Simple Summary
Sea Turtles have a unique immune system, which evolved over millions of years in the persistent host-pathogen arms race. As this species occupies a unique evolutionary and environmental niche, they provide an opportunity to gain insight into the evolution of immunity. We present an overview of the turtle immune system, including the cells and organs important for coordinating the immune response to pathogens, with a focus on pathogen recognition and inflammatory mediators, including Interferons. We highlight areas for future study and note which studies have investigated freshwater turtles and are lacking in sea turtles. We particularly focus on the Green Sea turtle (Chelonia mydas) as the juvenile turtles within this species are the most afflicted by the neoplastic tumorous disease, fibropapillomatosis (FP).
Abstract
The immune system of sea turtles is not completely understood. Sea turtles (as reptiles) bridge a unique evolutionary gap, being ectothermic vertebrates like fish and amphibians and amniotes like birds and mammals. Turtles are ectotherms; thus, their immune system is influenced by environmental conditions like temperature and season. We aim to review the turtle immune system and note what studies have investigated sea turtles and the effect of the environment on the immune response. Turtles rely heavily on the nonspecific innate response rather than the specific adaptive response. Turtles’ innate immune effectors include antimicrobial peptides, complement, and nonspecific leukocytes. The antiviral defense is understudied in terms of the diversity of pathogen receptors and interferon function. Turtles also mount adaptive responses to pathogens. Lymphoid structures responsible for lymphocyte activation and maturation are either missing in reptiles or function is affected by season. Turtles are a marker of health for their marine environment, and their immune system is commonly dysregulated because of disease or contaminants. Fibropapillomatosis (FP) is a tumorous disease that afflicts sea turtles and is thought to be caused by a virus and an environmental factor. We aim, by exploring the current understanding of the immune system in turtles, to aid the investigation of environmental factors that contribute to the pathogenesis of this disease and provide options for immunotherapy.
1. Introduction
The immune system has evolved to protect the host in the constant ‘host-pathogen arms race’. Pathogens evolve mechanisms to evade immunity, and the immune system must counterattack [1]. Our understanding of immunology is enriched by the study of a broad spectrum of species that encounter a diversity of microbial organisms. Many fundamental immunology concepts have been discovered through the study of non-mammalian or non-model organisms such as drosophila (discovery of Toll-like receptors [2]), starfish and sea urchins (concept of self-versus nonself recognition [3]), and chickens (discovery of T and B cell lineages and genetic mechanism of antibody diversity [4,5]). As sea turtles are long-lived ectotherms with a unique evolutionary history, further study of this ancient immune system could provide further insight into fundamental immunology that could lead to the development of new therapeutic approaches for a variety of diseases and potentially discover new antimicrobial effectors [6,7].
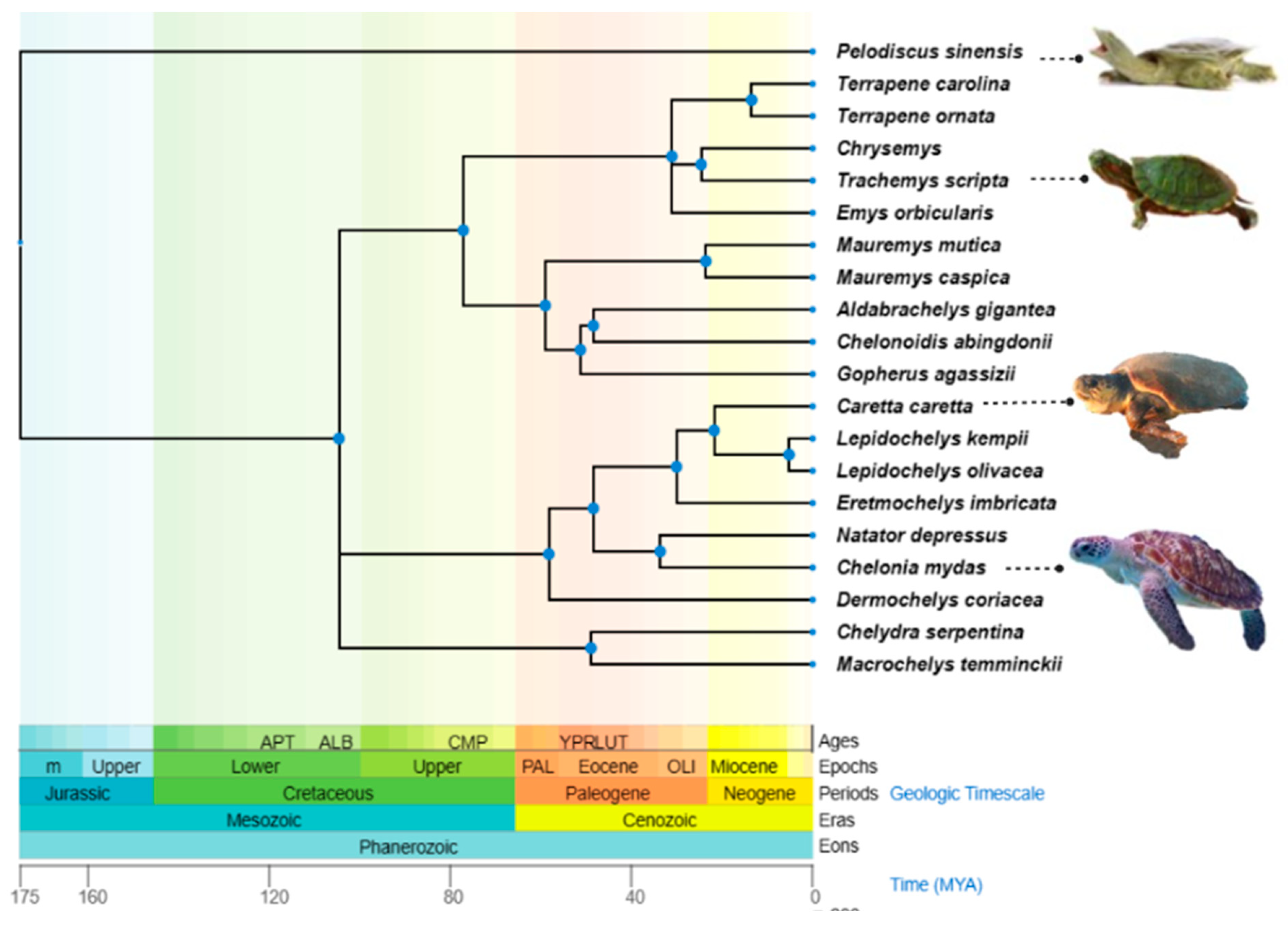
Out of the seven surviving sea turtle species, three are classed as ‘Vulnerable’; Loggerhead (Caretta caretta (Linnaeus, 1758)), Leatherback (Dermochelys coriacea (Vandelli, 1761)), and Olive Ridley (Lepidochelys olivacea, (Eschscholtz, 1829), one as ‘Endangered’; Green (Chelonia mydas (Linnaeus, 1758)), two as ‘Critically Endangered’; Kemp’s Ridley (Lepidochelys kempii (Garman, 1880)) and Hawksbill (Eretmochelys imbricata (Linnaeus, 1766)), and the flatback (Natator depressus (Garman, 1880)) is ‘data deficient’ according to the IUCN (International Union for Conservation of Nature and Natural Resources) [8]. The geographic distribution and phylogenetic relationships of the turtle species discussed in this review are outlined in Table 1 and Figure 1. Sea turtles live in a unique hyper-saline and microbe-laden marine environment. As a result, Sea turtles’ immune response has evolved its own unique approach to handling the highly conserved functions of host defence. Understanding how their environment has influenced variation in immune genes and regulation of immunity is important in developing long-term strategies to protect sea turtles from both existing and emerging infectious diseases.

Table 1.
Overview of Testudines habitat, ecosystem, and immune system studies reviewed.
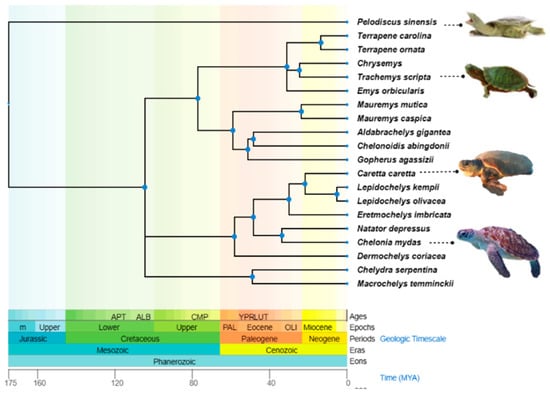
Figure 1.
The phylogenetic tree of Testudines species is mentioned in this review. The tree was constructed from the information provided by http://timetree.org/ (accessed on 20 December 2022).
The immune response of Testudines (turtles, terrapins, and tortoises) is the most studied of the surviving lineages in class Reptilia, which includes Crocodilia (crocodilians), Squamata (snakes, lizards, amphisbaenians) and Rhychocephalia (tuatara) [65]. Yet, these studies represent only 12% of the 365 species of Testudines [65]. Studies have focused on turtles’ heavy reliance upon the robust innate response to pathogens, leaving questions regarding the role of the adaptive response. The anti-viral response, in particular, is poorly understood [19,66,67]. Turtles’ lymphocytes are believed to respond to infection in a relatively nonspecific way with an altered antibody and memory response [66]. Large gaps in knowledge of the sea turtle immune system include; the mechanism of pathogen recognition through pattern recognition receptors (PRRs), the pathways and efficiency of antiviral effectors such as interferons (IFNs) and IFN-stimulated genes (ISGs), the role of cytotoxic T cells in killing infected or damaged host cells, and the mechanism and locations of T cell-mediated B cell activation [66].
Understanding these gaps will not only elucidate the evolution and diversity of the immune system but will also assist in the conservation of vulnerable sea turtle species from threats of chronic disease, infection, and a degrading marine environment. For example, fibropapillomatosis (FP) is a tumorous disease that affects sea turtles. The dramatic increase in FP prevalence since the 1980s is attributed to a virus, and an unknown environmental factor thought to be anthropogenic in nature [68,69,70]. Results from genomic [71], proteomic [72,73,74] and transcriptomic studies [70,75,76] have consistently identified immune genes as dysregulated between healthy and diseased turtles or by levels of exposure to marine contaminants [73]. Additionally, sea turtles are possible reservoirs for zoonotic viruses, as C. mydas, along with snakes and pangolins, have been suggested as potential intermediate hosts of SARS-CoV-2 [77]. Incidences of antibiotic-resistant bacterial infections in sea turtles have been increasing as antibiotics are continually released into the environment [34,78,79,80,81,82,83,84].
Multidisciplinary interest in sea turtle immunology is growing, guided by ecoimmunology and ‘One Health’ principles, and the application of omics techniques, protein isolation, and purification, microscopy, and histology will aid in the characterization of immune components [65,67,85]. The adaption, standardization, and application of these techniques to sea turtle research will continue to identify dysregulated and disease-related pathways, allowing the selection of potential precision medicine treatments [70,85]. However, the turtle immune response is influenced by individual and species-specific factors, including season, sex, and temperature [19,33,86,87]. Thus, further fundamental understanding and characterization of the immune response is required to achieve future successful therapies.
Studies of sea turtle immune function have been focused on the protection and conservation of turtle wildlife from threats such as FP, parasites, or environmental contaminants [42,49,50,51,52,53,54,55,56,73,75,76,88,89,90,91,92]. Functional studies of the turtle immune system have been carried out on red-eared slider turtles Trachemys scripta (Thunberg, 1792)) [19,20,21,22,23], Caspian turtle or striped-neck terrapin (Mauremys capsica (Gmelin, 1774)) [27,28,29,30,31] and Chinese soft-shelled turtle (Pelodiscus sinensis (Wiegmann, 1853)) [14,87,87,93]. In this review, we discuss the innate and adaptive immune systems of sea turtles, and we report what studies have examined the effects of the environment on the sea turtle immune function. We focus particularly on C. mydas. This is because, as juveniles, these turtles are most frequently afflicted by FP. Furthermore, the C. mydas genome was the first published and thus most investigated sea turtle genome.
2. Lymphoid Structures
Reptile lymphoid structures offer a first glance at the distinctiveness of their immune system compared to mammals. Missing or altered lymphoid structures highlight how the reptilian immune system is biased to the evolutionarily older innate response as a robust defence against infection [86]. Furthermore, seasonal changes in lymphoid structures provide evidence for an altered reliance upon the adaptive response [19,28,30,86].
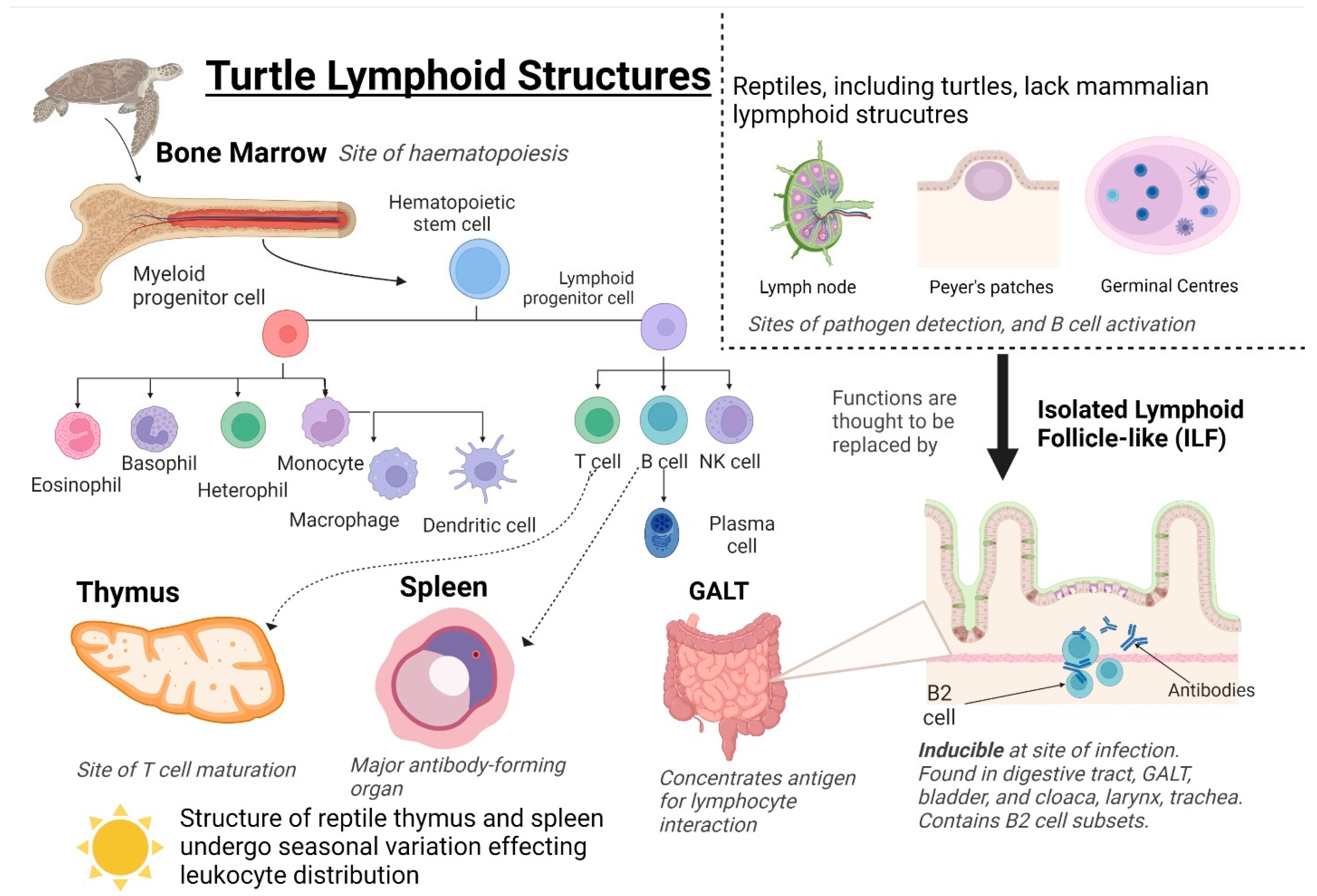
Lymphoid structures are organs in which immune effector cells originate, mature, detect antigens, and induce an immune response. Primary lymphoid structures in the turtle are the bone marrow and thymus (Figure 2) [86]. Bone marrow is the site of haematopoiesis, the production of red and white blood cells (erythrocytes and leukocytes) and plasma in blood. Leukocytes differentiate into innate effectors from myeloid progenitor cells (heterophils, eosinophils, basophils, monocytes) or adaptive effectors from lymphoid progenitor cells (T and B cells). (Natural killer (NK) cells are an exception as they are innate lymphocytes). As in mammals, turtle T cells migrate and mature in the thymus [59,63]. B cells migrate to the spleen, which is the largest antibody-forming organ in turtles. The spleen has been described in a snapping turtle (Chelydra serpentina, Linnaeus, 1758) [63], the Caspian turtle (M. capsica) [28,29] and the Chinese soft-shelled turtle (P. sinensis) [9].
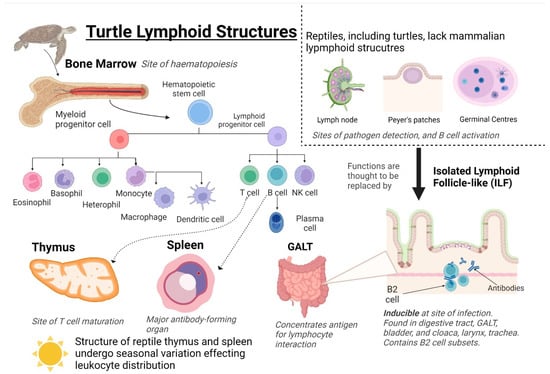
Figure 2.
