Females Are More Aggressive Than Males towards Same- and Opposite-Sex Intruders in the Blue Tit (Cyanistes caeruleus)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Field Methods
2.2. Simulated Territorial Intrusions
2.3. Statistical Methods
3. Results
3.1. Identification of Female and Male Behavioural Responses
3.2. Response to a Same- and Opposite-Sex Intruder
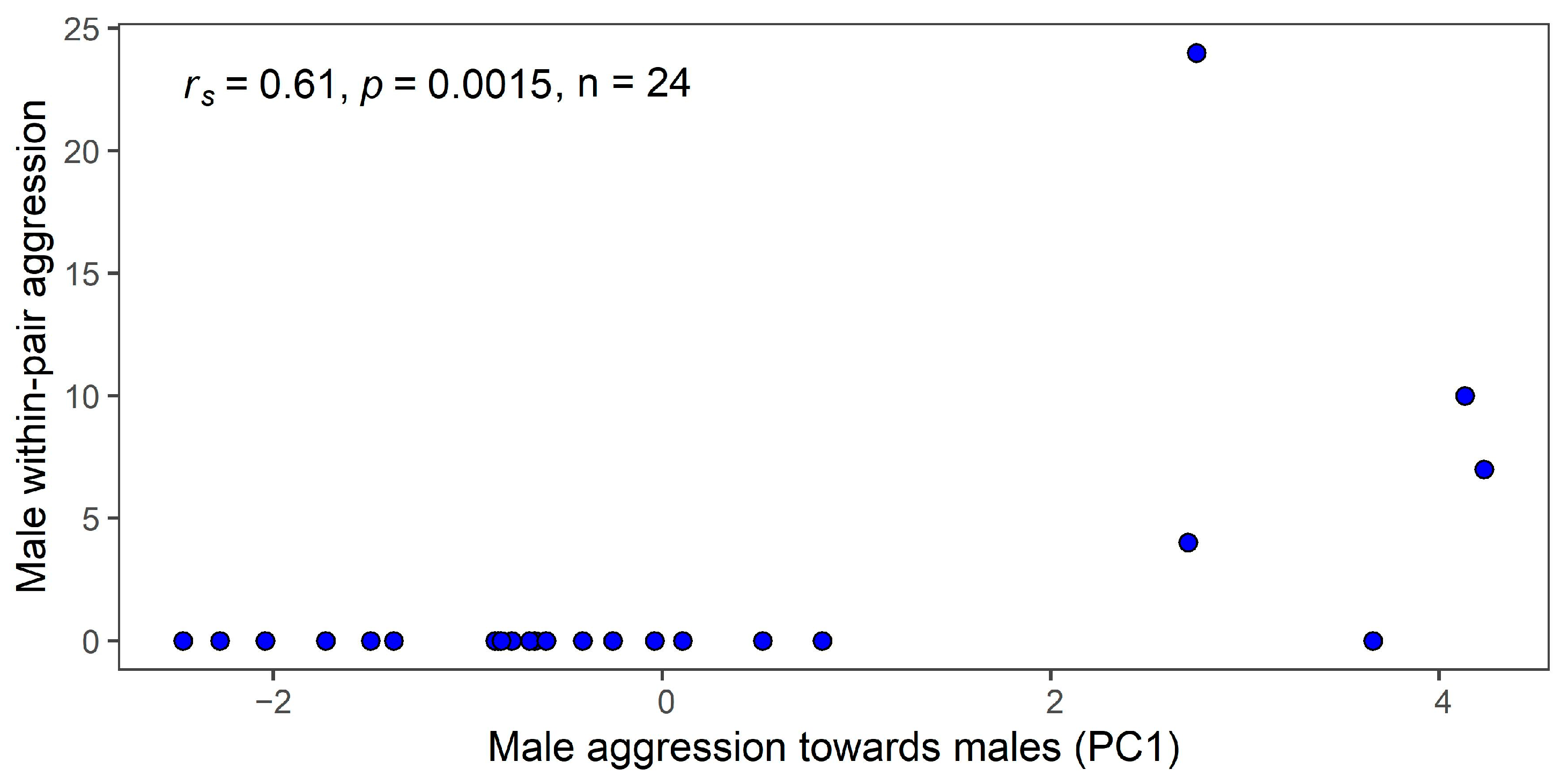
3.3. Within-Pair Aggression
3.4. Consistency in Same- and Opposite-Sex Aggression
3.5. Sex Differences in Aggressive Behaviour
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Female Decoy | Male Decoy | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable † | Sex | Mean ± SD | Min | Median | Max | Prop | Mean ± SD | Min | Median | Max | Prop |
| Pecks | Female | 17.58 ± 22.46 | 0 | 9 | 82 | 73.08 | 6.81 ± 7.47 | 0 | 5.5 | 24 | 73.08 |
| Male | 0.25 ± 0.68 | 0 | 0 | 3 | 16.67 | 12.54 ± 29.66 | 0 | 0 | 102 | 20.83 | |
| Time on decoy (s) | Female | 101.7 ± 75.25 | 0 | 91.5 | 226 | 96.15 | 84.15 ± 71.23 | 0 | 67.5 | 226 | 92.31 |
| Male | 19.08 ± 28.04 | 0 | 3.5 | 100 | 54.17 | 39.17 ± 71.87 | 0 | 0 | 218 | 41.67 | |
| Calls | Female | 1.65 ± 6.49 | 0 | 0 | 33 | 19.23 | 1.62 ± 5.73 | 0 | 0 | 29 | 23.08 |
| Male | 22.54 ± 25.18 | 0 | 14.5 | 90 | 75.00 | 24.79 | 0 | 23 | 61 | 83.33 | |
| Song | Female | 0 ± 0 | 0 | 0 | 0 | 0.00 | 0 ± 0 | 0 | 0 | 0 | 0.00 |
| Male | 0.04 ± 0.20 | 0 | 0 | 1 | 4.17 | 2.88 | 0 | 0 | 51 | 16.67 | |
| Minimum distance (m) | Female | 0.08 ± 0.39 | 0 | 0 | 2 | 96.15 | 0.08 ± 0.39 | 0 | 0 | 2 | 92.31 |
| Male | 0.72 ± 1.18 | 0 | 0.05 | 4 | 54.17 | 1.67 | 0 | 0.2 | 15 | 41.67 | |
| Time in entrance (s) | Female | 14.5 ± 23.23 | 0 | 1 | 98 | 50.00 | 14.73 ± 19.98 | 0 | 6.5 | 79 | 53.85 |
| Male | 7.83 ± 32.84 | 0 | 0 | 160 | 12.5 | 10.33 | 0 | 0 | 126 | 20.83 | |
| Time in nest box (s) | Female | 91.31 ± 110.44 | 0 | 50 | 297 | 61.54 | 107.4 ± 109.63 | 0 | 78.5 | 275 | 69.23 |
| Male | 0 ± 0 | 0 | 0 | 0 | 0.00 | 0 | 0 | 0 | 0 | 0.00 | |
| # on decoy | Female | 11.12 ± 10.48 | 0 | 9 | 42 | 96.15 | 9.58 ± 10.87 | 0 | 6.5 | 44 | 92.31 |
| Male | 3 ± 3.91 | 0 | 1 | 11 | 54.17 | 3.71 | 0 | 0 | 25 | 41.67 | |
| # in nest box | Female | 1.65 ± 2.58 | 0 | 1 | 12 | 61.54 | 1.5 ± 1.86 | 0 | 1 | 6 | 65.38 |
| Male | 0 ± 0 | 0 | 0 | 0 | 0.00 | 0 | 0 | 0 | 0 | 0.00 | |
| Decoy | Wilcoxon Signed Rank Test (Paired) | ||||
|---|---|---|---|---|---|
| Female (N = 26) | Male (N = 24) | ||||
| Variable † | Sex | Median | Median | V | Adjusted p-Value |
| Pecks | Female | 9 | 5.5 | 203 | 0.21 |
| Male | 0 | 0 | 10 | 1.0 | |
