| Streptococcus spp. | 81/12 | F: GAAGAATTGCTTGAATTGGTTGAAR: GGACGGTAGTTGTTGAAGAATGG | 559 |
| Traits | Age | C | T1 | T2 | T3 | p Value |
|---|---|---|---|---|---|---|
| M ± SEM | M ± SEM | M ± SEM | M ± SEM | |||
| Cumulative feed intake (g) | 1–7 d | 132 ± 0.57 | 131 ± 1.20 | 130 ± 0.66 | 131 ± 0.88 | 0.56 |
| 8–14 d | 492 ± 2.00 | 491 ± 2.40 | 495 ± 0.33 | 494 ± 1.16 | 0.37 | |
| 15–21 d | 1225 ± 5.78 | 1215 ± 6.51 | 1219 ± 0.88 | 1232 ± 6.35 | 0.21 | |
| 22–28 d | 2189 ± 8.57 | 2177 ± 9.49 | 2184 ± 2.52 | 2196 ± 10.17 | 0.48 | |
| 29–35 d | 3495 ± 18.27 | 3471 ± 12.12 | 3485 ± 0.88 | 3490 ± 7.53 | 0.55 | |
| Body weight (g) | 1–7 d | 148 b ± 0.58 | 148 b ± 0.33 | 152 a ± 0.58 | 147 b ± 0.58 | <0.05 |
| 8–14 d | 450 b ± 1.16 | 452 b ± 1.20 | 459 a ± 1.76 | 450 b ± 0.67 | <0.05 | |
| 15–21 d | 874 b ± 2.40 | 876 b ± 0.67 | 885 a ± 1.16 | 873 b ± 2.60 | <0.05 | |
| 22–28 d | 1464 b ± 2.08 | 1466 b ± 4.26 | 1496 a ± 3.18 | 1465 b ± 4.37 | <0.05 | |
| 29–35 d | 2018 b ± 9.39 | 2026 b ± 10.11 | 2097 a ± 10.26 | 2027 b ± 3.22 | <0.05 | |
| Cumulative feed conversion ratio (g/g gain) | 1–7 d | 894 a ± 6.51 | 887 ab ± 8.09 | 858 b ± 5.93 | 890 ab ± 8.54 | <0.05 |
| 8–14 d | 1092 ab ± 5.21 | 1086 ab ± 3.06 | 1080 b ± 4.06 | 1100 a ± 2.96 | <0.05 | |
| 15–21 d | 1400 ab ± 8.84 | 1387 ab ± 7.02 | 1377 b ± 1.20 | 1412 a ± 10.48 | <0.05 | |
| 22–28 d | 1495 a ± 3.84 | 1486 a ± 2.19 | 1457 b ± 1.45 | 1499 a ± 10.58 | <0.05 | |
| 29–35 d | 1732 a ± 14.85 | 1714 a ± 3.18 | 1660 b ± 7.54 | 1722 a ± 6.39 | <0.05 |
| Traits | C | T1 | T2 | T3 | p Value |
|---|---|---|---|---|---|
| M ± SEM | M ± SEM | M ± SEM | M ± SEM | ||
| Thigh | 209 ± 17.54 | 215 ± 42.73 | 232 ± 31.76 | 232 ± 23.61 | 0.28 |
| Drum sticks | 171 b ± 11.86 | 191 ab ± 20.52 | 201 a ± 19.48 | 190 ab ± 22.33 | <0.05 |
| Wings | 150 ± 12.99 | 151 ± 12.17 | 154 ± 14.75 | 154 ± 11.09 | 0.86 |
| Breast | 498 c ± 44.95 | 579 ab ± 37.35 | 632 a ± 90.31 | 545 bc ± 58.97 | <0.05 |
| Neck | 96 ± 9.19 | 98 ± 13.76 | 101 ± 8.14 | 102 ± 8.87 | 0.64 |
| Back | 199 ± 14.98 | 200 ± 18.77 | 211 ± 21.94 | 202 ± 21.91 | 0.59 |
| Legs | 74 ± 7.02 | 73 ± 9.29 | 77 ± 6.74 | 73 ± 7.78 | 0.61 |
| Head | 33 ± 2.86 | 35 ± 4.31 | 37 ± 4.26 | 37 ± 3.94 | 0.09 |
| Intestines | 99 c ± 4.71 | 107 b ± 7.27 | 108 b ± 2.10 | 124 a ± 8.58 | <0.05 |
| Heart | 9.14 ± 0.59 | 9.95 ± 1.42 | 10.23 ± 1.55 | 10.32 ± 1.46 | 0.23 |
| Liver | 41.97 b ± 3.85 | 43.41 ab ± 4.21 | 45.66 ab ± 1.42 | 47.19 a ± 4.34 | <0.05 |
| Gizzard | 28.47 b ± 1.90 | 29.17 ab ± 2.74 | 29.42 ab ± 2.89 | 31.9 a ± 2.62 | <0.05 |
| Crop | 3.88 b ± 0.81 | 3.96 b ± 0.47 | 3.98 b ± 0.61 | 4.88 a ± 0.52 | <0.05 |
| Proventriculus | 7.51 b ± 0.52 | 7.74 ab ± 1.08 | 8.27 ab ± 0.44 | 8.49 a ± 0.71 | <0.05 |
| Live weight | 1919 b ± 49.17 | 2031 ab ± 80.87 | 2109 a ± 172.95 | 2030 ab ± 109.78 | <0.05 |
| Carcass weight | 1432 b ± 39.49 | 1544 ab ± 106.09 | 1646 a ± 187.44 | 1535 ab ± 99.99 | <0.05 |
| Dressing % | 74.63 b ± 1.14 | 75.96 ab ± 3.07 | 77.89 a ± 2.55 | 75.61 ab ± 1.89 | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaazaa, A.; Mudalal, S.; Sabbah, M.; Altamimi, M.; Dalab, A.; Samara, M. Effects of Black Cumin Seed (Nigella sativa) and Coconut Meals (Cocos nucifera) on Broiler Performance and Cecal Microbiota. Animals 2023, 13, 535. https://doi.org/10.3390/ani13030535
Zaazaa A, Mudalal S, Sabbah M, Altamimi M, Dalab A, Samara M. Effects of Black Cumin Seed (Nigella sativa) and Coconut Meals (Cocos nucifera) on Broiler Performance and Cecal Microbiota. Animals. 2023; 13(3):535. https://doi.org/10.3390/ani13030535
Chicago/Turabian StyleZaazaa, Ahmed, Samer Mudalal, Mohammed Sabbah, Mohammad Altamimi, Abdelhafeed Dalab, and Maen Samara. 2023. "Effects of Black Cumin Seed (Nigella sativa) and Coconut Meals (Cocos nucifera) on Broiler Performance and Cecal Microbiota" Animals 13, no. 3: 535. https://doi.org/10.3390/ani13030535
APA StyleZaazaa, A., Mudalal, S., Sabbah, M., Altamimi, M., Dalab, A., & Samara, M. (2023). Effects of Black Cumin Seed (Nigella sativa) and Coconut Meals (Cocos nucifera) on Broiler Performance and Cecal Microbiota. Animals, 13(3), 535. https://doi.org/10.3390/ani13030535