Turtle Lymphoid Structures. Primary structures include the bone marrow and thymus, and secondary structures include the spleen, Gut-associated lymphoid tissue (GALT), and isolated lymphoid follicle-like (ILFs). Hematopoietic stem cells are generated from the bone marrow and differentiate into myeloid progenitor cells or lymphoid progenitor cells. Myeloid progenitor cells differentiate into innate effector cells; granulocytes: basophils and eosinophils, and phagocytes: heterophils, monocytes, and macrophages. Lymphoid progenitor cells differentiate into adaptive immune effector cells: T and B cells. The distribution and differentiation of leukocytes are affected by seasonal variations in the structure of the spleen and thymus. Created using BioRender.
Other than the spleen, secondary lymphoid structures in turtles include gut-associated lymphoid tissue (GALT) and isolated lymphoid follicle-like structures (ILFs) (Figure 2). The GALT and ILFs concentrate antigens to interact with lymphocytes in tissue and induce an immune response. Murine ILFs are organized lymphoid structures found in the GALT that contain lymphocytes, predominately antibody-producing B-2 subtype B cells [94]. ILFs have a unique inducible nature, and changes in the local microbial community influence distribution [94,95]. B cells containing ILF-like aggregates were discovered in the intestine of the red-eared slider turtle (T. scripta) [21] and in the digestive tract, urinary bladder, cloaca, larynx, and trachea of C. mydas [59].
Reptiles lack both lymph nodes and Peyer’s patches, both of which are key sites for the initiation of the mammalian adaptive response (Figure 2). Lymph nodes are areas of high lymphocyte density where lymphatic fluid is filtered, and antigens are presented to lymphocytes. Germinal centers are found here and are the site of B lymphocyte maturation [96]. ILFs are thought to represent the ancestor of mammalian lymph nodes and replace the missing function of lymph nodes [63,86]. Peyer’s patches are located in the GALT and consist of isolated or aggregated lymphoid follicles with a function to induce immune tolerance or defense against pathogens by the interplay between the immune cells and the follicle-associated epithelium [97]. ILFs are suggested to trap and process antigens for presentation to cells of the immune response, are possible sites of B cell maturation, and play a role in mucosal immunity [21,59]. The mechanisms regulating immune cell trafficking to and from ILFs and the cell: cell interactions within the ILFs required for optimal immunity have not been fully described.
Seasonal Changes of Lymphoid Structures
The structure of the reptilian spleen and thymus is season-dependent [86]. Seasonal involution of the thymus and spleen results from neuroendocrine interactions that occur in all ectothermic vertebrates. This has been studied in lizards, snakes, and freshwater turtles [28,30,98,99]. The key effector cells of the adaptive immune system, T and B cells, mature and are activated in ILFs. Thus, reptiles cannot rely on adaptive immunity in the same manner as mammals all year round [86]. The precise patterns of seasonal variations in the structure are species-specific [86]. To our knowledge, this effect is yet to be discussed, specifically in sea turtles. However, a study of the terrapin Mauremys capsica shows that the spleen and thymus undergo seasonal variation affecting lymphocyte density and differentiation [28,30]. As Winter gives way to Spring, the thymus gland increases in size, and the cortex begins to develop. By the end of Spring, the thymus reached its maximum size with a well-developed medulla and cortex, and adaptive lymphocytes are most abundant. In Summer, the thymus involutes greatly, and lymphocyte density declines to its lowest in Autumn. The thymus undergoes a small increase in Autumn, only to gradually decrease again during Winter [28,30]. Innate immune effectors, heterophils, basophils, and monocytes, reach their highest levels in Autumn [30]. In addition, Muñoz et al. reported a sex difference in the distribution of lymphocytes; females had higher proportions of lymphocytes than males in the thymus during Summer and in the spleen during Spring [30]. Variation in lymphoid structure implies that turtles rely more heavily upon the innate immune system in Autumn and Winter. Adaptive immunity bolsters defense against infection during Spring providing enhanced protection during mating and nesting [19].
3. Innate Immune System
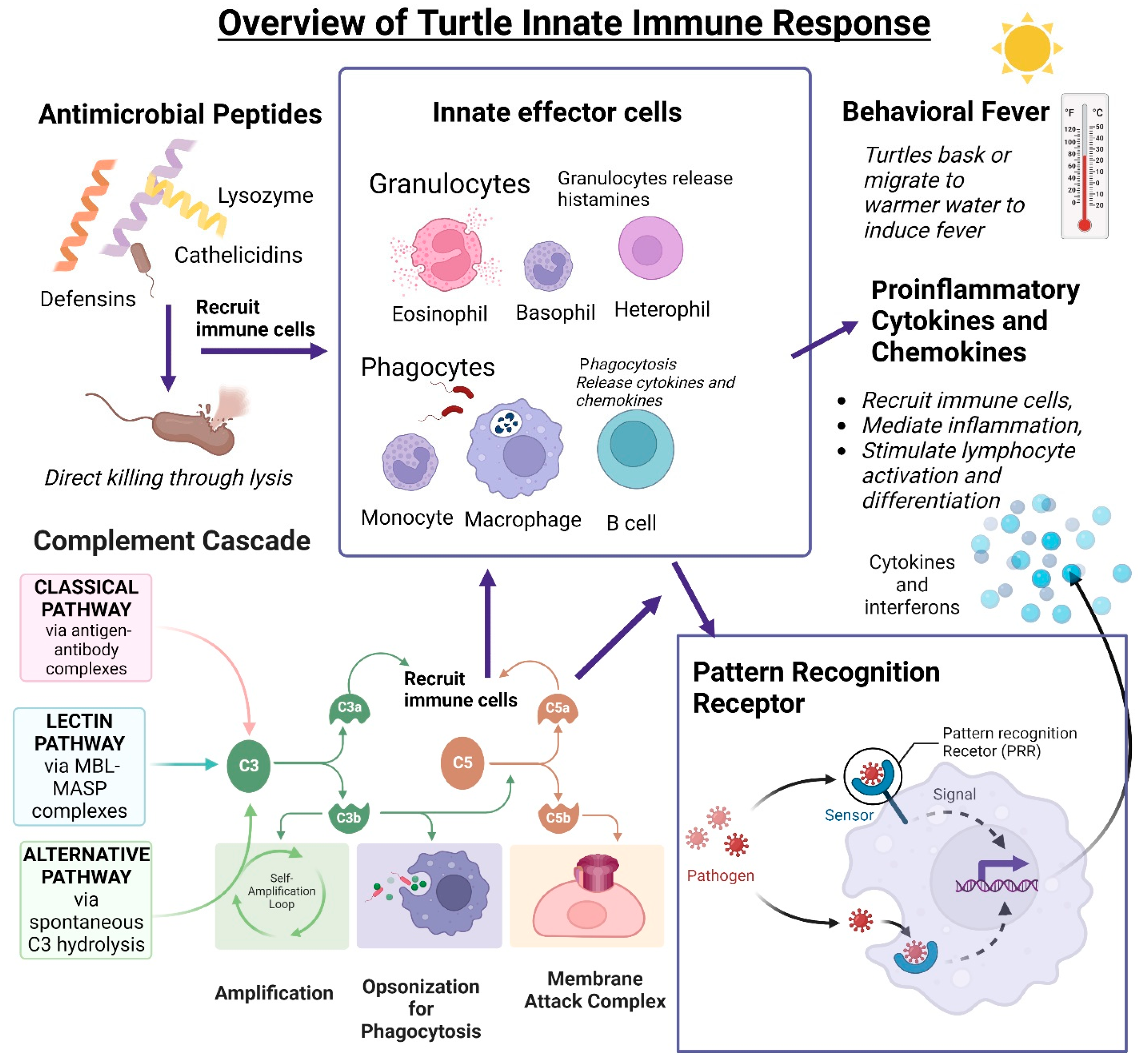
Reptiles rely on their robust innate immune system to protect themselves from infection. The innate immune system is an organism’s first line of defense against invading pathogens. It is non-specific and needs no previous exposure to a pathogen. The first barrier against microbial invasion is a turtle’s thick, keratinized outer layer of skin [100]. This antimicrobial epidermal barrier is surveilled by Langerhans cells and is aided by antimicrobial peptides (AMPs) [101]. If a pathogen succeeds in breaching this barrier, the innate immune system mobilizes a broad spectrum of non-specific responses, including inflammation, behavioral fever, innate effectors, and non-specific leukocytes, and activates the adaptive response. [67]. Inflammatory mediators signal the recruitment of heterophils, not neutrophils, as in mammals, to the site of infection and begin the immune response [86]. In this section, we discuss what is known about the innate immune system in the turtle (summarized in Figure 3).
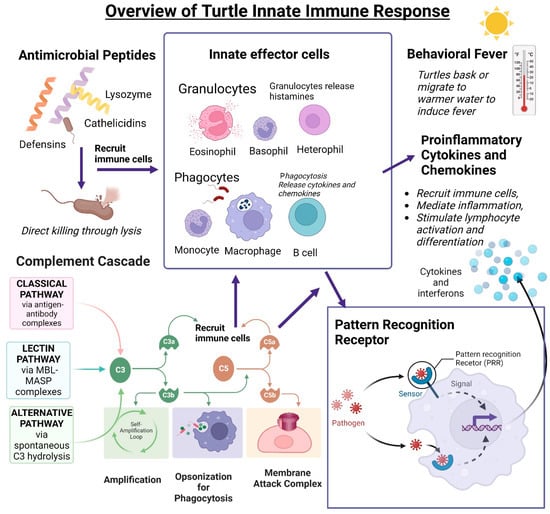
Figure 3.
Overview of turtle innate immune functions. Nonspecific innate responses require no previous exposure to microorganisms to ensure a robust response. Antimicrobial peptides (lysozymes, cathelicidins, and defensins) can directly kill microorganisms by cell wall permeabilization and induction of lysis. Innate effector cells include granulocytes and phagocytes. Granulocytes are heterophils, basophils, and eosinophils and can release histamines when activated by microorganisms. Reptiles, including C. mydas, have phagocytic B cells. The complement cascade activates C3 through the classical, lectin, or alternative pathways, which results in the opsonization of microorganisms for phagocytosis or lyse cell wall through the membrane attack complex (MAC) formation. Cleaved C3a and C5a recruit nonspecific effector cells. Innate effector cells recognize microorganisms through the binding of pattern recognition receptors which begins a downstream signaling cascade ending in the release of cytokines and interferons. Cytokines and chemokines can recruit immune cells and mediate inflammation and lymphocyte activation and differentiation. Created using BioRender.
Innate Effector Cells
Although reptiles and mammals as vertebrates have similarities in innate immune genes, there are frequent differences in their function [67]. As interest in the field of eco-immunology grows and the list of published reptilian genomes gets longer, comparative studies reveal the diverse evolution and mechanisms of host defense [102,103,104]. In reptiles, the dividing line between innate and adaptive immunity is blurred. For example, adaptive cells take part in traditional innate functions like phagocytosis. Even in mammals, innate effector cells undergo ‘trained immunity’ where the second exposure to an antigen can induce a stronger response which is the traditional adaptive characteristic of memory. (Trained immunity is the heritable epigenetic changes, first described as in monocytes and macrophages, that induce a state of hyper- or hypo-responsiveness to future infection [105,106,107,108,109]). Turtle innate effector cells consist of non-specific leukocytes: monocytes, macrophages, dendritic cells, NK cells, heterophils, eosinophils, and basophils [110,111].
Phagocytosis is an extremely conserved function across many species and is one of the fundamental functions of innate immune effector cells. After phagocytosis of a pathogen, a phagocyte releases cytokines to modulate the immune response, and professional antigen-presenting cells can initiate the adaptive response through the activation of T cells. Turtles have a wide variety of phagocytes, including monocytes, macrophages, melanomacrophages, heterophils, eosinophils, thrombocytes, and B cells [89,110,111,112]. The major phagocytic effectors of the innate immune response in sea turtles are reported to be heterophils and lymphocytes [111,113]. C. mydas lymphocytes were identified as major phagocytes, although it is unclear if this includes both T and B cells [111].
Turtle granulocytes include heterophils, eosinophils, and basophils. Heterophils are large round granulocytes with an unlobed nucleus, thought to replace the function of mammalian neutrophils. These cells assist in suppressing microbial invasion by the release of antimicrobial peptides and are involved in the inflammatory response [86]. Eosinophils are larger in sea turtles compared to freshwater turtles [114]. They are phagocytic cells involved in the removal of parasitic pathogens. High numbers in blood are associated with innate immune stimulation and parasitic infection [114]. A study in snapping turtles reported eosinophils could phagocytize immune complexes [62]. Basophils are one of the only leukocytes in turtles, not yet described to have phagocytic properties. Basophils have variable morphology in sea turtles but are usually small in size with a centrally located nucleus [114]. Basophils have a parallel immune function to that of mammalian basophils or mast cells, both containing 5-hydroxytryptamine and histamine. Basophil cell surface contains antigen-specific immunoglobulins that, when activated, induce degranulation and release of histamine [114]. The level of histamine released increases as the concentration of antigen increases [115]. This reaction time in turtles lasts significantly longer (40–60 min) in comparison to humans (2 min) [64].
Monocytes can differentiate into macrophages and dendritic cells [116]. Turtle monocytes have the most variable morphology of the leukocytes and can be rhomboid, oval, or round in shape and contain basophilic cytoplasm [114]. Monocytes are phagocytic, antigen-presenting cells (APCs) and are involved with inflammatory disease through the release of cytokines [19]. Generally, monocytes are found at a low count in C. mydas [114]. However, count increases during chronic inflammation, bacterial or parasitic diseases, and chronic antigenic stimulation [113]. Langerhans cells are a dendritic cell subtype particularly found in the human epidermis and mucosal surfaces, and were reported in C. mydas epidermis [101]. These Langerin+ cells adopted a pleomorphic morphology and were observed around ulcerative dermatitis ulcers.
Respiratory burst is an ancient method of killing ingested microorganisms by toxic molecules observed in birds, reptiles, and fish [86,113]. After phagocytosis of the pathogen, phagocytes NADPH generate intracellular reactive oxygen species (ROS) via NADPH oxidase, which eliminates microorganisms from the host. Antioxidants are expressed in turtle blood as a defense against the physiological stress caused by excessive ROS production. Lymphocytes are suggested to be the major effectors of respiratory burst in loggerhead sea turtles, C. caretta [113].
4. Behaviorally Induced Fever
Fever is an integral part of the innate immune response in homeotherms. As ectotherms, turtles cannot self-regulate their body temperature. Instead, they increase their body temperature in response to infection by moving to warmer waters or basking (Figure 3) [86]. Behavioral fever activity raises body temperature by approximately 4 °C in response to infection or handling stress in a freshwater turtle (Chrysemys picta, Schneider, 1783), two terrestrial turtles (Terrapene carolina carolina (Linnaeus, 1758) and Clemmys insculpta (Le Conte, 1830)), and a tortoise (Gopherus polyphemus, Daudin, 1801) [18,117,118]. Temperature fluctuation can have a direct effect on innate immunity. Turtles’ innate effector cell function is influenced by temperature [64,86,119]. Snapping turtle basophils released histamine in a range of 10–37 °C, with an optimum of 27 °C [19,64]. In pond slider turtle (T. scripta), the macrophage-mediated respiratory burst was optimum between 25–30 °C [119].
Certain C. mydas populations in the Pacific Ocean, including Hawaii, engage in terrestrial basking behavior, due to cold winter sea surface temperature [120]. A 2006 study observed a relationship between FP and basking behavior in C. mydas. Only turtles with FP were observed basking, resulting in a 2.9 °C increase above ambient body temperature [121]. Heat-seeking behavior has been reported in murine cancer models, and cold stress is associated with accelerated tumor growth [122,123]. Further study is needed to define the relationship between infection and mechanisms governing behavioral fever in sea turtles and to understand if warming oceans will impact the fever response and how this will affect the immune response to infection and the incidence of cancer.
5. Innate Immune Effectors
Studies of reptilian innate immune effectors, including lysozyme, complement, and antimicrobial peptides, suggest these molecules have a broader range of activity in comparison to their mammalian counterparts (Figure 3) [115].
- (1)
- Lysozyme
Lysozymes are enzymes that kill bacteria by catalyzing the hydrolysis of the cell wall [124]. Lysozymes are released by phagocytes into mucus, saliva, and plasma in response to foreign microorganisms. Reptilian lysozyme activity can vary on water temperature, pH, toxicants, sex, age, season, and stressors [124].
Lysozyme C (chicken) and G (goose) genes have been identified in the C. mydas genome [125]. Lysozyme C is the major lysozyme type, ubiquitously and constitutively expressed in vertebrates and all tissue types, respectively [126]. Lysozyme G has been described in mammals, birds, fish, and insects with activity against Gram-positive and Gram-negative bacteria. Lysozyme G is not constitutively expressed and is highly upregulated in response to infection. In fish, lysozyme G expression is upregulated after challenge with lipopolysaccharide (LPS), Vibrio alginolyticus, Aermonas hydrophila, and Aphanomyces invadans [126,127,128,129,130,131,132].
C. mydas lysozyme C has a dual pH optima at pH 6.0 and pH 8.0, contrasting with single pH optima of pH 6.0 in lysozyme isolated from soft-shelled turtles [133]. C. mydas lysozyme C had high activity against the Gram-positive bacteria; Micrococcus luteus, moderate activity to two Gram-positive and two Gram-negative bacteria; Staphylococcus aureus and Staphylococcus epidermidis, and Pseudomonas aeruginosa and Vibrio cholerae, respectively. Gram-negative bacteria Pseudomonas spp. and Vibrio spp. are associated with inflammatory diseases, including ulcerative dermatitis and ulcerative stomatitis. Interestingly, C. mydas lysozyme C showed mild activity against Gram-negative bacteria that are identified as hazardous bacterial threats to sea turtles. Escherichia coli and Salmonella typhi are opportunistic pathogens with zoonotic potential, while Klebsiella pneumoniae has been associated with regional mass mortalities. Turtles are vulnerable to disease when the innate response is inadequate to kill these pathogens.