| Time on decoy | Female | 91.5 | 67.5 | 217 | 1.0 |
| Male | 3.5 | 0 | 47 | 1.0 | |
| Calls | Female | 0 | 0 | 30.5 | 1.0 |
| Male | 14.5 | 23 | 96.5 | 1.0 | |
| Song | Female | / | / | / | / |
| Male | 0 | 0 | 1.5 | 0.95 | |
| Minimum distance | Female | 0 | 0 | 2.5 | 1.0 |
| Male | 0.05 | 0.2 | 54.5 | 1.0 | |
| Time in entrance | Female | 1 | 6.5 | 69.5 | 1.0 |
| Male | 0 | 0 | 6 | 1.0 | |
| Time in nest box | Female | 50 | 78.5 | 85 | 1.0 |
| Male | / | / | / | / | |
| # on decoy | Female | 9 | 6.5 | 217.5 | 1.0 |
| Male | 1 | 0 | 66.5 | 1.0 | |
| # in nest box | Female | 1 | 1 | 74.5 | 1.0 |
| Male | / | / | / | / | |
| Sex | Mann–Whitney U Test | |||||
|---|---|---|---|---|---|---|
| Female (N = 26) | Male (N = 24) | |||||
| Variable † | Decoy | Median | Median | U | Adjusted p-Value | |
| Pecks | Female | 9 | 0 | 525 | 1.0 × 10−4 | *** |
| Male | 5.5 | 0 | 428 | 0.064 | ||
| Time on decoy | Female | 91.5 | 3.5 | 525.5 | 3.4 × 10−4 | *** |
| Male | 67.5 | 0 | 479.5 | 0.0059 | ** | |
| Calls | Female | 0 | 14.5 | 108.5 | 2.1 × 10−4 | *** |
| Male | 0 | 23 | 82 | 3.9 × 10−5 | *** | |
| Song | Female | 0 | 0 | 299 | 0.32 | |
| Male | 0 | 0 | 260 | 0.10 | ||
| Minimum distance | Female | 0 | 0.05 | 171 | 0.0034 | ** |
| Male | 0 | 0.2 | 149 | 0.0014 | ** | |
| Time in entrance | Female | 1 | 0 | 427.5 | 0.035 | * |
| Male | 6.5 | 0 | 408 | 0.10 | ||
| Time in nest box | Female | 50 | 0 | 504 | 1.0 × 10−4 | *** |
| Male | 78.5 | 0 | 528 | 2.0 × 10−5 | *** | |
| # on decoy | Female | 9 | 1 | 494 | 0.0034 | ** |
| Male | 6 | 0 | 483.5 | 0.0050 | ** | |
| # in nest box | Female | 1 | 0 | 504 | 1.0 × 10−4 | *** |
| Male | 1 | 0 | 516 | 4.0 × 10−5 | *** | |
References
- Rosvall, K.A. Sexual Selection on Aggressiveness in Females: Evidence from an Experimental Test with Tree Swallows. Anim. Behav. 2008, 75, 1603–1610. [Google Scholar] [CrossRef]
- Pandolfi, M.; Scaia, M.F.; Fernandez, M.P. Sexual Dimorphism in Aggression: Sex-Specific Fighting Strategies Across Species. Front. Behav. Neurosci. 2021, 15, 659615. [Google Scholar] [CrossRef]
- Cain, K.E.; Rich, M.S.; Ainsworth, K.; Ketterson, E.D. Two Sides of the Same Coin? Consistency in Aggression to Conspecifics and Predators in a Female Songbird. Ethology 2011, 117, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Slagsvold, T. Female-Female Aggression and Monogamy in Great Tits Parus major. Ornis Scand. Scand. J. Ornithol. 1993, 24, 155–158. [Google Scholar] [CrossRef]