Plasma lysozyme levels can act as an innate immune response biomarker and can help indicate the general state of health of a turtle [113]. Lysozyme activity in turtles decreases upon exposure to plastic pollution [35], heavy metals [36], fluctuating temperature [87], brevotoxins [22,37,53,134], and organochlorine contaminants [135]. Conversely, P. sinensis lysozyme activity is enhanced by increased dietary protein intake [136], linking good nutrition to immunity.
Lysozyme plays a role in the protection of turtle eggs from infection. Egg-white lysozyme has been characterized in C. mydas [133,137], L. olivacea [47], and soft-shelled turtles P. sinensis and T. gangeticus [138,139]. Interestingly, the egg white of loggerheads (C. caretta) is reported to lack lysozyme, and its antibacterial activity is suggested to be replaced by a small cationic β-defensin-like protein named TEWP (turtle egg white protein) [140].
- (2)
- Antimicrobial Peptides (AMPs)
Antimicrobial peptides (AMPs), also known as host defense peptides (HDPs), in turtles include defensins and cathelicidins. Interestingly, turtle genomes encode ‘avian-like’ defensin-type peptides, yet their cathelicidins are ‘snake-like’ [125].
Defensins are one of the major classes of cationic antimicrobial peptides and are expressed in response to bacterial or viral infection [141]. Defensins are investigated for their powerful antimicrobial and immunomodulatory properties as a potential therapy against antibiotic-resistant bacteria. Defensins can modulate autophagy, apoptosis, pro- and anti-inflammation responses, wound healing, chemoattraction, and cellular differentiation [141,142]. Defensins are divided into three main sub-classes; α, β and θ. Turtles express β-defensins, humans express both α and β, while primates express θ [125]. Three defensins have been described thus far in turtles, turtle β-defensin 1 (TBD-1), pelovaterin, and turtle egg white protein (TEWP). The antimicrobial activity of some turtle defensins has been investigated, although their mechanisms of immune modulation are not yet characterized to our knowledge.
A 40-residue peptide, called TBD-1, was the first defensin isolated from reptilian leukocytes in 2008 from a European pond turtle (Emys orbicularis, Linnaeus, 1758) [143]. TBD-1 showed high antimicrobial activity against E. coli, L. monocytogenes and low activity against S. aureus and C. albicans, a similar antimicrobial activity as the control human and pig defensins included in the study [143]. TBD-1 has activity against both gram-positive and negative bacteria and has antifungal activity. Turtle eggshell contains antimicrobial peptides. Pelovaterin is the major intracrystalline component in Chinese soft-shelled (P. sinensis) eggshells. Pelovaterin has a similar tertiary structure to mammalian β-defensins, yet very low sequence identity and a distinctive anionic charge. Lakshminarayanan et al. demonstrated pelovaterins strong antimicrobial activity against two gram-negative bacteria Pseudomonas aeruginosa and Proteus vulgaris, moderate activity against gram-positive and Staphylococcus aureus and poor activity against gram-negative E. coli and Enterobacter aerogenes. [93]. TEWP is an ovodensin, a cysteine-rich AMP abundantly found in the egg white of reptiles and birds with varying degrees of antimicrobial activity. Vertebrate defensins are characterized by the presence of a conserved six-cysteine motif forming three disulfide bridges. However, chicken and C. mydas ovodefensins exhibit different disulfide bridge patterns [140,144,145]. TEWP is present in C. caretta egg white and displays anti-microbial activity against E. coli, Salmonella spp., and Chandipura virus. As previously mentioned, the loggerhead turtle egg white lacks lysozyme, TEWP plays a particularly important role in the protection of these eggs from infection [140].
Further understanding of the diverse role of turtle defensins in protection from infection and disease could have therapeutic potential. Defensins are investigated for both pro- and anti-tumor properties [146,147]. In mammals, Ultraviolet (UV) exposure can stimulate the innate response and suppress the adaptive response [148,149]. UV-B exposure increased the expression of human defensins, including β-defensins, from keratinocytes [149]. In C. mydas, UV exposure improved FP prognosis and increased vitamin D levels [56]. However, UV exposure in humans is characteristically known for its immunosuppressive qualities on the adaptive response [148]. As turtles rely on their innate response, the study of sea turtle defensins and how the expression is influenced by the environment may have therapeutic potential for prevalent diseases or as a method to boost natural immunity against antibiotic-resistant bacteria [150].
Cathelicidins are another major class of cationic antimicrobial peptides with broad-spectrum activity against bacteria, fungi, and enveloped viruses and the ability to trigger the innate immune response [151]. Four cathelicidins have been described in C. mydas and six in P. sinensis [7,13]. C. mydas cathelicidins (Cm-CATH1-4) are both constitutively expressed and inducible in response to in vivo bacterial challenges [7]. Cm-CATH2 can directly kill pathogens via membrane permeabilization, and other family members boost the immune response by activation of MAPKs and NF-kB-dependent signaling pathways. C. mydas cathelicidins have been suggested as a candidate for novel antimicrobial drug development due to their potent, broad spectrum, and rapid bactericidal and anti-biofilm activities. Cm-CATH2 boosted immune response by inducing the trafficking of nonspecific leukocytes to the infection site. However, CmCATH2 could also block the toll-like receptor (TLR) complex to prevent the LPS-mediated induction of proinflammatory cytokines in an in vitro cell culture assay [7]. While this finding should be validated in vivo, this study suggests that this class of AMP has a range of anti-microbial and immunomodulatory properties in sea turtles that merit further study.
- (3)
- Complement System
The complement system has potent anti-microbial and pro-inflammatory properties [86,152]. The complement cascade involves a series of proteins in plasma that, when activated, can lead to the formation of the membrane attack complex (MAC) that causes lysis when inserted into microbial cell walls. In addition, complement activation initiates the recruitment of leukocytes to the site of infection, phagocytosis, and expression of pro-inflammatory cytokines (Figure 3) [61,152].
All three complement pathways, classical, alternative and lectin, have been observed in reptiles [67]. The classical pathway is a link between innate and adaptive immunity by involving the recognition and binding of antigens by antibodies [61]. The alternative pathway is activated in response to viral or bacterial particles such as LPS. Mannose residues on the surface of bacteria activate the lectin pathway [152]. Only in recent years have all three complement pathways been reported as being a part of the turtle immune response to bacterial challenge. Key immune response genes, including C1q (classic), MASP2 (lectin), CFB, and CFD (alternative), and complement C3 attack complex members were significantly upregulated in turtle liver after Edwardsiella tarda infection [15]. Complement C3 mRNA levels increased in soft-shelled turtle liver after infection with Aeromonas hydrophilia in a similar manner to that reported in humans, mice, and zebrafish [153,154].
Turtle complement is activated in response to environmental stressors, including the plastic-derived contaminant PBDE-47 [35] and nitrite [10]. At the same time, complement activity is suppressed in response to temperature fluctuation [27,155,156], the pesticide diazinon, and the herbicide atrazine [24,31]. Complement proteins have an optimal temperature range, and complement cascade proteins (C3, C4, C6-C9, and MASP1/2) expression was significantly downregulated at 32 °C compared to 28 °C in the pond turtle M. mutica (Cantor, 1842) [27]. In an investigation of the effects of different sunlamp-based lighting on captive soft-shelled turtles, turtles exposed to light in the basking area showed a significant increase in C3 in comparison to those without light exposure. These results demonstrate how turtles respond to temperature and pollutants in their environment by modulation of the complement cascade [157].
Similar turtle species rely on their innate immune response in an individual and species-specific manner. A study of the complement activity of two species of snapping turtles, the Common Snapping Turtle (CST, C. serpentina) and the Alligator Snapping Turtle (AST, Macrochelys temminckii, Troost, 1835), showed that CST relied on the alternative pathway. However, the AST relied upon the Lectin-activated pathway [61]. In a similar experiment comparing eastern (EBT) and ornate (OBT) box turtles, EBT relied on the lectin pathway and OBD, the alternative pathway [17]. Both turtles display bactericidal capacity. However, OBT displayed greater bactericidal capacity at a broader range (20–40 °C) against Salmonella typhimurium, Escherichia coli, Enterobacter cloacae, Citrobacter freundi, Bacillus subtilis, Staphylococcus epidermidis, and Staphylococcus aureus [17]. The results are unique as serum complement pathways are assumed to be conserved within clades and highlight the need for avoiding the generalization of immune functions between turtle species [61]. Regarding sea turtles, complement proteins (C3, C4, C5) have been highlighted as a potential biomarker of C. caretta [38] and C. mydas [73] health when recovering in rehabilitation facilities as they serve as good indicators of stress, infection, or exposure to pollutants.
6. Natural Abs
Natural antibodies (NAbs) are a broad first line of defense and are mediators between innate and adaptive responses. They are germline-encoded, polyreactive to PAMPs, and have a low-binding affinity [86]. NAbs can stimulate both the innate and adaptive immune system through the activation of complement and specific lymphocytes [86]. Reptilian humoral responses are slower and longer lasting than those of mammals. Following immunization, both mammals and reptiles have detectable antibody levels after about one week. In mammals, peak antibody production is after two weeks, while in reptiles, the production peaks between 6–8 weeks post-immunization [115]. However, reptiles’ responses are long-lasting responses with antibodies detectable up to 34 weeks post-immunization [115]. It is suggested that the longer-lasting NAb response in reptiles is a ‘trade-off’ when T cell proliferation is diminished due to seasonal changes of the spleen and thymus [67,158].
Mucosal NAbs in the red-eared slider turtles (Trachemys scripta) are non-specific, respond equivalently to new and previously exposed antigens, and are thought to be of the IgM subset produced by B-1 cells [19,159]. NAbs agglutination was not affected by exposure to the harmful algae toxin microcystin in the painted turtle (Chrysemys picta) [160]. NAbs are thought to play a key role in the robustness of the reptilian innate response. The B1 subset has a long half-life, is expressed throughout plasma and mucus, and can mount a specific and non-specific immune response. This is thought to protect long-lived turtles as levels of NAbs in mucus increases with age [159]. Further study of NAbs and the cells that produce them will further our understanding of the link between innate and adaptive immunity in turtles.
7. Mucosal Immunoglobulin
Mucosal immunity is an important arm of the innate immune defense. Polymeric immunoglobulin receptor (pIgR) is critical on mucosal surfaces to defend against invading pathogens through the transportation of polymeric Immunoglobulin (Ig) across epithelial tight junctions and the release of secretory Igs (Sigs) [161,162,163]. pIgR has been characterized in P. sinensis as closely related to avian and reptile pIgRs, with four Ig-like domains, and basal levels are upregulated in the oesophagus and intestines. P. sinensis pIgR expression was upregulated in vivo in response to LPS and Aermonas sobria challenge in the gastrointestinal tract [163]. IgA is the most abundant Ig isotype in the mammalian mucosal immune system. As turtles lack IgA, further characterization of the role of antibodies in mucosal immunity is required. For instance, P. sinensis strengthens mucosal immune functions in Winter when adaptive responses are lowered, with an increase in the expression of TLR2 and TLR4, and Muc2 by goblet cells [164]. Compensatory mechanisms are likely to exist in other species and understanding the impact of seasonality on mucosal antibodies across different species of turtle needs to be more fully explored.
8. Acute Phase Proteins
Acute phase proteins are used in wildlife diagnostics as biomarkers of immune response activity. The acute phase response is a systemic reaction of the host to local or systemic disturbances in homeostasis due to tissue injury, trauma, neoplastic growth, infection, or immunological disorders [165]. At the site of damage (infection or injury), pro-inflammatory cytokines are released from activated inflammatory cells, and the vascular system is activated. The activation of the innate immune effectors in immediate responses leads to the production of more cytokines and inflammatory mediators in blood and the extracellular fluid compartment. A systemic reaction is induced through activated cytokine receptors on various target cells, leading to the activation of the hypothalamic-pituitary-adrenal gland axis, reduction of growth hormones, and physical changes characterized by fever, anorexia, negative nitrogen balance, and catabolism of muscle cells [165]. The acute phase response can be measured in the blood through markers including higher levels of leukocytes, adrenocorticotrophic hormone (ACTH) and glucocorticoids, complement activation and blood coagulation, decreased levels of calcium, zinc, iron, vitamin A, and changes in concentration of plasma proteins [165].
Acute phase proteins have been measured in investigations for potential uses as biomarkers of immune response in C. mydas [73,74] and C. caretta [38,39]. Chaousis et al. employed a mass-spectrometry-based non-targeted proteomics approach and highlighted its potential for biomarker discovery in wildlife toxicology [73]. In this comparison study of green sea turtles from three different foraging sites in Australia, seven acute phase proteins were highly dysregulated; Alpha-1-antitrypsin, Alpha-2-macroglobulin-like, Ceruloplasmin, Complement C4, Complement factor, fibrinogen alpha chain, and haptoglobin [73]. Validation of simple assays to measure these proteins cheaply in small sample volumes would be an important step to understanding the impact of different environmental stressors and infections on the health of different populations of sea turtles. These assays would help to establish normal ranges for each protein incorporating change due to temperature and season. If adopted, the use of these markers in routine monitoring of sea turtles would be able to tell us important information about the impact of changes in their environment on turtle health.
9. Pattern Recognition Receptors (PPRs)
The innate immune system relies on pattern recognition receptors (PRRs) to identify conserved pathogen-associated molecular patterns (PAMPs), damaged senescent cells, and apoptotic host cells. PRRs distinguish these infectious microbial products and damage-associated molecular patterns (DAMPs) from healthy ‘self’ host cells [166,167]. Activated PRRs initiate two important immune responses; a cascade of signaling events to produce cytokines that begin nonspecific anti-infection and antitumor effects and begin the process for antigen-presenting cells (APCs) to activate antigen-specific adaptive immunity. There are five classes of PRRs: Toll-like receptors (TLRs), Retinoic-acid inducible gene I (RIG-I)-like receptors (RLRs), Nod-like receptors (NLRs), and C-type lectin receptors (CLRs), and cytosolic DNA sensors (cGAS, AIM2, IFI16) [167].
9.1. Toll-like Receptors
Toll-like receptors are type-1 transmembrane glycoproteins that are expressed either on the cell membrane or intracellular vesicles [168]. Ligation of TLRs triggers a downstream signaling cascade that activates the innate and then the adaptive immune response. The discovery of TLRs in Drosophila melanogaster was a landmark discovery for the understanding of innate immunity, and subsequently, TLRs were found to be highly conserved across vertebrates and some invertebrates [2,169,170]. Humans have 10 (TLR1-10) [171], fish have over 20 [172] and C. mydas has 11 TLRs [168]. The C. mydas genome contains TLR1, TLR2, TLR18, TLR3, TLR4, TLR5, TLR7, TLR8, TLR9, TLR21, and TLR22 genes [168]. Of these, mammals do not have TLR18, TLR21, or TLR22. However, these genes have been described in fish [173,174]. We will discuss this gene family in C. mydas and highlight duplicated genes or pseudogenes.
Gene duplication events can facilitate the evolution and expansion of a gene family’s function, specialized sub-functionalization, or differences in gene expression [175,176,177]. However, gene loss through mutations or pseudogenization can also be an adaptive evolutionary force depending on gene dispensability, mutational robustness, and environmental conditions [178]. The genomic diversity of TLRs in non-model species is a very interesting area of research, indicating evolution through both positive and negative selection because of infectious or environmental pressure. TLR members in different species can recognize different pathogenic ligands, thus differing in function. For example, human TLR5 only recognizes flagellin, while TLR5 in fish can recognize both bacterial and viral PAMPs, flagellin, and dsRNA, respectively [179]. TLRs are a result of positive selection and are constantly driven by evolutionary forces. More research is required to understand the specific patterns and pathways of innate defense that sea turtle PRRs have evolved.
TLR2 and TLR8 genes are duplicated pseudogenes in the C. mydas genome [168]. TLR2 is expressed in a variety of cells, including keratinocytes and sebaceous glands, which can produce bactericidal sebum to protect from skin infections. C. mydas and P. sinensis display a different number of TLR2 gene copies, perhaps highlighting the need for semi-aquatic turtles to have a more robust defense due to a thinner epidermis and increased exposure to pathogens [180,181]. TLR8 is an endosomal receptor that recognizes single-stranded RNA viruses, and this gene displays an interesting evolutionary pattern within C. mydas. Birds and squamate reptiles have no TLR8 ortholog, and mammals have one TLR8 gene. A duplication event (TLR8B) occurred in crocodilians and testudines, with a second duplication event occurring specific to testudines (TLR8C).
TLR8C is a pseudogene within C. mydas and a Mojave desert tortoise [177]. TLR8 gene duplication and loss events could represent a change in subspecialized function or an adaption to an abrupt environmental change.
We will simplify and discuss C. mydas TLRs in two groups; nonviral (TLR1, TLR2, TLR18, TLR4, and TLR5) and viral (TLR3, TLR7, TLR8, TLR9, TLR21, and TLR22). However, some TLRs can recognize both viral and bacterial-derived PAMPS and -induced DAMPS, respectively [168,182,183].
Nonviral. TLR1, TLR2, and TLR18 are in the TLR1 subfamily in C. mydas. These TLRs form heterodimers to recognize triacylated lipoprotein and peptidoglycan from bacteria [183]. TLR1 and TLR2-dependent pro-inflammatory pathways are also upregulated by C. caretta in response to organic ultraviolet filters found in sunscreen agents [184]. TLR18 is widely expressed by aquatic animals. Fish increase TLR18 expression in response to challenges with Aeromonas hydrophilia, LPS, flagellin, and polyinosinic polycytidylic acid (poly(I:C)) [174,185,186]. The role of TLR18 in turtles is yet to be characterized.
TLR2 and TLR4 play a role in both the immune and reproductive systems of P. sinensis. TLR4 is the first discovered TLR and binds to MD-2 (myeloid differentiation factor 2), recognizing LPS molecules and components of C. albicans, Trypanosoma, and viruses [187]. TLR2, TLR4, and associated downstream target genes (MyD88, TNFα, IL-1β, and IL-6) were significantly upregulated in the Pelodiscus sinensis spleen. After the challenge with Aeromonas hydrophilia infection [66]. Turtle TLR expression is also regulated by estrogen in oviducts. Inflammatory pathways downstream of TLR2 and TLR4 were significantly downregulated in mated turtle oviducts in comparison to unmated ones. Estrogen receptor 1 (ESR1) and progesterone receptor (PGR) expression were upregulated, indicating an increase in hormone signaling can suppress the innate immune response to facilitate the long-term storage of spermatozoa [188]. As TLR2 and TLR4 are important PRRs for the initiation of the immune response to bacterial pathogens, the crosstalk between sex hormones and the regulation of pathogen recognition is an interesting and important area for future research.
Viral. TLR7, TLR8, and TLR9 comprise the TLR7 subfamily in C. mydas. TLR9 is found in mammals and in some chelonian genomes, including C. mydas and P. sinensis, but not in crocodilian or squamate [168]. TLR9, TLR21, and TLR22 recognize DNA with CpG motifs. However, reptilian TLR21 and TLR22 ligands have not been fully characterized [177].
Turtle TLRs have been investigated for their role in mediating the effect of diet on immune function. Bacillis subtilis B10 has been investigated as a pre-biotic to improve P. sinensis turtle health due to its immunomodulatory and antioxidant effects. Upregulation of the TLR8-dependent pathway and intestinal tight junction proteins in the gut and TLR5 in the liver was observed in animals on this diet [14]. This study suggests that dietary supplements and pre/probiotics that maintain gut microbial homeostasis can influence gut health and immunity in turtles. We are only at the beginning of understanding the diversity of the microbial communities that live within different niches of turtles’ gut/skin. We need to catalog these microbial communities and examine how they relate to health and disease. A major research priority is to understand how these microbes interact with each other and how these communities interact with their hosts.
The four other classes of PRRs, RLRs, NLRS, CLRs, and cytosolic receptors are lacking significant studies in reptiles, turtles included. TLRs can interact with RLRs and NLRs to coordinate an effective immune response. As evolutionary older organisms rely more heavily upon innate than adaptive responses [86,189], we speculate the PRRs in reptiles may have a more diverse function than in mammals in co-ordinating immune responses and other cellular functions of differentiation, proliferation, apoptosis, and autophagy.
9.2. RIG-I-like Receptors (RLRs)
RIG-I, melanoma differentiation-associated gene 5 (MDA5; also known as IFIH1), and laboratory of genetics and physiology 2 (LGP2) are RLRs. RIG-I and MDA5 detect cytosolic RNA, activate downstream signaling cascades resulting in phosphorylation of NF-κB and IRF3, and induce proinflammatory cytokines and interferons (IFN), respectively [190]. RIG-I is recognized for its diverse roles beyond innate immune activation, such as cellular proliferation and differentiation and cancer development [191]. RIG-I and MDA5 share similar structural homology but can respond differently to viruses. RLRs are conserved throughout reptile evolution, and MDA5 is reported to be under positive selection for the evolution of semi-aquatic reptiles [192]. The C. mydas genome contains LGP2, MDA5, and RIG-I [192].
There are few functional studies on reptilian RIG-I/MDA-5. However, one study of Chinese giant salamander RIG-I and MDA5 (adRIG-I and adMDA5) found that there was a high degree of homology with C. mydas genes as determined through BLAST analysis. RIG-I and MDA-5 gene expression was upregulated following iridovirus infection of Chinese giant salamanders [193]. Quantitative expression analysis of adRIG-I and adMDA5 revealed the highest expression in the spleen and lowest in the skin, and adRIG-I is slightly higher expressed in muscle, heart, kidney, lung, and liver than adMDA5 [193]. Reptile RLRs deserve further study to understand their role in the antiviral response.
9.3. Nod-like Receptors (NLRs)
NLRs are cytoplasmic receptors in immune cells (macrophages, dendritic cells, lymphocytes) and nonimmune cells (epithelial). NLRs are a highly expanded group with over 20 members in humans and 30 in mice [194]. NOD1 and NOD2 are very well described for their roles in peptidoglycan recognition and immune and apoptosis regulation. Notably, NOD2 is absent in all reptiles [192]. Reptile NLR gene structure and evolution have been described. However, functional characterization or role in infection has not been examined. C. mydas has differing functional domains within its NLR repertoire, NOD1, NLRX1, and NLRC3 when compared to other reptiles [195]. It is unknown what the significance of this is in terms of response to infection and immune regulation.
9.4. C-Type Lectin Receptors Are Primarily Expressed by Myeloid Cells
They mediate pathogen recognition by binding to specific microbial carbohydrates. This, in turn, mediates the inflammatory response and plays a key role in defense against viral and fungal infection [196]. There are no reports on turtle C-type lectin receptors. However, studies of bearded dragon, snake, and alligator genomes highlight the expanded repertoire of reptile CLR domains [197,198,199].
10. Interferons (IFN)
PRRs recognize their specific ligand and initiate a downstream signaling cascade resulting in the expression of proinflammatory cytokines, chemokinesand/or interferons (IFNs). IFNs are an essential part of the antiviral response, so named as they ‘interfere’ with viral replication [200]. IFNs induce the expression of a multitude of antiviral IFN-stimulated genes (ISGs) in a wide variety of cells and tissues and are critical for the induction of the antiviral response. Of the three subfamilies of IFN, IFN type I and II genes have been described in P. sinensis, while IFN type III has been described in green anole lizard (Anolis carolinsis) [11,201,202]. IFN-like activity has been observed in the kidney and peritoneal cells of tortoises in response to viral infection [203]. An IFN produced in response to the Saint Louis encephalitis virus was found in turtle heart cells that are chemically and physically similar to mammalian and avian IFN [204]. IFN-g gene has been described in P. sinensis [11].
IFN-g gene is constitutively expressed across most tissues [11]. IFN-γ gene expression was induced by LPS and Poly(I:C) in vitro, and transcription factors IRF1, IRF7, and STAT1 were upregulated. IFN-g stimulation induced ISGs, OAS, and PKR similar to the mammalian response [11]. IFN-g is produced by innate lymphoid cells, Natural killer (NK) cells, T lymphocytes, and plasmacytoid dendritic cells [205]. Type I IFN responses have not been described in C. mydas.
Interferon Regulatory Factors (IRFs)
Interferon Regulatory Factors (IRF) are transcription factors that regulate the expression of type I IFN [183]. IRFs play a role in many immune responses, including apoptosis regulation, antimicrobial defense, and hematopoietic differentiation [200,206]. Eleven IRFs have been described in vertebrates, with 9 in humans and 6 in C. mydas (IRF1, IRF2, IRF4, IRF5, IRF6, and IRF8) [207]. IRF3, IRF7, and IRF9 are not described by Liu et al. These are important factors in producing type I IFN in mammalian cells and how turtles compensate for their absence needs to be elucidated [207].
11. Adaptive Immunity
The classic view of adaptive immunity is that T and B cells encode variable receptors that will bind to a specific antigen and instigate cytotoxic and memory responses. Reptiles’ adaptive response has been recently reviewed by Zimmerman [66]. Adaptive immunity involves antigen-specific immune responses and requires previous exposure to mount a full response. It is slower than the innate immune response, targets very specific antigens, and forms immunological memory to control re-infections [66] There is an important dialogue between innate and adaptive immune responses. The nature of the pathogen will dictate how it is sensed by the PRRs and the resulting innate immune response will stimulate either humoral or cell-mediated adaptive immunity.
12. Cell-Mediated Immunity
Cell-mediated immunity is predominantly dependent on T cell recognition and destruction of antigens, either by direct killing mechanisms or the secretion of cytokines to enhance the phagocytic ability of phagocytes [208]. T cells can recognize specific antigens of bacterial, viral, or parasitic origin or tumor cells [208]. T cell-mediated immunity can also result in autoimmune conditions due to aberrant recognition of self-antigens. The T-cell receptor (TCR), which contains CD3+ peptide chains, binds the specific antigenic peptide presented by the major histocompatibility complex (MHC) on APCs (antigen-presenting cells). The TCR/CD3 complex is found in all jawed vertebrates [209]. Dependent upon cytokines in the cellular environment, T-helper cells (CD4+) differentiate into five T-cell subtypes (Th1, Th2, Th17, Treg, or Th9) and produce cytokines to stimulate T-cell effector functions and B-cell antibody production or form memory T-cells. Cytotoxic T cells (CD8+) release cytotoxic granules upon antigen recognition that kill virally infected or tumor cells [208].
CD3+ T lymphocytes have been identified in the blood and tissue of C. mydas [209]. In this study, the authors found that an antibody raised against human CD3 cross-reacted with CD3 expressed by turtle T cells. This antibody recognized a conserved region of the epsilon chain of CD3. These are assumed to be helper and cytotoxic T cells. However, we do not yet understand if these cells acquire different effector functions that align with murine or human T cell subsets [66,67]. The study of cellular immunology in non-model organisms is limited by a dearth of reagents that recognize and discriminate different cell types. Understanding how key molecules are conserved across species will allow the identification of further antibodies that can be used to study the function of T cells in species such as turtles. For example, the genetic components for CD4+ and CD8+ T-cells are described in the western painted turtle (Chrysemys p. bellii) and giant tortoises (Chelonoidis abingdonii, (Quoy and Gaimard, 1824) and Aldabrachelys gigantea (Schweigger, 1812)) [32]. Further comparative analysis of these T cell receptor/T cell signaling pathways across reptiles and mammals will further our understanding of how the cellular response is regulated in turtles and potentially increase access to tools to undertake functional studies.
Lymphocyte proliferation is measured as a biomarker for turtle adaptive immune health. Concanavalin A (ConA) and phytohemagglutinin (PHA) have been examined as T-cell mitogens and LPS as a B-cell mitogen. Turtles have a decreased T and B cell mitogen response in comparison to mammals [50]. T cell-mediated immunity is compromised in turtles with severe FP. Thus, immunosuppression is a marker of late-stage disease [88,210]. In FP-afflicted turtles, both T and B cell proliferation in response to mitogens was reduced in turtles with moderate and severe FP [49,50,53,88]. In early-stage tumors, there is a measurable immune response, an increase of CD3+ lymphocytes, and upregulation of key immune pathways (leukocyte and lymphocyte pathways). In late-stage tumors, there were fewer CD3+ T-cells with downregulation of immune and apoptotic genes [211].
T cell lymphocyte proliferation is affected by environmental contaminants such as organochlorides and mercury in C. caretta [36,135]. The effects of heavy metal accumulation (Cd, Cr, Cu, Pb, and Hg) on red-eared slider turtle lymphocyte proliferation were examined with a PHA skin test. A PHA skin test stimulates T-cell proliferation, differentiation, and cytokine production with inflammation and swelling at the injection site. The accumulation of lead (Pb) in kidneys may be immunosuppressive and disrupt T-lymphocyte proliferation [212].
In mammals, regulatory T cells (Tregs) are responsible for immune suppression to avoid overreaction of the immune system causing damage to an organism’s tissues [67,208]. The transcription factor FoxP3 is required for the differentiation of Treg precursors and needs further study in reptiles. It is thought that if FoxP3 is not functional in reptiles, however the TGF-β cytokine may play a role in inducing T-cell tolerance [67]. As C. mydas lack germinal centers and lymph nodes, T cells may play a different role in immunity than mammals [115]. T cell proliferation is seasonally dependent, as the thymus and spleen change during the seasons [86]. Turtles are likely to have unique mechanisms underpinning T-cell proliferation, differentiation, and memory responses. As we develop better methods to assess cellular immunity in non-model species, we will gain insight into the evolution of adaptive immunity.
13. Humoral Immunity
The antibodies produced by B cells are the backbone of the humoral immune response. Antibodies can neutralize toxins, prevent pathogen internalization, and opsonize targets for phagocytosis. B cells are activated and differentiate into antibody-secreting plasma cells in the presence of an antigen and are assisted by helper CD4+ T cells [213]. In mammals, there are two subtypes of B cells, the evolutionarily older B-1 subset, which produces natural antibodies (Nabs) before and after antigen stimulation, and the B-2 subset, which produces highly variable and specific antibodies after stimulation by an antigen. B-1 cells in turtles are similar to fish B-1 cells, and evidence for the B-2 cell subset is lacking, and it is suggested that turtles do not have them [66,110]. However, a memory response is induced, and the role of B cells in reptiles deserves further study.
Turtle B-1-like cells with ‘like’ functions of phagocytosis, lack of senescence, and production of non-specific, polyreactive antibodies have been described in red-eared slider turtle (T. scripta) [20] and Mojave desert [158] and Gopher tortoises [214] (Gopherus agassizii (Cooper, 1861) and Gopherus polyphemus (Daudin, 1801)) [66]. As mammalian B cells are found in secondary lymphoid structures not found in reptiles, such as lymph nodes and germinal centers formed by activated B-2 cells (previously discussed, see Figure 2), it is suggested that reptilian B cells are not limited to anatomical locations such as peritoneal and pleural cavities [66].
Phagocytic B cells in fish have microbicidal activity and can function as antigen-presenting cells to stimulate the adaptive immune response [215]. B lymphocytes in C. mydas have phagocytic activity, and this ability is not affected by FP [51,111]. B cell characteristics described in fish that are lacking studies in turtles include lack of proliferation upon antigen stimulation of the B-cell receptor (BCR), expression of B-1 specific cell markers and innate receptors, and activation in T-dependent and T-independent manner [66,216,217]. Further study is required on the function of phagocytic B cells in both C. mydas and all reptiles [66,67]. Furthermore, the regulatory role of B cells is not well understood. A study of desert tortoises suggests that B cells play a role in polarizing the immune system to avoid a dangerous inflammatory response [158]. This study suggests that B cells play a role in controlling inflammation.
Immunoglobulin (Ig) molecules are composed of one heavy (H) and one light (L) protein chain joined by disulfide bonds [213]. Mammals have five classes of Ig (M, D, G, E, and A) based on their heavy chain isotypes, and each class displays a differentiated function (complement fixation, unclear, opsonization, parasite infection, mucosal immunity, respectively). Diversity in Ig isotypes represents an evolution in immune function [218]. IgE and IgG in mammals are derived from IgY genes [219].
IgM, IgD, and IgY genes have been described in Chinese soft-shelled turtles, pond slider turtles, and red-eared sliders (P. sinensis, Pseudemys scripta, and Trachemys scripta elegans) [25,220,221,222,223]. The genomic structure of four turtle Ig classes has been described in P. sinensis and Chrysemys picta belli, with one gene each for IgD and IgM and multiple genes for IgY and IgD2-like) [223]. P. sinensis and C. picta have 17 and 13 sequences, respectively, coding for Ig isotypes [223]. C. mydas has 30 immunoglobulin genes, with one gene each for IgM and IgD and 14 genes each for IgD2 and IgY [218].
C. mydas have three described Ig structures [58,60]. Turtle Igs include a presumed pentamer 17S IgM and two monomeric IgYs: 7S IgY and 5.7S IgY. The 5.7S IgY is composed of two equally sized heavy and light chains with a truncated Fc portion, which is also found in ducks [58,224,225]. The 7S IgY has similar functions to human IgG, contains two differently sized heavy chains bound to light chains, and is presumed noncovalently associated with a 90-kDa moiety that is secreted during chronic antigenic stimulation [58]. Unlike mammals, reptile IgY is thought to lack a hinge region due to a lack of molecular evidence for one. However, the flexibility of the Fab arms is maintained [58]. Work et al. reported C. mydas to mount a response to glycoprotein antigens of ChHV5 using 7S and 5.7S IgY antibodies [226].
Monoclonal antibodies for the detection of turtle immunoglobulins via competitive ELISA have been developed for C. caretta [40] and C. mydas. Reference intervals have been established for Ig in C. mydas (0.40–0.85 g/dL) and C. carreta (0.38–0.94 g/dL) [227]. These assays can be used to monitor antibody responses in turtles and further refined to measure antigen-specific antibody titers. This would allow us to gain further insight into the importance of antibodies in the immune response in turtles.
14. Conclusions
In conclusion, sea turtles’ immune system is a robust defense capable of protecting the host from microorganisms or cancer. However, they relied upon innate response is highly sensitive to natural and anthropogenic environmental conditions. The lack of reliance upon the adaptive immune response is less understood. Characterization or confirmation of T and B cell subsets would elucidate the importance of the adaptive response. PRRs and the interferon response have more than one function of monitoring for PAMPS or DAMPS. They exert control over cellular processes like apoptosis, proliferation, and differentiation. The antiviral defense is less understood, and knowledge of interferons, their regulatory functions, and ISG expression could be employed to assist cancer treatments. Turtles’ antiviral and anticancer immune functions are yet to be fully characterized.
Sea turtles are a sentinel species, as they are long-lived obligatory inhabitants of near-shore ecosystems [228,229,230,231,232]. The health of a sentinel species is monitored as a measure of the health of the marine environment [230,233]. Sea turtle immune function is sensitive to fluctuations in temperature [87] and to pollutants in the environment, such as brevotoxins [22,37,52,134] and chemical disruptions [24,31]. Rapid changes in the marine environment are thought to make marine life more vulnerable to diseases and infections. However, this exact mechanism of action is poorly understood [230,231].
Earth is on track to enter a sixth mass extinction event as the current loss of biodiversity caused by anthropogenic actions is at a quicker rate than any other extinction event caused by natural phenomena [234,235]. Testudines are particularly vulnerable, and it is estimated that if the current rate of extinction continues that massive losses of turtle biodiversity will occur in this lifetime, and the entire group could disappear in a few short centuries [236,237]. Turtles have survived at least two mass extinction events and have proven adaptability to new extreme environments [237,238]. Ecological opportunities such as recovery from mass extinction, invasion of new habitats, loss of predators, or development of key phenotypic traits can trigger periods of adaptive radiations in which fast evolutionary rates facilitate expansion and loss of clades. However, this occurs on timescales encompassing millions of years [234,239,240]. Testudines have the potential to recover from modern extinction rates through reducing the risk of disease to current species and populations. Increased knowledge of the sea, freshwater, and terrestrial turtle immune systems will aid this aim, provide insight into the ancient evolution of host defense against pathogens, and potentially inform future precision medicine therapies.