- Petrie, M.; Kempenaers, B. Extra-Pair Paternity in Birds: Explaining Variation between Species and Populations. Trends Ecol. Evol. 1998, 13, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Cain, K.E.; Ketterson, E.D. Costs and Benefits of Competitive Traits in Females: Aggression, Maternal Care and Reproductive Success. PLoS ONE 2013, 8, e77816. [Google Scholar] [CrossRef]
- Stockley, P.; Campbell, A. Female Competition and Aggression: Interdisciplinary Perspectives. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130073. [Google Scholar] [CrossRef] [PubMed]
- Lawson, S.L.; Enos, J.K.; Antonson, N.D.; Gill, S.A.; Hauber, M.E. Chapter Two—Do Hosts of Avian Brood Parasites Discriminate Parasitic vs. Predatory Threats? A Meta-Analysis. In Advances in the Study of Behavior; Naguib, M., Barrett, L., Healy, S.D., Podos, J., Simmons, L.W., Zuk, M., Eds.; Academic Press: Cambridge, MA, USA, 2021; Volume 53, pp. 63–95. [Google Scholar]
- Appleby, B.M.; Yamaguchi, N.; Johnson, P.J.; Macdonald, D.W. Sex-Specific Territorial Responses in Tawny Owls Strix aluco. Ibis 1999, 141, 91–99. [Google Scholar] [CrossRef]
- Kempenaers, B.; Verheyen, G.R.; den Broeck, M.V.; Burke, T.; Broeckhoven, C.V.; Dhondt, A. Extra-Pair Paternity Results from Female Preference for High-Quality Males in the Blue Tit. Nature 1992, 357, 494–496. [Google Scholar] [CrossRef]
- Lipshutz, S.E.; Rosvall, K.A. Nesting Strategy Shapes Territorial Aggression but Not Testosterone: A Comparative Approach in Female and Male Birds. Horm. Behav. 2021, 133, 104995. [Google Scholar] [CrossRef]
- Mays, H.L.; Hopper, K.R. Differential Responses of Yellow-Breasted Chats, Icteria virens, to Male and Female Conspecific Model Presentations. Anim. Behav. 2004, 67, 21–26. [Google Scholar] [CrossRef]
- Pärn, H.; Lindström, K.M.; Sandell, M.; Amundsen, T. Female Aggressive Response and Hormonal Correlates—An Intrusion Experiment in a Free-Living Passerine. Behav. Ecol. Sociobiol. 2008, 62, 1665–1677. [Google Scholar] [CrossRef]
- Sandell, M.I. Female Aggression and the Maintenance of Monogamy: Female Behaviour Predicts Male Mating Status in European Starlings. Proc. R. Soc. Lond. B Biol. Sci. 1998, 265, 1307–1311. [Google Scholar] [CrossRef]
- Schlicht, E.; Kempenaers, B. Origin and Outcome of Social Polygyny in the Blue Tit. Ardea 2021, 109, 91–118. [Google Scholar] [CrossRef]
- Van Dongen, W.F.D. Mate Guarding and Territorial Aggression Vary with Breeding Synchrony in Golden Whistlers (Pachycephala pectoralis). Naturwissenschaften 2008, 95, 537–545. [Google Scholar] [CrossRef]
- Gowaty, P.A. Aggression of Breeding Eastern Bluebirds (Sialia sialis) toward Their Mates and Models of Intra-and Interspecific Intruders. Anim. Behav. 1981, 29, 1013–1027. [Google Scholar] [CrossRef]