Author Contributions
Conceptualization, A.N. and E.J.R.; Writing—Original Draft Preparation, A.N.; Writing—Review & Editing, E.J.R. All authors have read and agreed to the published version of the manuscript.
Funding
A.N. is funded by a Faculty of Science and Engineering scholarship awarded by the University of Limerick.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank David Duffy, The Whitney Laboratory for Marine Bioscience, University of Florida, and Liam Whitmore, University of Limerick for helpful discussions and for introducing us to the fascinating world of Sea Turtle Immunology.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fumagalli, M.; Sironi, M.; Pozzoli, U.; Ferrer-Admetlla, A.; Ferrer-Admettla, A.; Pattini, L.; Nielsen, R. Signatures of environmental genetic adaptation pinpoint pathogens as the main selective pressure through human evolution. PLoS Genet. 2011, 7, e1002355. [Google Scholar] [CrossRef]
- Hoffmann, J.A. The immune response of Drosophila. Nature 2003, 426, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Metchnikoff, E. Lectures on the Comparative Pathology of Inflammation Delivered at the Pasteur Institute in 1891; Ripol Klassik: Moscow, Russia, 1893; ISBN 5-87712-856-6. [Google Scholar]
- Cooper, M.D.; Peterson, R.D.; Good, R.A. Delineation of the thymic and bursal lymphoid systems in the chicken. Nature 1965, 205, 143–146. [Google Scholar] [CrossRef]
- Reynaud, C.-A.; Anquez, V.; Grimal, H.; Weill, J.-C. A hyperconversion mechanism generates the chicken light chain preimmune repertoire. Cell 1987, 48, 379–388. [Google Scholar] [CrossRef]
- Litman, G.W.; Cooper, M.D. Why study the evolution of immunity? Nat. Immunol. 2007, 8, 547–548. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Yang, H.; Gao, J.; Zhang, F.; Chu, P.; Yang, Y.; Zhang, M.; Wang, Y.; Yu, H. Diversity, immunoregulatory action and structure-activity relationship of green sea turtle cathelicidins. Dev. Comp. Immunol. 2019, 98, 189–204. [Google Scholar] [CrossRef] [PubMed]
- The IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org/en (accessed on 20 November 2022).
- Field, E.K.; Hartzheim, A.; Terry, J.; Dawson, G.; Haydt, N.; Neuman-Lee, L.A. Reptilian Innate Immunology and Ecoimmunology: What Do We Know and where Are We Going? Integr. Comp. Biol. 2022, 62, 1557–1571. [Google Scholar] [CrossRef]
- Zimmerman, L.M. Adaptive immunity in reptiles: Conventional components but unconventional strategies. Integr. Comp. Biol. 2022, 62, 1557–1571. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, L.M.; Paitz, R.T.; Vogel, L.A.; Bowden, R.M. Variation in the seasonal patterns of innate and adaptive immunity in the red-eared slider (Trachemys scripta). J. Exp. Biol. 2010, 213, 1477–1483. [Google Scholar] [CrossRef]
- Zimmerman, L.M. The reptilian perspective on vertebrate immunity: 10 years of progress. J. Exp. Biol. 2020, 223, jeb.214171. [Google Scholar] [CrossRef]
- Herbst, L.; Ene, A.; Su, M.; Desalle, R.; Lenz, J. Tumor outbreaks in marine turtles are not due to recent herpesvirus mutations. Curr. Biol. 2004, 14, R697–R699. [Google Scholar] [CrossRef]
- Jones, K.; Ariel, E.; Burgess, G.; Read, M. A review of fibropapillomatosis in Green turtles (Chelonia mydas). Vet. J. 2016, 212, 48–57. [Google Scholar] [CrossRef]
- Duffy, D.J.; Schnitzler, C.; Karpinski, L.; Thomas, R.; Whilde, J.; Eastman, C.; Yang, C.; Krstic, A.; Rollinson, D.; Zirkelbach, B.; et al. Sea turtle fibropapilloma tumors share genomic drivers and therapeutic vulnerabilities with human cancers. Commun. Biol. 2018, 1, 1–13. [Google Scholar] [CrossRef]
- Banerjee, S.M.; Stoll, J.A.; Allen, C.D.; Lynch, J.M.; Harris, H.S.; Kenyon, L.; Connon, R.E.; Sterling, E.J.; Naro-Maciel, E.; McFadden, K.; et al. Species and population specific gene expression in blood transcriptomes of marine turtles. BMC Genom. 2021, 22, 346. [Google Scholar] [CrossRef]
- Chaousis, S.; Leusch, F.D.L.; Nouwens, A.; Melvin, S.D.; van de Merwe, J.P. Changes in global protein expression in sea turtle cells exposed to common contaminants indicates new biomarkers of chemical exposure. Sci. Total Environ. 2021, 751, 141680. [Google Scholar] [CrossRef]
- Chaousis, S.; Leusch, F.D.L.; Limpus, C.J.; Nouwens, A.; Weijs, L.J.; Weltmeyer, A.; Covaci, A.; van de Merwe, J.P. Non-targeted proteomics reveals altered immune response in geographically distinct populations of green sea turtles (Chelonia mydas). Environ. Res. 2023, 216, 114352. [Google Scholar] [CrossRef]
- Marancik, D.P.; Perrault, J.R.; Komoroske, L.M.; Stoll, J.A.; Kelley, K.N.; Manire, C.A. Plasma proteomics of green turtles (Chelonia mydas) reveals pathway shifts and potential biomarker candidates associated with health and disease. Conserv. Physiol. 2021, 9, coab018. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, N.B.; Leandro, A.C.; Nahvi, N.; Devlin, M.A.; Leandro, M.; Escobedo, I.M.; Peralta, J.M.; George, J.; Stacy, B.A.; deMaar, T.W.; et al. Transcriptomic Profiling of Fibropapillomatosis in Green Sea Turtles (Chelonia mydas) From South Texas. Front. Immunol. 2021, 12, 630988. [Google Scholar] [CrossRef] [PubMed]
- Kane, R.A.; Christodoulides, N.; Jensen, I.M.; Becker, D.J.; Mansfield, K.L.; Savage, A.E. Gene expression changes with tumor disease and leech parasitism in the juvenile green sea turtle skin transcriptome. Gene 2021, 800, 145800. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xiao, X.; Wei, X.; Li, J.; Yang, J.; Tan, H.; Zhu, J.; Zhang, Q.; Wu, J.; Liu, L. Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS-CoV-2. J. Med. Virol. 2020, 92, 595–601. [Google Scholar] [CrossRef]
- Foti, M.; Giacopello, C.; Bottari, T.; Fisichella, V.; Rinaldo, D.; Mammina, C. Antibiotic resistance of gram negatives isolates from loggerhead sea turtles (Caretta caretta) in the central Mediterranean Sea. Mar. Pollut. Bull. 2009, 58, 1363–1366. [Google Scholar] [CrossRef]
- Ahasan, M.S.; Picard, J.; Elliott, L.; Kinobe, R.; Owens, L.; Ariel, E. Evidence of antibiotic resistance in Enterobacteriales isolated from green sea turtles, Chelonia mydas on the Great Barrier Reef. Mar. Pollut. Bull. 2017, 120, 18–27. [Google Scholar] [CrossRef]
- Tsai, T.-T.; Huang, C.-Y.; Chen, C.-A.; Shen, S.-W.; Wang, M.-C.; Cheng, C.-M.; Chen, C.-F. Diagnosis of Tuberculosis Using Colorimetric Gold Nanoparticles on a Paper-Based Analytical Device. ACS Sens. 2017, 2, 1345–1354. [Google Scholar] [CrossRef] [PubMed]
- Drane, K.; Huerlimann, R.; Power, M.; Whelan, A.; Ariel, E.; Sheehan, M.; Kinobe, R. Testudines as Sentinels for Monitoring the Dissemination of Antibiotic Resistance in Marine Environments: An Integrative Review. Antibiotics 2021, 10, 775. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.; Grilo, M.L.; Carneiro, C.; Cunha, E.; Tavares, L.; Patino-Martinez, J.; Oliveira, M. Antibiotic Resistance and Virulence Profiles of Gram-Negative Bacteria Isolated from Loggerhead Sea Turtles (Caretta caretta) of the Island of Maio, Cape Verde. Antibiotics 2021, 10, 771. [Google Scholar] [CrossRef]
- Trotta, A.; Cirilli, M.; Marinaro, M.; Bosak, S.; Diakoudi, G.; Ciccarelli, S.; Paci, S.; Buonavoglia, D.; Corrente, M. Detection of multi-drug resistance and AmpC β-lactamase/extended-spectrum β-lactamase genes in bacterial isolates of loggerhead sea turtles (Caretta caretta) from the Mediterranean Sea. Mar. Pollut. Bull. 2021, 164, 112015. [Google Scholar] [CrossRef]
- Trotta, A.; Marinaro, M.; Sposato, A.; Galgano, M.; Ciccarelli, S.; Paci, S.; Corrente, M. Antimicrobial Resistance in Loggerhead Sea Turtles (Caretta caretta): A Comparison between Clinical and Commensal Bacterial Isolates. Animals 2021, 11, 2435. [Google Scholar] [CrossRef]
- Carini, A.D.P.; Ariel, E.; Picard, J.; Elliott, L. Antibiotic Resistant Bacterial Isolates from Captive Green Turtles and In Vitro Sensitivity to Bacteriophages. Int. J. Microbiol. 2017, 2017, 5798161. [Google Scholar] [CrossRef]
- Duffy, D.J.; Martindale, M.Q. Perspectives on the expansion of human precision oncology and genomic approaches to sea turtle fibropapillomatosis. Commun. Biol. 2019, 2, 54. [Google Scholar] [CrossRef] [PubMed]
- Leceta, J.; Zapata, A. Seasonal changes in the thymus and spleen of the turtle, Mauremys caspica. A morphometrical, light microscopical study. Dev. Comp. Immunol. 1985, 9, 653–668. [Google Scholar] [CrossRef]
- Lu, H.; Jin, J.; Fan, H.; Dang, W. The magnitude of incubation temperature fluctuation affects the immunity of Chinese soft-shelled turtle (Pelodiscus sinensis) hatchlings. Aquac. Res. 2021, 52, 5229–5238. [Google Scholar] [CrossRef]
- Baker, S.; Kessler, E.; Darville-Bowleg, L.; Merchant, M. Different mechanisms of serum complement activation in the plasma of common (Chelydra serpentina) and alligator (Macrochelys temminckii) snapping turtles. PLoS ONE 2019, 14, e0217626. [Google Scholar] [CrossRef]
- Adamovicz, L.; Baker, S.J.; Merchant, M.; Darville, L.; Allender, M.C. Plasma complement activation mechanisms differ in ornate (Terrapene ornata ornata) and eastern box turtles (Terrapene carolina carolina). J. Exp. Zool. Part Ecol. Integr. Physiol. 2020, 333, 720–731. [Google Scholar] [CrossRef] [PubMed]
- Work, T.M.; Balazs, G.H.; Rameyer, R.A.; Chang, S.P.; Berestecky, J. Assessing humoral and cell-mediated immune response in Hawaiian green turtles, Chelonia mydas. Vet. Immunol. Immunopathol. 2000, 74, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Work, T.M.; Rameyer, R.A.; Balazs, G.H.; Cray, C.; Chang, S.P. IMMUNE STATUS OF FREE-RANGING GREEN TURTLES WITH FIBROPAPILLOMATOSIS FROM HAWAII. J. Wildl. Dis. 2001, 37, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Cray, C.; Varella, R.; Bossart, G.D.; Lutz, P. Altered in vitro Immune Responses in Green Turtles (Chelonia mydas) with Fibropapillomatosis. J. Zoo Wildl. Med. 2001, 32, 436–440. [Google Scholar]
- Rossi, S.; Sá-Rocha, V.M.; Kinoshita, D.; Genoy-Puerto, A.; Zwarg, T.; Werneck, M.R.; Sá-Rocha, L.C.; Matushima, E.R. Flow cytometry as a tool in the evaluation of blood leukocyte function in Chelonia mydas (Linnaeus, 1758) (Testudines, Cheloniidae). Braz. J. Biol. Rev. Brasleira Biol. 2009, 69, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; de Queiroz Hazarbassanov, N.G.T.; Sánchez-Sarmiento, A.M.; Prioste, F.E.S.; Matushima, E.R. Immune Response of Green Sea Turtles with and without Fibropapillomatosis: Evaluating Oxidative Burst and Phagocytosis via Flow Cytometry. Chelonian Conserv. Biol. 2016, 15, 273–278. [Google Scholar] [CrossRef]
- Swarthout, R.F.; Keller, J.M.; Peden-Adams, M.; Landry, A.M.; Fair, P.A.; Kucklick, J.R. Organohalogen contaminants in blood of Kemp’s ridley (Lepidochelys kempii) and green sea turtles (Chelonia mydas) from the Gulf of Mexico. Chemosphere 2010, 78, 731–741. [Google Scholar] [CrossRef]
- Komoroske, L.M.; Lewison, R.L.; Seminoff, J.A.; Deheyn, D.D.; Dutton, P.H. Pollutants and the health of green sea turtles resident to an urbanized estuary in San Diego, CA. Chemosphere 2011, 84, 544–552. [Google Scholar] [CrossRef]
- Perrault, J.R.; Stacy, N.I.; Lehner, A.F.; Mott, C.R.; Hirsch, S.; Gorham, J.C.; Buchweitz, J.P.; Bresette, M.J.; Walsh, C.J. Potential effects of brevetoxins and toxic elements on various health variables in Kemp’s ridley (Lepidochelys kempii) and green (Chelonia mydas) sea turtles after a red tide bloom event. Sci. Total Environ. 2017, 605–606, 967–979. [Google Scholar] [CrossRef]
- Perrault, J.R.; Levin, M.; Mott, C.R.; Bovery, C.M.; Bresette, M.J.; Chabot, R.M.; Gregory, C.R.; Guertin, J.R.; Hirsch, S.E.; Ritchie, B.W.; et al. Insights on Immune Function in Free-Ranging Green Sea Turtles (Chelonia mydas) with and without Fibropapillomatosis. Animals 2021, 11, 861. [Google Scholar] [CrossRef] [PubMed]
- Pace, A.; Rinaldi, L.; Ianniello, D.; Borrelli, L.; Cringoli, G.; Fioretti, A.; Hochscheid, S.; Dipineto, L. Gastrointestinal investigation of parasites and Enterobacteriaceae in loggerhead sea turtles from Italian coasts. BMC Vet. Res. 2019, 15, 370. [Google Scholar] [CrossRef] [PubMed]
- Shaw, K.R.; Lynch, J.M.; Balazs, G.H.; Jones, T.T.; Pawloski, J.; Rice, M.R.; French, A.D.; Liu, J.; Cobb, G.P.; Klein, D.M. Trace Element Concentrations in Blood and Scute Tissues from Wild and Captive Hawaiian Green Sea Turtles (Chelonia mydas). Environ. Toxicol. Chem. 2021, 40, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Sposato, P.; Keating, P.; Lutz, P.L.; Milton, S.L. EVALUATION OF IMMUNE FUNCTION IN TWO POPULATIONS OF GREEN SEA TURTLES (CHELONIA MYDAS) IN A DEGRADED VERSUS A NONDEGRADED HABITAT. J. Wildl. Dis. 2021, 57, 761–772. [Google Scholar] [CrossRef]
- Bastos, K.V.; Machado, L.P.; Joyeux, J.-C.; Ferreira, J.S.; Militão, F.P.; Fernandes, V.d.O.; Santos, R.G. Coastal degradation impacts on green turtle’s (Chelonia mydas) diet in southeastern Brazil: Nutritional richness and health. Sci. Total Environ. 2022, 823, 153593. [Google Scholar] [CrossRef] [PubMed]
- Garefino, V.E.; Milton, S.L. Influence of Sunlight on Vitamin D and Health Status in Green (Chelonia mydas) Sea Turtles with Fibropapillomatosis. Animals 2022, 12, 488. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, L.M.; Bowden, R.M.; Vogel, L.A. Red-Eared Slider Turtles Lack Response to Immunization with Keyhole Limpet Hemocyanin but Have High Levels of Natural Antibodies. ISRN Zool. 2013, 2013, 858941. [Google Scholar] [CrossRef]
- Ashford, M.A.; Palackdharry, S.M.; Sadd, B.M.; Bowden, R.M.; Vogel, L.A. Intestinal B cells in the red-eared slider turtle, Trachemys scripta: Anatomical distribution and implications for ecological interactions with pathogenic microbes. J. Exp. Zool. Part Ecol. Integr. Physiol. 2019, 331, 407–415. [Google Scholar] [CrossRef]
- Walsh, C.J.; Cocilova, C.; Restivo, J.; Flewelling, L.; Milton, S. Immune function in Trachemys scripta following exposure to a predominant brevetoxin congener, PbTx-3, as a model for potential health impacts for sea turtles naturally exposed to brevetoxins. Ecotoxicology 2019, 28, 1085–1104. [Google Scholar] [CrossRef]
- Ding, L.; Li, W.; Liang, L.; Huang, Z.; Li, N.; Zhang, J.; Shi, H.; Storey, K.B.; Hong, M. Modulation of the intestinal barrier adaptive functions in red-eared slider (Trachemys scripta elegans) invading brackish waters. Sci. Total Environ. 2021, 751, 141744. [Google Scholar] [CrossRef] [PubMed]
- Leceta, J.; Zapata, A.G. White pulp compartments in the spleen of the turtle Mauremys caspica: A light-microscopic, electron-microscopic, and immuno-histochemical study. Cell Tissue Res. 1991, 266, 605–613. [Google Scholar] [CrossRef]
- Muñoz, F.J.; de la Fuente, M. Seasonal Changes in Lymphoid Distribution of the Turtle Mauremys caspica. Copeia 2004, 2004, 178–183. [Google Scholar] [CrossRef]
- Gao, Y.; Wei, Y.; Cao, D.; Ge, Y.; Gong, S. Transcriptome analysis reveals decreased immunity under heat stress in Mauremys mutica. Aquaculture 2021, 531, 735894. [Google Scholar] [CrossRef]
- Soltanian, S.; Fallahi, R.; Fereidouni, M.S. Effects of diazinon on some innate resistance parameters in the Caspian pond turtle (Mauremys caspica caspica). Bulg. J. Vet. Med. 2018, 21, 212–223. [Google Scholar]
- Lakshminarayanan, R.; Vivekanandan, S.; Samy, R.P.; Banerjee, Y.; Chi-Jin, E.O.; Teo, K.W.; Jois, S.D.S.; Kini, R.M.; Valiyaveettil, S. Structure, Self-Assembly, and Dual Role of a β-Defensin-like Peptide from the Chinese Soft-Shelled Turtle Eggshell Matrix. J. Am. Chem. Soc. 2008, 130, 4660–4668. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Q.; Wang, F.; Li, X.; Wang, B.; Zhou, Y.; Zou, P.; Tang, L.; Yu, D.; Li, W. Improved immune function of Chinese soft-shelled turtles (Pelodiscus sinensis) through oral probiotics via the TLR signaling pathway. Aquaculture 2022, 555, 738126. [Google Scholar] [CrossRef]
- Bao, H.-J.; Li, M.-Y.; Wang, J.; Qin, J.-H.; Xu, C.-S.; Hei, N.-N.; Yang, P.; Gandahi, J.; Chen, Q.-S. Architecture of the Blood-Spleen Barrier in the Soft-Shelled Turtle, Pelodiseus Sinensis. Anat. Rec. 2009, 292, 1079–1087. [Google Scholar] [CrossRef]
- Feng, R.; Zhang, Z.; Guan, Y. Physiological and transcriptional analysis of Chinese soft-shelled turtle (Pelodiscus sinensis) in response to acute nitrite stress. Aquat. Toxicol. 2021, 237, 105899. [Google Scholar] [CrossRef]
- Fu, J.P.; Chen, S.N.; Zou, P.F.; Huang, B.; Guo, Z.; Zeng, L.B.; Qin, Q.W.; Nie, P. IFN-γ in turtle: Conservation in sequence and signalling and role in inhibiting iridovirus replication in Chinese soft-shelled turtle Pelodiscus sinensis. Dev. Comp. Immunol. 2014, 43, 87–95. [Google Scholar] [CrossRef]
- Fu, J.; Liu, W.; Cui, H.; Song, C.; Liu, Y.; Wei, L. Characterization and functional analysis of liver-expressed antimicrobial peptide-2 (LEAP-2) in Pelodiscus sinensis. Aquaculture 2019, 511, 734263. [Google Scholar] [CrossRef]
- Shi, N.; Cai, S.; Gao, J.; Qiao, X.; Yang, H.; Wang, Y.; Yu, H. Roles of polymorphic cathelicidins in innate immunity of soft-shell turtle, Pelodiscus sinensis. Dev. Comp. Immunol. 2019, 92, 179–192. [Google Scholar] [CrossRef]
- Liang, Q.; Zhu, N.; Zheng, X.; Ding, X.; He, R.; Xu, H.; Cao, F.; Xue, H.; Zhou, F.; Zheng, T. Transcriptome Analysis of Immune Responses and Metabolic Regulations of Chinese Soft-Shelled Turtle (Pelodiscus sinensis) against Edwardsiella tarda Infection. Fishes 2022, 7, 79. [Google Scholar] [CrossRef]
- Liu, T.; Han, Y.; Chen, S.; Zhao, H. Genome-wide identification of Toll-like receptors in the Chinese soft-shelled turtle Pelodiscus sinensis and expression analysis responding to Aeromonas hydrophila infection. Fish Shellfish Immunol. 2019, 87, 478–489. [Google Scholar] [CrossRef]
- Monagas, W.R.; Gatten, R.E. Behavioural fever in the turtles Terrapene carolina and Chrysemys picta. J. Therm. Biol. 1983, 8, 285–288. [Google Scholar] [CrossRef]
- Soltanian, S. Effect of atrazine on immunocompetence of red-eared slider turtle (Trachemys scripta). J. Immunotoxicol. 2016, 13, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, T.; Sun, Y.; Cheng, G.; Yang, H.; Wei, Z.; Wang, P.; Hu, X.; Ren, L.; Meng, Q.; et al. Extensive Diversification of IgD-, IgY-, and Truncated IgY(ΔFc)-Encoding Genes in the Red-Eared Turtle (Trachemys scripta elegans). J. Immunol. 2012, 189, 3995–4004. [Google Scholar] [CrossRef]
- Romanova, E.B.; Stolyarova, I.A.; Bakiev, A.G.; Gorelov, R.A. Leukocyte blood composition of Emys orbicularis and Mauremys capsica (Reptilia: Testudines: Emydidae, Geoemydidae) at syntopy. Povolzhskiy J. Ecol. 2022, 0, 79–93. [Google Scholar] [CrossRef]
- Shaffer, H.B.; Minx, P.; Warren, D.E.; Shedlock, A.M.; Thomson, R.C.; Valenzuela, N.; Abramyan, J.; Amemiya, C.T.; Badenhorst, D.; Biggar, K.K.; et al. The western painted turtle genome, a model for the evolution of extreme physiological adaptations in a slowly evolving lineage. Genome Biol. 2013, 14, R28. [Google Scholar] [CrossRef]
- Xu, C.; Dolby, G.A.; Drake, K.K.; Esque, T.C.; Kusumi, K. Immune and sex-biased gene expression in the threatened Mojave desert tortoise, Gopherus agassizii. PLoS ONE 2020, 15, e0238202. [Google Scholar] [CrossRef]
- Bianchi, L.; Casini, S.; Vantaggiato, L.; Di Noi, A.; Carleo, A.; Shaba, E.; Armini, A.; Bellucci, F.; Furii, G.; Bini, L.; et al. A Novel Ex Vivo Approach Based on Proteomics and Biomarkers to Evaluate the Effects of Chrysene, MEHP, and PBDE-47 on Loggerhead Sea Turtles (Caretta caretta). Int. J. Environ. Res. Public Health 2022, 19, 4369. [Google Scholar] [CrossRef]
- Day, R.D.; Segars, A.L.; Arendt, M.D.; Lee, A.M.; Peden-Adams, M.M. Relationship of Blood Mercury Levels to Health Parameters in the Loggerhead Sea Turtle (Caretta caretta). Environ. Health Perspect. 2007, 115, 1421–1428. [Google Scholar] [CrossRef]
- Perrault, J.R.; Bauman, K.D.; Greenan, T.M.; Blum, P.C.; Henry, M.S.; Walsh, C.J. Maternal transfer and sublethal immune system effects of brevetoxin exposure in nesting loggerhead sea turtles (Caretta caretta) from western Florida. Aquat. Toxicol. 2016, 180, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Jakšić, Ž.; Mrljak, V.; Horvatić, A.; Gelemanović, A.; Mičić, M. Loggerhead sea turtle Caretta caretta plasma biochemistry and proteome profile modulation during recovery. J. Proteom. 2022, 252, 104433. [Google Scholar] [CrossRef]
- Dickey, M.; Cray, C.; Norton, T.; Murray, M.; Barysauskas, C.; Arheart, K.L.; Nelson, S.; Rodriguez, M. ASSESSMENT OF HEMOGLOBIN BINDING PROTEIN IN LOGGERHEAD SEA TURTLES (Caretta caretta) UNDERGOING REHABILITATION. J. Zoo Wildl. Med. 2014, 45, 700–703. [Google Scholar] [CrossRef]
- Rodgers, M.L.; Toline, C.A.; Rice, C.D. Humoral Immune Responses to Select Marine Bacteria in Loggerhead Sea Turtles Caretta caretta and Kemp’s Ridley Sea Turtles Lepidochelys kempii from the Southeastern United States. J. Aquat. Anim. Health 2018, 30, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Stacy, N.I.; Lynch, J.M.; Arendt, M.D.; Avens, L.; McNeill, J.B.; Cray, C.; Day, R.D.; Harms, C.A.; Lee, A.M.; Peden-Adams, M.M.; et al. Chronic debilitation in stranded loggerhead sea turtles (Caretta caretta) in the southeastern United States: Morphometrics and clinicopathological findings. PLoS ONE 2018, 13, e0200355. [Google Scholar] [CrossRef] [PubMed]
- Hunt, K.E.; Innis, C.; Rolland, R.M. CORTICOSTERONE AND THYROXINE IN COLD-STUNNED KEMP’S RIDLEY SEA TURTLES (Lepidochelys kempii). J. Zoo Wildl. Med. 2012, 43, 479–493. [Google Scholar] [CrossRef]
- Gregory, L.F.; Schmid, J.R. Stress Responses and Sexing of Wild Kemp’s Ridley Sea Turtles (Lepidochelys kempii) in the Northeastern Gulf of Mexico. Gen. Comp. Endocrinol. 2001, 124, 66–74. [Google Scholar] [CrossRef]
- Valverde, R.A.; Owens, D.W.; MacKenzie, D.S.; Amoss, M.S. Basal and stress-induced corticosterone levels in olive ridley sea turtles (Lepidochelys olivacea) in relation to their mass nesting behavior. J. Exp. Zool. 1999, 284, 652–662. [Google Scholar] [CrossRef]
- Praja, R.N.; Yudhana, A.; Haditanojo, W.; Oktaviana, V. Short Communication: Antimicrobial properties in cloacal fluid of olive ridley sea turtle (Lepidochelys olivacea). Biodiversitas J. Biol. Divers. 2022, 13, 930629. [Google Scholar] [CrossRef]
- López-Hurtado, M.; Castro-González, M.I.; Guerra-Infante, F.M. Actividad antibacteriana de la clara de huevo de la tortuga marina Lepidochelys olivacea. Rev. Biol. Mar. Oceanogr. 2010, 45, 353–357. [Google Scholar] [CrossRef]
- Jessop, T.S.; Sumner, J.M.; Limpus, C.J.; Whittier, J.M. Interplay between plasma hormone profiles, sex and body condition in immature hawksbill turtles (Eretmochelys imbricata) subjected to a capture stress protocol. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2004, 137, 197–204. [Google Scholar] [CrossRef]
- Muñoz, F.A.; Estrada-Parra, S.; Romero-Rojas, A.; Gonzalez-Ballesteros, E.; Work, T.M.; Villaseñor-Gaona, H.; Estrada-Garcia, I. IMMUNOLOGICAL EVALUATION OF CAPTIVE GREEN SEA TURTLE (CHELONIA MYDAS) WITH ULCERATIVE DERMATITIS. J. Zoo Wildl. Med. 2013, 44, 837–844. [Google Scholar] [CrossRef]
- Work, T.M.; Dagenais, J.; Breeden, R.; Schneemann, A.; Sung, J.; Hew, B.; Balazs, G.H.; Berestecky, J.M. Green Turtles (Chelonia mydas) Have Novel Asymmetrical Antibodies. J. Immunol. 2015, 195, 5452–5460. [Google Scholar] [CrossRef]
- Collins, B.R.; Peck, B.; Buergelt, C.; Moreland, A. The Lymphoid Structure of the Green Turtle (Chelonia mydas); VIN.Com: Davis, CA, USA, 1986. [Google Scholar]
- Benedict, A.A.; Pollard, L.W. Three classes of immunoglobulins found in the sea turtle, Chelonia mydas. Folia Microbiol. 1972, 17, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Mead, K.F.; Borysenko, M.; Findlay, S.R. Naturally abundant basophils in the snapping turtle, Chelydra serpentina, possess cytophilic surface antibody with reaginic function. J. Immunol. 1983, 130, 334–340. [Google Scholar] [CrossRef]
- Borysenko, M.; Cooper, E.L. Lymphoid tissue in the snapping turtle, Chelydra serpentina. J. Morphol. 1972, 138, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Sypek, J.P.; Borysenko, M.; Findlay, S.R. Anti-immunoglobulin induced histamine release from naturally abundant basophils in the snapping turtle, Chelydra serpentina. Dev. Comp. Immunol. 1984, 8, 359–366. [Google Scholar] [CrossRef]
- Zimmerman, L.M.; Vogel, L.A.; Bowden, R.M. Understanding the vertebrate immune system: Insights from the reptilian perspective. J. Exp. Biol. 2010, 213, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, R.G.; Newberry, R.D. Isolated lymphoid follicles can function as sites for induction of mucosal immune responses. Ann. N. Y. Acad. Sci. 2004, 1029, 44–57. [Google Scholar] [CrossRef]
- Kiss, E.A.; Vonarbourg, C.; Kopfmann, S.; Hobeika, E.; Finke, D.; Esser, C.; Diefenbach, A. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science 2011, 334, 1561–1565. [Google Scholar] [CrossRef]
- Klein, U.; Dalla-Favera, R. Germinal centres: Role in B-cell physiology and malignancy. Nat. Rev. Immunol. 2008, 8, 22–33. [Google Scholar] [CrossRef]
- Jung, C.; Hugot, J.-P.; Barreau, F. Peyer’s Patches: The Immune Sensors of the Intestine. Int. J. Inflamm. 2010, 2010, 823710. [Google Scholar] [CrossRef]
- Hussein, M.F.; Badir, N.; el-Ridi, R.; Akef, M. Effect of seasonal variation on lymphoid tissues of the lizards, Mabuya quinquetaeniata Licht. and Uromastyx aegyptia Forsk. Dev. Comp. Immunol. 1978, 2, 469–478. [Google Scholar] [CrossRef] [PubMed]
- el Ridi, R.; Zada, S.; Afifi, A.; el Deeb, S.; el Rouby, S.; Farag, M.; Saad, A.H. Cyclic changes in the differentiation of lymphoid cells in reptiles. Cell Differ. 1988, 24, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Matoltsy, A.G.; Huszar, T. Keratinization of the reptilian epidermis: An ultrastructural study of the turtle skin. J. Ultrastruct. Res. 1972, 38, 87–101. [Google Scholar] [CrossRef]
- Tenería, F.A.M.; Calderón-Amador, J.; Negrete-Philippe, A.C.; Flores-Romo, L. A subpopulation of green turtle suprabasal epidermal cells are Langerin+ and migrate under in vitro stimulation of the chemokine CCL21. Vet. Res. Commun. 2022, 46, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, A. Immunolocalization of a beta-defensin (Tu-BD-1) in the skin and subdermal granulocytes of turtles indicate the presence of an antimicrobial skin barrier. Ann. Anat.-Anat. Anz. 2013, 195, 554–561. [Google Scholar] [CrossRef]
- Rimer, J.; Cohen, I.R.; Friedman, N. Do all creatures possess an acquired immune system of some sort? BioEssays 2014, 36, 273–281. [Google Scholar] [CrossRef]
- Viney, M.E.; Riley, E.M. From immunology to eco-immunology: More than a new name. In Eco-Immunology; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–19. [Google Scholar]
- Zimmerman, L.M.; Bowden, R.M.; Vogel, L.A. A vertebrate cytokine primer for eco-immunologists. Funct. Ecol. 2014, 28, 1061–1073. [Google Scholar] [CrossRef]
- van der Heijden, C.D.; Noz, M.P.; Joosten, L.A.; Netea, M.G.; Riksen, N.P.; Keating, S.T. Epigenetics and trained immunity. Antioxid. Redox Signal. 2018, 29, 1023–1040. [Google Scholar] [CrossRef]
- Netea, M.G.; Domínguez-Andrés, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.; van der Meer, J.W.; Mhlanga, M.M.; Mulder, W.J. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef]
- Divangahi, M.; Aaby, P.; Khader, S.A.; Barreiro, L.B.; Bekkering, S.; Chavakis, T.; van Crevel, R.; Curtis, N.; DiNardo, A.R.; Dominguez-Andres, J. Trained immunity, tolerance, priming and differentiation: Distinct immunological processes. Nat. Immunol. 2021, 22, 2–6. [Google Scholar] [CrossRef]
- Katzmarski, N.; Domínguez-Andrés, J.; Cirovic, B.; Renieris, G.; Ciarlo, E.; Le Roy, D.; Lepikhov, K.; Kattler, K.; Gasparoni, G.; Händler, K. Transmission of trained immunity and heterologous resistance to infections across generations. Nat. Immunol. 2021, 22, 1382–1390. [Google Scholar] [CrossRef]
- Tomalka, J.A.; Suthar, M.S.; Diamond, M.S.; Sekaly, R.P. Innate antiviral immunity: How prior exposures can guide future responses. Trends Immunol. 2022, 43, 696–705. [Google Scholar] [CrossRef]
- Zimmerman, L.M.; Vogel, L.A.; Edwards, K.A.; Bowden, R.M. Phagocytic B cells in a reptile. Biol. Lett. 2010, 6, 270–273. [Google Scholar] [CrossRef]
- Muñoz, F.A.; Franco-Noguez, S.Y.; Gonzalez-Ballesteros, E.; Negrete-Philippe, A.C.; Flores-Romo, L. Characterisation of the green turtle’s leukocyte subpopulations by flow cytometry and evaluation of their phagocytic activity. Vet. Res. Commun. 2014, 38, 123–128. [Google Scholar] [CrossRef]
- Pellizzon, C.H.; Lunardi, L.O. Endocytic activity in the thrombocytes of the turtle Phrynopys hilarii (freshwater South American species). J. Submicrosc. Cytol. Pathol. 2000, 32, 651–656. [Google Scholar]
- Caliani, I.; Poggioni, L.; D’Agostino, A.; Fossi, M.C.; Casini, S. An immune response-based approach to evaluate physiological stress in rehabilitating loggerhead sea turtle. Vet. Immunol. Immunopathol. 2019, 207, 18–24. [Google Scholar] [CrossRef]
- Feiyan, Z.; Hexiang, G.; Pipeng, L. A Review of Chelonian Hematology: A Review of Chelonian Hematology. Asian Herpetol. Res. 2011, 2, 12–20. [Google Scholar] [CrossRef]
- Rios, F.M.; Zimmerman, L.M. Immunology of Reptiles. In eLS; American Cancer Society: Atlanta, GA, USA, 2015; pp. 1–7. ISBN 978-0-470-01590-2. [Google Scholar]
- León, B.; López-Bravo, M.; Ardavín, C. Monocyte-derived dendritic cells. Semin. Immunol. 2005, 17, 313–318. [Google Scholar] [CrossRef]