- Kempenaers, B. Polygyny in the Blue Tit: Intra- and Inter-Sexual Conflicts. Anim. Behav. 1995, 49, 1047–1064. [Google Scholar] [CrossRef]
- Lamers, K.P.; Nicolaus, M.; Rakhimberdiev, E.; Nilsson, J.-Å.; Both, C. Descriptive and Experimental Evidence for Timing-Mediated Polygyny Risk in a Pied Flycatcher Ficedula hypoleuca Population. J. Avian Biol. 2020, 51, e02190. [Google Scholar] [CrossRef]
- Santoro, S.; Fernández-Díaz, P.; Canal, D.; Camacho, C.; Garamszegi, L.Z.; Martínez-Padilla, J.; Potti, J. High Frequency of Social Polygyny Reveals Little Costs for Females in a Songbird. Sci. Rep. 2022, 12, 277. [Google Scholar] [CrossRef]
- Kempenaers, B. Polygyny in the Blue Tit: Unbalanced Sex Ratio and Female Aggression Restrict Mate Choice. Anim. Behav. 1994, 47, 943–957. [Google Scholar] [CrossRef]
- Pinxten, R.; Eens, M. Polygyny in the European Starling: Effect on Female Reproductive Success. Anim. Behav. 1990, 40, 1035–1047. [Google Scholar] [CrossRef]
- Searcy, W.A. Are Female Red-Winged Blackbirds Territorial? Anim. Behav. 1986, 34, 1381–1391. [Google Scholar] [CrossRef]
- Naďo, L.; Kašová, M.; Krištín, A.; Kaňuch, P. Cooperative Nest-Defence Behaviour and Territory Quality in a Resident and Socially Monogamous Passerine. Ethology 2018, 124, 514–526. [Google Scholar] [CrossRef]
- Thys, B.; Pinxten, R.; Raap, T.; De Meester, G.; Rivera-Gutierrez, H.F.; Eens, M. The Female Perspective of Personality in a Wild Songbird: Repeatable Aggressiveness Relates to Exploration Behaviour. Sci. Rep. 2017, 7, 7656. [Google Scholar] [CrossRef]
- Van Staaden, M.J.; Searcy, W.A.; Hanlon, R.T. Chapter 3—Signaling Aggression. In Advances in Genetics; Huber, R., Bannasch, D.L., Brennan, P., Eds.; Aggression; Academic Press: San Diego, CA, USA, 2011; Volume 75, pp. 23–49. [Google Scholar]
- Briffa, M.; Sneddon, L.U.; Wilson, A.J. Animal Personality as a Cause and Consequence of Contest Behaviour. Biol. Lett. 2015, 11, 20141007. [Google Scholar] [CrossRef]
- Réale, D.; Reader, S.M.; Sol, D.; McDougall, P.T.; Dingemanse, N.J. Integrating Animal Temperament within Ecology and Evolution. Biol. Rev. Camb. Philos. Soc. 2007, 82, 291–318. [Google Scholar] [CrossRef]
- Caramaschi, D.; de Boer, S.F.; de Vries, H.; Koolhaas, J.M. Development of Violence in Mice through Repeated Victory along with Changes in Prefrontal Cortex Neurochemistry. Behav. Brain Res. 2008, 189, 263–272. [Google Scholar] [CrossRef]
- Palanza, P.; Rodgers, R.J.; Ferrari, P.F.; Parmigiani, S. Effects of Chlordiazepoxide on Maternal Aggression in Mice Depend on Experience of Resident and Sex of Intruder. Pharmacol. Biochem. Behav. 1996, 54, 175–182. [Google Scholar] [CrossRef]