- Cabanac, M.; Bernieri, C. Behavioral rise in body temperature and tachycardia by handling of a turtle (Clemmys insculpta). Behav. Process. 2000, 49, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Goessling, J.M.; Guyer, C.; Mendonça, M.T. More than Fever: Thermoregulatory Responses to Immunological Stimulation and Consequences of Thermoregulatory Strategy on Innate Immunity in Gopher Tortoises (Gopherus polyphemus). Physiol. Biochem. Zool. 2017, 90, 484–493. [Google Scholar] [CrossRef]
- Pasmans, F.; Herdt, P.D.; Nerom, A.V.; Haesebrouck, F. Induction of the respiratory burst in turtle peritoneal macrophages by Salmonella muenchen. Dev. Comp. Immunol. 2001, 25, 159–168. [Google Scholar] [CrossRef]
- Van Houtan, K.S.; Halley, J.M.; Marks, W. Terrestrial basking sea turtles are responding to spatio-temporal sea surface temperature patterns. Biol. Lett. 2015, 11, 20140744. [Google Scholar] [CrossRef]
- Swimmer, J.Y. Relationship Between Basking and Fibropapillomatosis in Captive Green Turtles (Chelonia mydas). Chelonian Conserv. Biol. 2006, 5, 305–309. [Google Scholar] [CrossRef]
- Kokolus, K.M.; Capitano, M.L.; Lee, C.-T.; Eng, J.W.-L.; Waight, J.D.; Hylander, B.L.; Sexton, S.; Hong, C.-C.; Gordon, C.J.; Abrams, S.I.; et al. Baseline tumor growth and immune control in laboratory mice are significantly influenced by subthermoneutral housing temperature. Proc. Natl. Acad. Sci. USA 2013, 110, 20176–20181. [Google Scholar] [CrossRef]
- Evans, S.S.; Repasky, E.A.; Fisher, D.T. Fever and the thermal regulation of immunity: The immune system feels the heat. Nat. Rev. Immunol. 2015, 15, 335–349. [Google Scholar] [CrossRef]
- Saurabh, S.; Sahoo, P.K. Lysozyme: An important defense molecule of fish innate immune system. Aquac. Res. 2008, 39, 223–239. [Google Scholar] [CrossRef]
- van Hoek, M.L. Antimicrobial Peptides in Reptiles. Pharmaceuticals 2014, 7, 723–753. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, A.; Parida, S.; Mohanty, J.; Sahoo, P.K. Identification and functional characterization of a g-type lysozyme gene of Labeo rohita, an Indian major carp species. Dev. Comp. Immunol. 2019, 92, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.-X.; He, J.-G.; Deng, W.-X.; Chan, S.-M. Molecular cloning, expression of orange-spotted grouper goose-type lysozyme cDNA, and lytic activity of its recombinant protein. Dis. Aquat. Organ. 2003, 55, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Cantizano, R.M.; Infante, C.; Martin-Antonio, B.; Ponce, M.; Hachero, I.; Navas, J.I.; Manchado, M. Molecular characterization, phylogeny, and expression of c-type and g-type lysozymes in brill (Scophthalmus rhombus). Fish Shellfish. Immunol. 2008, 25, 57–65. [Google Scholar] [CrossRef]
- Ye, X.; Zhang, L.; Tian, Y.; Tan, A.; Bai, J.; Li, S. Identification and expression analysis of the g-type and c-type lysozymes in grass carp Ctenopharyngodon idellus. Dev. Comp. Immunol. 2010, 34, 501–509. [Google Scholar] [CrossRef]
- Buonocore, F.; Randelli, E.; Trisolino, P.; Facchiano, A.; de Pascale, D.; Scapigliati, G. Molecular characterization, gene structure and antibacterial activity of a g-type lysozyme from the European sea bass (Dicentrarchus labrax L.). Mol. Immunol. 2014, 62, 10–18. [Google Scholar] [CrossRef]
- Wei, S.; Huang, Y.; Huang, X.; Cai, J.; Wei, J.; Li, P.; Ouyang, Z.; Qin, Q. Molecular cloning and characterization of a new G-type lysozyme gene (Ec-lysG) in orange-spotted grouper, Epinephelus coioides. Dev. Comp. Immunol. 2014, 46, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Kumaresan, V.; Bhatt, P.; Ganesh, M.-R.; Harikrishnan, R.; Arasu, M.; Al-Dhabi, N.A.; Pasupuleti, M.; Marimuthu, K.; Arockiaraj, J. A novel antimicrobial peptide derived from fish goose type lysozyme disrupts the membrane of Salmonella enterica. Mol. Immunol. 2015, 68, 421–433. [Google Scholar] [CrossRef]
- Thammasirirak, S.; Ponkham, P.; Preecharram, S.; Khanchanuan, R.; Phonyothee, P.; Daduang, S.; Srisomsap, C.; Araki, T.; Svasti, J. Purification, characterization and comparison of reptile lysozymes. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2006, 143, 209–217. [Google Scholar] [CrossRef]
- Walsh, C.J.; Leggett, S.R.; Carter, B.J.; Colle, C. Effects of brevetoxin exposure on the immune system of loggerhead sea turtles. Aquat. Toxicol. 2010, 97, 293–303. [Google Scholar] [CrossRef]
- Keller, J.M.; McClellan-Green, P.D.; Kucklick, J.R.; Keil, D.E.; Peden-Adams, M.M. Effects of organochlorine contaminants on loggerhead sea turtle immunity: Comparison of a correlative field study and in vitro exposure experiments. Environ. Health Perspect. 2006, 114, 70–76. [Google Scholar] [CrossRef]
- Kou, H.; Hu, J.; Wang, A.-L.; Pan, X.; Miao, Y.; Lin, L. Impacts of dietary zinc on growth performance, haematological indicators, transaminase activity and tissue trace mineral contents of soft-shelled turtle (Pelodiscus sinensis). Aquac. Nutr. 2021, 27, 2182–2194. [Google Scholar] [CrossRef]
- Chijiiwa, Y.; Kawamura, S.; Torikata, T.; Araki, T. Amino Acid Sequence and Activity of Green Turtle (Chelonia mydas) Lysozyme. Protein J. 2006, 25, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Gayen, S.K.; Som, S.; Sinha, N.K.; Sen, A. Lysozyme in egg whites of tortoises and turtle: Purification and properties of egg white lysozyme of Trionyx gangeticus cuvier. Arch. Biochem. Biophys. 1977, 183, 432–442. [Google Scholar] [CrossRef]
- Araki, T.; Yamamoto, T.; Torikata, T. Reptile Lysozyme: The Complete Amino Acid Sequence of Soft-Shelled Turtle Lysozyme and Its Activity. Biosci. Biotechnol. Biochem. 1998, 62, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Sinha, N.K.; Banerjee, S.; Roy, D.; Chattopadhyay, D.; Roy, S. Small cationic protein from a marine turtle has β-defensin-like fold and antibacterial and antiviral activity. Proteins Struct. Funct. Bioinforma. 2006, 64, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Hilchie, A.L.; Wuerth, K.; Hancock, R.E.W. Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nat. Chem. Biol. 2013, 9, 761–768. [Google Scholar] [CrossRef]
- Mansour, S.C.; Pena, O.M.; Hancock, R.E.W. Host defense peptides: Front-line immunomodulators. Trends Immunol. 2014, 35, 443–450. [Google Scholar] [CrossRef]
- Stegemann, C.; Kolobov, A., Jr.; Leonova, Y.F.; Knappe, D.; Shamova, O.; Ovchinnikova, T.V.; Kokryakov, V.N.; Hoffmann, R. Isolation, purification and de novo sequencing of TBD-1, the first beta-defensin from leukocytes of reptiles. Proteomics 2009, 9, 1364–1373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, D.; Yu, L.; Wei, Y.; Li, J.; Zhou, C. Genome-wide analysis of the ovodefensin gene family: Monophyletic origin, independent gene duplication and presence of different selection patterns. Infect. Genet. Evol. 2019, 68, 265–272. [Google Scholar] [CrossRef]
- Guyot, N.; Landon, C.; Monget, P. The Two Domains of the Avian Double-β-Defensin AvBD11 Have Different Ancestors, Common with Potential Monodomain Crocodile and Turtle Defensins. Biology 2022, 11, 690. [Google Scholar] [CrossRef]
- Agarwal, S.; Chauhan, A.; Singh, K.; Kumar, K.; Kaur, R.; Masih, M.; Gautam, P.K. Immunomodulatory effects of β-defensin 2 on macrophages induced immuno-upregulation and their antitumor function in breast cancer. BMC Immunol. 2022, 23, 53. [Google Scholar] [CrossRef]
- Conejo-Garcia, J.R.; Benencia, F.; Courreges, M.-C.; Kang, E.; Mohamed-Hadley, A.; Buckanovich, R.J.; Holtz, D.O.; Jenkins, A.; Na, H.; Zhang, L.; et al. Tumor-infiltrating dendritic cell precursors recruited by a β-defensin contribute to vasculogenesis under the influence of Vegf-A. Nat. Med. 2004, 10, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, T. 25 years of UV-induced immunosuppression mediated by T cells—From disregarded T suppressor cells to highly respected regulatory T cells. Photochem. Photobiol. 2008, 84, 10–18. [Google Scholar] [CrossRef]
- Gläser, R.; Navid, F.; Schuller, W.; Jantschitsch, C.; Harder, J.; Schröder, J.M.; Schwarz, A.; Schwarz, T. UV-B radiation induces the expression of antimicrobial peptides in human keratinocytes in vitro and in vivo. J. Allergy Clin. Immunol. 2009, 123, 1117–1123. [Google Scholar] [CrossRef]
- Le, M.N.-T.; Kawada-Matsuo, M.; Komatsuzawa, H. Efficiency of Antimicrobial Peptides Against Multidrug-Resistant Staphylococcal Pathogens. Front. Microbiol. 2022, 13, 930629. [Google Scholar] [CrossRef]
- Kościuczuk, E.M.; Lisowski, P.; Jarczak, J.; Strzałkowska, N.; Jóźwik, A.; Horbańczuk, J.; Krzyżewski, J.; Zwierzchowski, L.; Bagnicka, E. Cathelicidins: Family of antimicrobial peptides. A review. Mol. Biol. Rep. 2012, 39, 10957–10970. [Google Scholar] [CrossRef] [PubMed]
- Sarma, J.V.; Ward, P.A. The complement system. Cell Tissue Res. 2011, 343, 227–235. [Google Scholar] [CrossRef]
- Lin, B.; Chen, S.; Cao, Z.; Lin, Y.; Mo, D.; Zhang, H.; Gu, J.; Dong, M.; Liu, Z.; Xu, A. Acute phase response in zebrafish upon Aeromonas salmonicida and Staphylococcus aureus infection: Striking similarities and obvious differences with mammals. Mol. Immunol. 2007, 44, 295–301. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, L.; Feng, H.; Guo, Q.; Dai, H. Acute phase response in Chinese soft-shelled turtle (Trionyx sinensis) with Aeromonas hydrophila infection. Dev. Comp. Immunol. 2011, 35, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Freedberg, S.; Greives, T.J.; Ewert, M.A.; Demas, G.E.; Beecher, N.; Nelson, C.E. Incubation Environment Affects Immune System Development in a Turtle with Environmental Sex Determination. J. Herpetol. 2008, 42, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Niu, C.; Chen, B.; Storey, K.B. Digital Gene Expression Profiling reveals transcriptional responses to acute cold stress in Chinese soft-shelled turtle Pelodiscus sinensis juveniles. Cryobiology 2018, 81, 43–56. [Google Scholar] [CrossRef]
- Li, H.; Zhao, J.; Ji, B.; Ye, Z.; Zhu, S.; Zhou, C. Effects of Sunlamp-Based Lighting Mode on Growth Performance, Survival Rate, Stress Response, and Oxidative Stress of Juvenile Chinese Soft-Shelled Turtles (Pelodiscus sinensis) in a Greenhouse; ASABE: St. Joseph, MI, USA, 2021; Volume 64, pp. 1269–1276. [Google Scholar]
- Sandmeier, F.C.; Tracy, C.R.; Dupré, S.; Hunter, K. A trade-off between natural and acquired antibody production in a reptile: Implications for long-term resistance to disease. Biol. Open 2012, 1, 1078–1082. [Google Scholar] [CrossRef]
- Stromsland, K.; Zimmerman, L.M. Relationships between parasitic infection and natural antibodies, age, and sex in a long-lived vertebrate. J. Exp. Zool. Part Ecol. Integr. Physiol. 2017, 327, 407–412. [Google Scholar] [CrossRef]
- Refsnider, J.M.; Garcia, J.A.; Holliker, B.; Hulbert, A.C.; Nunez, A.; Streby, H.M. Effects of harmful algal blooms on stress levels and immune functioning in wetland-associated songbirds and reptiles. Sci. Total Environ. 2021, 788, 147790. [Google Scholar] [CrossRef] [PubMed]
- Kulseth, M.A.; Krajci, P.; Myklebost, O.; Rogne, S. Cloning and Characterization of Two Forms of Bovine Polymeric Immunoglobulin Receptor cDNA. DNA Cell Biol. 1995, 14, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Stadtmueller, B.M.; Yang, Z.; Huey-Tubman, K.E.; Roberts-Mataric, H.; Hubbell, W.L.; Bjorkman, P.J. Biophysical and Biochemical Characterization of Avian Secretory Component Provides Structural Insights into the Evolution of the Polymeric Ig Receptor. J. Immunol. 2016, 197, 1408–1414. [Google Scholar] [CrossRef]
- Xu, J.; Wu, Y.; Xu, C.; Munang’andu, H.M.; Xu, H. Characterization of the Pelodiscus sinensis polymeric immunoglobulin receptor (P. sinensis pIgR) and its response to LPS and Aeromonas sobria. Dev. Comp. Immunol. 2021, 121, 104072. [Google Scholar] [CrossRef]
- Shi, Y.; Vistro, W.A.; Bai, X.; Wu, R.; Chen, C.; Huang, Y.; Fazlani, S.A.; Tarique, I.; Yang, P.; Chen, Q. Effect of seasonal variance on intestinal epithelial barriers and the associated innate immune response of the small intestine of the Chinese soft-shelled turtles. Fish Shellfish Immunol. 2020, 97, 173–181. [Google Scholar] [CrossRef]
- Gruys, E.; Toussaint, M.J.M.; Niewold, T.A.; Koopmans, S.J. Acute phase reaction and acute phase proteins. J. Zhejiang Univ. Sci. B 2005, 6, 1045–1056. [Google Scholar] [CrossRef]
- Janeway, C.A.; Medzhitov, R. Innate Immune Recognition. Annu. Rev. Immunol. 2002, 20, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015, 16, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Maldonado, E.; Silva, L.; Almeida, D.; Johnson, W.E.; O’Brien, S.J.; Zhang, G.; Jarvis, E.D.; Gilbert, M.T.P.; Antunes, A. The Vertebrate TLR Supergene Family Evolved Dynamically by Gene Gain/Loss and Positive Selection Revealing a Host–Pathogen Arms Race in Birds. Diversity 2019, 11, 131. [Google Scholar] [CrossRef]
- Anderson, K.V.; Bokla, L.; Nüsslein-Volhard, C. Establishment of dorsal-ventral polarity in the Drosophila embryo: The induction of polarity by the Toll gene product. Cell 1985, 42, 791–798. [Google Scholar] [CrossRef]
- Medzhitov, R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 2001, 1, 135–145. [Google Scholar] [CrossRef]
- Fore, F.; Budipranama, M.; Destiawan, R.A. TLR10 and Its Role in Immunity. In Toll-Like Receptors in Health and Disease; Kumar, V., Ed.; Handbook of Experimental Pharmacology; Springer International Publishing: Cham, Switzerland, 2022; pp. 161–174. ISBN 978-3-031-06512-5. [Google Scholar]
- Huo, R.; Zhao, X.; Han, J.; Xu, T. Genomic organization, evolution and functional characterization of soluble toll-like receptor 5 (TLR5S) in miiuy croaker (Miichthys miiuy). Fish Shellfish Immunol. 2018, 80, 109–114. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, X.; Zhou, C.; Li, L.; Nie, G.; Li, X. Toll-like receptor recognition of bacteria in fish: Ligand specificity and signal pathways. Fish Shellfish Immunol. 2014, 41, 380–388. [Google Scholar] [CrossRef]
- Shan, S.; Liu, D.; Liu, R.; Zhu, Y.; Li, T.; Zhang, F.; An, L.; Yang, G.; Li, H. Non-mammalian Toll-like receptor 18 (Tlr18) recognizes bacterial pathogens in common carp (Cyprinus carpio L.): Indications for a role of participation in the NF-κB signaling pathway. Fish Shellfish Immunol. 2018, 72, 187–198. [Google Scholar] [CrossRef]
- Zhang, Y.E.; Landback, P.; Vibranovski, M.D.; Long, M. Accelerated Recruitment of New Brain Development Genes into the Human Genome. PLoS Biol. 2011, 9, e1001179. [Google Scholar] [CrossRef]
- Ranz, J.M.; Parsch, J. Newly evolved genes: Moving from comparative genomics to functional studies in model systems. BioEssays 2012, 34, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Dolby, G.A.; Morales, M.; Webster, T.H.; DeNardo, D.F.; Wilson, M.A.; Kusumi, K. Discovery of a New TLR Gene and Gene Expansion Event through Improved Desert Tortoise Genome Assembly with Chromosome-Scale Scaffolds. Genome Biol. Evol. 2020, 12, 3917–3925. [Google Scholar] [CrossRef]
- Albalat, R.; Cañestro, C. Evolution by gene loss. Nat. Rev. Genet. 2016, 17, 379–391. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, M.; Tang, X.; Xu, D.; Chi, C.; Lv, Z.; Liu, H. Characterization of a novel Toll-like receptor 13 homologue from a marine fish Nibea albiflora, revealing its immunologic function as PRRs. Dev. Comp. Immunol. 2023, 139, 104563. [Google Scholar] [CrossRef] [PubMed]
- Georgel, P.; Crozat, K.; Lauth, X.; Makrantonaki, E.; Seltmann, H.; Sovath, S.; Hoebe, K.; Du, X.; Rutschmann, S.; Jiang, Z.; et al. A toll-like receptor 2-responsive lipid effector pathway protects mammals against skin infections with gram-positive bacteria. Infect. Immun. 2005, 73, 4512–4521. [Google Scholar] [CrossRef]
- Shang, S.; Zhong, H.; Wu, X.; Wei, Q.; Zhang, H.; Chen, J.; Chen, Y.; Tang, X.; Zhang, H. Genomic evidence of gene duplication and adaptive evolution of Toll like receptors (TLR2 and TLR4) in reptiles. Int. J. Biol. Macromol. 2018, 109, 698–703. [Google Scholar] [CrossRef]
- Cervantes, J.L.; Weinerman, B.; Basole, C.; Salazar, J.C. TLR8: The forgotten relative revindicated. Cell. Mol. Immunol. 2012, 9, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, H.; Zhao, C.; Zhang, H. Evolutionary History of the Toll-Like Receptor Gene Family across Vertebrates. Genome Biol. Evol. 2019, 12, 3615–3634. [Google Scholar] [CrossRef] [PubMed]
- Cocci, P.; Mosconi, G.; Palermo, F.A. Organic UV Filters Induce Toll-like-Receptors and Related Signaling Pathways in Peripheral Blood Mononuclear Cells of Juvenile Loggerhead Sea Turtles (Caretta caretta). Animals 2022, 12, 594. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-J.; Shen, Y.; Xu, X.-Y.; Hu, M.-Y. Identification and characterization of the TLR18 gene in grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2015, 47, 681–688. [Google Scholar] [CrossRef]
- Wang, K.-L.; Ji, W.; Zhang, G.-R.; Wei, K.-J.; Shi, Z.-C.; Zhang, X.-T.; Zheng, H.; Fan, Q.-X. Molecular characterization and expression analysis of three TLR genes in yellow catfish (Pelteobagrus fulvidraco): Responses to stimulation of Aeromonas hydrophila and TLR ligands. Fish Shellfish Immunol. 2017, 66, 466–479. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Yeh, W.-C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef]
- Cai, W.; Chen, W.; Wang, Y.; Bu, X.; Xia, X.; Nie, L.; Cai, W.; Chen, W.; Wang, Y.; Bu, X.; et al. Sperm storage in the oviduct of the Chinese pond turtle Mauremys reevesii depends on oestrogen-based suppression of the TLR2/4 immune pathway. Reprod. Fertil. Dev. 2021, 33, 736–745. [Google Scholar] [CrossRef]
- Rauta, P.R.; Nayak, B.; Das, S. Immune system and immune responses in fish and their role in comparative immunity study: A model for higher organisms. Immunol. Lett. 2012, 148, 23–33. [Google Scholar] [CrossRef]
- Iurescia, S.; Fioretti, D.; Rinaldi, M. The Innate Immune Signalling Pathways: Turning RIG-I Sensor Activation against Cancer. Cancers 2020, 12, 3158. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wan, H.; Nie, L.; Shao, T.; Xiang, L.; Shao, J. RIG-I: A multifunctional protein beyond a pattern recognition receptor. Protein Cell 2018, 9, 246–253. [Google Scholar] [CrossRef]
- Chen, J.; Shang, S.; Wu, X.; Zhong, H.; Zhao, C.; Wei, Q.; Zhang, H.; Xia, T.; Chen, Y.; Zhang, H.; et al. Genomic analysis and adaptive evolution of the RIG-I-like and NOD-like receptors in reptiles. Int. J. Biol. Macromol. 2019, 134, 1045–1051. [Google Scholar] [CrossRef]
- Meng, Y.; Fan, Y.; Zhou, Y.; Jiang, N.; Xue, M.; Liu, W.; Li, Y.; Zeng, L. Identification and comparative expression analysis of RIG-I and MDA5 in Chinese giant salamander Andrias davidianus. Aquac. Res. 2020, 51, 4575–4582. [Google Scholar] [CrossRef]
- Inohara, N.; Nuñez, G. The NOD: A signaling module that regulates apoptosis and host defense against pathogens. Oncogene 2001, 20, 6473–6481. [Google Scholar] [CrossRef]
- Priyam, M.; Tripathy, M.; Rai, U.; Ghorai, S.M. Tracing the evolutionary lineage of pattern recognition receptor homologues in vertebrates: An insight into reptilian immunity via de novo sequencing of the wall lizard splenic transcriptome. Vet. Immunol. Immunopathol. 2016, 172, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Drouin, M.; Saenz, J.; Chiffoleau, E. C-Type Lectin-Like Receptors: Head or Tail in Cell Death Immunity. Front. Immunol. 2020, 11, 251. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.-H.; Pan, S.-K.; Hu, L.; Zhu, Y.; Xu, P.-W.; Xia, J.-Q.; Chen, H.; He, G.-Y.; He, J.; Ni, X.-W.; et al. Genome analysis and signature discovery for diving and sensory properties of the endangered Chinese alligator. Cell Res. 2013, 23, 1091–1105. [Google Scholar] [CrossRef]
- Aird, S.D.; Da Silva, N.J.; Qiu, L.; Villar-Briones, A.; Saddi, V.A.; de Campos Telles, M.P.; Grau, M.L.; Mikheyev, A.S. Coralsnake Venomics: Analyses of Venom Gland Transcriptomes and Proteomes of Six Brazilian Taxa. Toxins 2017, 9, 187. [Google Scholar] [CrossRef]
- Pénzes, J.J.; Szirovicza, L.; Harrach, B. The complete genome sequence of bearded dragon adenovirus 1 harbors three genes encoding proteins of the C-type lectin-like domain superfamily. Infect. Genet. Evol. 2020, 83, 104321. [Google Scholar] [CrossRef] [PubMed]
- Graber, J.J.; Dhib-Jalbut, S. Interferons. In Encyclopedia of the Neurological Sciences, 2nd ed.; Aminoff, M.J., Daroff, R.B., Eds.; Academic Press: Oxford, UK, 2014; pp. 718–723. ISBN 978-0-12-385158-1. [Google Scholar]
- Chen, S.N.; Zhang, X.W.; Li, L.; Ruan, B.Y.; Huang, B.; Huang, W.S.; Zou, P.F.; Fu, J.P.; Zhao, L.J.; Li, N.; et al. Evolution of IFN-λ in tetrapod vertebrates and its functional characterization in green anole lizard (Anolis carolinensis). Dev. Comp. Immunol. 2016, 61, 208–224. [Google Scholar] [CrossRef]
- Chen, S.N.; Huang, L.; Fu, J.P.; Pang, A.N.; Wang, K.L.; Nie, P. Gene synteny, evolution and antiviral activity of type I IFNs in a reptile species, the Chinese soft-shelled turtle Pelodiscus sinensis. Dev. Comp. Immunol. 2022, 134, 104461. [Google Scholar] [CrossRef] [PubMed]
- Galabov, A.S. [28] Induction and characterization of tortoise interferon. In Methods in Enzymology; Interferons Part A; Academic Press: Cambridge, MA, USA, 1981; Volume 78, pp. 196–208. [Google Scholar]
- Mathews, J.H.; Vorndam, A.V. Interferon-mediated persistent infection of Saint Louis encephalitis virus in a reptilian cell line. J. Gen. Virol. 1982, 61, 177–186. [Google Scholar] [CrossRef]
- Fleming, S.B. Viral Inhibition of the IFN-Induced JAK/STAT Signalling Pathway: Development of Live Attenuated Vaccines by Mutation of Viral-Encoded IFN-Antagonists. Vaccines 2016, 4, 23. [Google Scholar] [CrossRef] [PubMed]
- Alphonse, N.; Dickenson, R.E.; Odendall, C. Interferons: Tug of War Between Bacteria and Their Host. Front. Cell. Infect. Microbiol. 2021, 11, 624094. [Google Scholar] [CrossRef]
- Liu, T.; Han, Y.; Chen, S.; Zhao, H. Global characterization and expression analysis of interferon regulatory factors in response to Aeromonas hydrophila challenge in Chinese soft-shelled turtle (Pelodiscus sinensis). Fish Shellfish Immunol. 2019, 92, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Broere, F.; van Eden, W. T Cell Subsets and T Cell-Mediated Immunity. In Nijkamp and Parnham’s Principles of Immunopharmacology; Parnham, M.J., Nijkamp, F.P., Rossi, A.G., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 23–35. ISBN 978-3-030-10809-0. [Google Scholar]
- Muñoz, F.A.; Estrada-Parra, S.; Romero-Rojas, A.; Work, T.M.; Gonzalez-Ballesteros, E.; Estrada-Garcia, I. Identification of CD3+ T lymphocytes in the green turtle Chelonia mydas. Vet. Immunol. Immunopathol. 2009, 131, 211–217. [Google Scholar] [CrossRef]
- Aguirre, A.A.; Balazs, G.H.; Spraker, T.R.; Gross, T.S. Adrenal and Hematological Responses to Stress in Juvenile Green Turtles (Chelonia mydas) with and without Fibropapillomas. Physiol. Zool. 1995, 68, 831–854. [Google Scholar] [CrossRef]
- Yetsko, K.; Farrell, J.; Stammnitz, M.R.; Whitmore, L.; Whilde, J.; Eastman, C.B.; Ramia, D.R.; Thomas, R.; Krstic, A.; Linser, P.; et al. Mutational, transcriptional and viral shedding dynamics of the marine turtle fibropapillomatosis tumor epizootic. bioRxiv 2020. [Google Scholar] [CrossRef]
- Yu, S.; Halbrook, R.S.; Sparling, D.W.; Colombo, R. Metal accumulation and evaluation of effects in a freshwater turtle. Ecotoxicology 2011, 20, 1801–1812. [Google Scholar] [CrossRef]
- Charles, A.; Janeway, J.; Travers, P.; Walport, M.; Shlomchik, M.J. The structure of a typical antibody molecule. In Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Science: New York, NY, USA, 2001. [Google Scholar]
- Goessling, J.M.; Koler, S.A.; Overman, B.D.; Hiltbold, E.M.; Guyer, C.; Mendonça, M.T. Lag of Immunity Across Seasonal Acclimation States in Gopher Tortoises (Gopherus Polyphemus). J. Exp. Zool. Part Ecol. Integr. Physiol. 2017, 327, 235–242. [Google Scholar] [CrossRef]
- Wu, L.; Qin, Z.; Liu, H.; Lin, L.; Ye, J.; Li, J. Recent Advances on Phagocytic B Cells in Teleost Fish. Front. Immunol. 2020, 11, 824. [Google Scholar] [CrossRef] [PubMed]
- Abós, B.; Bird, S.; Granja, A.G.; Morel, E.; Bayona, J.A.M.; Barreda, D.R.; Tafalla, C. Identification of the First Teleost CD5 Molecule: Additional Evidence on Phenotypical and Functional Similarities between Fish IgM+ B Cells and Mammalian B1 Cells. J. Immunol. 2018, 201, 465–480. [Google Scholar] [CrossRef]
- Xing, J.; Luo, K.; Xiao, Y.; Tang, X.; Zhan, W. Influence of CD4-1+, CD4-2+ and CD8+ T lymphocytes subpopulations on the immune response of B lymphocytes in flounder (Paralichthys olivaceus) immunized with thymus-dependent or thymus-independent antigen. Fish Shellfish Immunol. 2019, 84, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, D.N.; Mirete-Bachiller, S.; Gambón-Deza, F. Insights into the evolution of IG genes in Amphibians and reptiles. Dev. Comp. Immunol. 2021, 114, 103868. [Google Scholar] [CrossRef] [PubMed]
- Leslie, G.A.; Clem, L.W. Phylogeny of immunoglobulin structure and function. VI. 17S, 7.5S and 5.7S anti-DNP of the turtle, Pseudamys scripta. J. Immunol. Baltim. 1972, 108, 1656–1664. [Google Scholar] [CrossRef]
- Turchin, A.; Hsu, E. The generation of antibody diversity in the turtle. J. Immunol. 1996, 156, 3797–3805. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, G.L.; Nie, P. IgM, IgD and IgY and their expression pattern in the Chinese soft-shelled turtle Pelodiscus sinensis. Mol. Immunol. 2009, 46, 2124–2132. [Google Scholar] [CrossRef]
- Magadán-Mompó, S.; Sánchez-Espinel, C.; Gambón-Deza, F. Immunoglobulin genes of the turtles. Immunogenetics 2013, 65, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Magor, K.E.; Warr, G.W.; Middleton, D.; Wilson, M.R.; Higgins, D.A. Structural relationship between the two IgY of the duck, Anas platyrhynchos: Molecular genetic evidence. J. Immunol. Baltim. 1992, 149, 2627–2633. [Google Scholar] [CrossRef]
- Herbst, L.H.; Klein, P.A. Monoclonal antibodies for the measurement of class-specific antibody responses in the green turtle, Chelonia mydas. Vet. Immunol. Immunopathol. 1995, 46, 317–335. [Google Scholar] [CrossRef]
- Work, T.M.; Dagenais, J.; Willimann, A.; Balazs, G.; Mansfield, K.; Ackermann, M. Differences in Antibody Responses against Chelonid Alphaherpesvirus 5 (ChHV5) Suggest Differences in Virus Biology in ChHV5-Seropositive Green Turtles from Hawaii and ChHV5-Seropositive Green Turtles from Florida. J. Virol. 2020, 94, e01658-19. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.J.; Stacy, N.I.; Jacobson, E.; Le-Bert, C.R.; Nollens, H.H.; Origgi, F.C.; Green, L.G.; Bootorabi, S.; Bolten, A.; Hernandez, J.A. Development and validation of a competitive enzyme-linked immunosorbent assay for the measurement of total plasma immunoglobulins in healthy loggerhead sea (Caretta caretta) and green turtles (Chelonia mydas). J. Vet. Diagn. Investig. 2016, 28, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, A.A.; Ostfeld, R.S.; Tabor, G.M.; House, C.; Pearl, M.C. Conservation Medicine: Ecological Health in Practice; Oxford University Press: Oxford, UK, 2002; ISBN 978-0-19-534862-0. [Google Scholar]
- Aguirre, A.A.; Lutz, P.L. Marine Turtles as Sentinels of Ecosystem Health: Is Fibropapillomatosis an Indicator? EcoHealth 2004, 1, 275–283. [Google Scholar] [CrossRef]
- Aguirre, A.A.; Tabor, G.M. Introduction: Marine Vertebrates as Sentinels of Marine Ecosystem Health. EcoHealth 2004, 1, 236–238. [Google Scholar] [CrossRef]
- Bossart, G.D. Marine Mammals as Sentinel Species for Oceans and Human Health. Vet. Pathol. 2011, 48, 676–690. [Google Scholar] [CrossRef]
- Pace, A.; Dipineto, L.; Fioretti, A.; Hochscheid, S. Loggerhead sea turtles as sentinels in the western Mediterranean: Antibiotic resistance and environment-related modifications of Gram-negative bacteria. Mar. Pollut. Bull. 2019, 149, 110575. [Google Scholar] [CrossRef] [PubMed]
- Rapport, D.J. What Constitutes Ecosystem Health? Perspect. Biol. Med. 1989, 33, 120–132. [Google Scholar] [CrossRef]
- Barnosky, A.D.; Matzke, N.; Tomiya, S.; Wogan, G.O.U.; Swartz, B.; Quental, T.B.; Marshall, C.; McGuire, J.L.; Lindsey, E.L.; Maguire, K.C.; et al. Has the Earth’s sixth mass extinction already arrived? Nature 2011, 471, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Ceballos, G.; Ehrlich, P.R.; Raven, P.H. Vertebrates on the brink as indicators of biological annihilation and the sixth mass extinction. Proc. Natl. Acad. Sci. USA 2020, 117, 13596–13602. [Google Scholar] [CrossRef] [PubMed]
- Stanford, C.B.; Iverson, J.B.; Rhodin, A.G.J.; Paul van Dijk, P.; Mittermeier, R.A.; Kuchling, G.; Berry, K.H.; Bertolero, A.; Bjorndal, K.A.; Blanck, T.E.G.; et al. Turtles and Tortoises Are in Trouble. Curr. Biol. 2020, 30, R721–R735. [Google Scholar] [CrossRef] [PubMed]
- McCallum, M.L. Turtle biodiversity losses suggest coming sixth mass extinction. Biodivers. Conserv. 2021, 30, 1257–1275. [Google Scholar] [CrossRef]
- Li, C.; Fraser, N.C.; Rieppel, O.; Wu, X.-C. A Triassic stem turtle with an edentulous beak. Nature 2018, 560, 476–479. [Google Scholar] [CrossRef]
- Stroud, J.T.; Losos, J.B. Ecological Opportunity and Adaptive Radiation. Annu. Rev. Ecol. Evol. Syst. 2016, 47, 507–532. [Google Scholar] [CrossRef]
- Simões, T.R.; Vernygora, O.; Caldwell, M.W.; Pierce, S.E. Megaevolutionary dynamics and the timing of evolutionary innovation in reptiles. Nat. Commun. 2020, 11, 3322. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).