- Rosvall, K.A. Intrasexual Competition in Females: Evidence for Sexual Selection? Behav. Ecol. 2011, 22, 1131–1140. [Google Scholar] [CrossRef]
- Colquhoun, M.K. Notes on the Social Behaviour of Blue Tits. Br. Birds 1942, 35, 234–240. [Google Scholar]
- Kempenaers, B.; Verheyen, G.R.; Dhondt, A.A. Mate Guarding and Copulation Behaviour in Monogamous and Polygynous Blue Tits: Do Males Follow a Best-of-a-Bad-Job Strategy? Behav. Ecol. Sociobiol. 1995, 36, 33–42. [Google Scholar] [CrossRef]
- Velasco, A.C.; Ferrer, E.S.; Sanz, J.J. Conspecific Aggression Strategies Are Conditioned by Environmental, Social and Intrinsic Variables in Breeding Blue Tits Cyanistes caeruleus. Behaviour 2021, 159, 133–169. [Google Scholar] [CrossRef]
- Kempenaers, B.; Dhondt, A.A. Competition between Blue and Great Tit for Roosting Sites in Winter: An Aviary Experiment. Ornis Scand. 1991, 22, 73. [Google Scholar] [CrossRef]
- Minot, E.O.; Perrins, C.M. Interspecific Interference Competition--Nest Sites for Blue and Great Tits. J. Anim. Ecol. 1986, 55, 331–350. [Google Scholar] [CrossRef]
- Sun, J.; Raap, T.; Pinxten, R.; Eens, M. Artificial Light at Night Affects Sleep Behaviour Differently in Two Closely Related Songbird Species. Environ. Pollut. 2017, 231, 882–889. [Google Scholar] [CrossRef]
- Stenning, M. The Blue Tit; Bloomsbury Publishing: London, UK, 2018; ISBN 978-1-4729-5480-0. [Google Scholar]
- Dauwe, T.; Janssens, E.; Pinxten, R.; Eens, M. The Reproductive Success and Quality of Blue Tits (Parus caeruleus) in a Heavy Metal Pollution Gradient. Environ. Pollut. 2005, 136, 243–251. [Google Scholar] [CrossRef]
- Bijnens, L.; Dhondt, A. Vocalizations in a Belgian Blue Tit Parus c. caeruleus Population. Le Gerfaut 1984, 74, 243–269. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Revelle, W. Psych: Procedures for Psychological, Psychometric, and Personality Research. Northwestern University: Evanston, IL, USA, 2022. [Google Scholar]
- Holm, S. A Simple Sequentially Rejective Multiple Test Procedure. Scand. J. Stat. 1979, 6, 65–70. [Google Scholar]
- Sierro, J.; de Kort, S.R.; Riebel, K.; Hartley, I.R. Female Blue Tits Sing Frequently: A Sex Comparison of Occurrence, Context, and Structure of Song. Behav. Ecol. 2022, 33, 912–925. [Google Scholar] [CrossRef]
- Rogers, A.C.; Langmore, N.E.; Mulder, R.A. Function of Pair Duets in the Eastern Whipbird: Cooperative Defense or Sexual Conflict? Behav. Ecol. 2007, 18, 182–188. [Google Scholar] [CrossRef]
- Cain, K.E.; Cockburn, A.; Langmore, N.E. Female Song Rates in Response to Simulated Intruder Are Positively Related to Reproductive Success. Front. Ecol. Evol. 2015, 3, 119. [Google Scholar] [CrossRef]
- Duckworth, R.A. Behavioral Correlations across Breeding Contexts Provide a Mechanism for a Cost of Aggression. Behav. Ecol. 2006, 17, 1011–1019. [Google Scholar] [CrossRef]
- Réale, D.; Garant, D.; Humphries, M.M.; Bergeron, P.; Careau, V.; Montiglio, P.-O. Personality and the Emergence of the Pace-of-Life Syndrome Concept at the Population Level. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 4051–4063. [Google Scholar] [CrossRef]
- Barrientos, R.; Bueno-Enciso, J.; Serrano-Davies, E.; Sanz, J.J. Facultative Interspecific Brood Parasitism in Tits: A Last Resort to Coping with Nest-Hole Shortage. Behav. Ecol. Sociobiol. 2015, 69, 1603–1615. [Google Scholar] [CrossRef]
- Potti, J.; Camacho, C.; Canal, D.; Martínez-Padilla, J. Three Decades of Crimes and Misdemeanours in the Nest Box Life of European Pied Flycatchers Ficedula hypoleuca. Ardeola 2021, 68, 315–333. [Google Scholar] [CrossRef]
- Vedder, O.; Komdeur, J.; Van Der Velde, M.; Magrath, M.J.L. Conclusive Evidence for Conspecific Brood Parasitism in the Blue Tit Cyanistes caeruleus: A Reply to Griffith et Al. J. Avian Biol. 2010, 41, 348–349. [Google Scholar] [CrossRef]
- Slagsvold, T.; Wiebe, K.L. Interspecific Aggression and Defence of Extra Nest Sites in Two Species of Songbirds. Ethology 2021, 127, 294–301. [Google Scholar] [CrossRef]
- Hansen, B.T.; Slagsvold, T. Rival Imprinting: Interspecifically Cross-Fostered Tits Defend Their Territories against Heterospecific Intruders. Anim. Behav. 2003, 65, 1117–1123. [Google Scholar] [CrossRef]



| Female (n = 26) | |||
|---|---|---|---|
| PC1 | PC2 | PC3 | |
| Eigenvalue | 3.24 | 1.98 | 1.02 |
| Variance % | 40.53 | 24.76 | 12.80 |
| Variable | Loadings | Loadings | Loadings |
| Pecks | 0.45 | 0.064 | 0.18 |
| Time on decoy | 0.50 | 0.087 | −0.18 |
| Calls | −0.072 | 0.40 | 0.59 |
| Minimum distance | 0.30 | −0.34 | −0.47 |
| Time in entrance | 0.41 | 0.037 | 0.30 |
| Time in nest box | −0.14 | −0.62 | 0.24 |
| Decoy frequency | 0.50 | 0.052 | 0.092 |
| Nest-box frequency | 0.12 | −0.57 | 0.46 |
| Male (n = 24) | |||
|---|---|---|---|
| PC1 | PC2 | PC3 | |
| Eigenvalue | 2.60 | 1.11 | 1.06 |
| Variance % | 43.25 | 18.55 | 17.74 |
| Variable | Loadings | Loadings | Loadings |
| Pecks | 0.50 | 0.35 | 0.14 |
| Time on decoy | 0.57 | 0.0035 | 0.015 |
| Calls | −0.12 | 0.19 | 0.88 |
| Song | −0.15 | 0.73 | −0.42 |
| Minimum distance | 0.41 | −0.46 | 0.15 |
| Time in entrance | 0.48 | 0.31 | 0.048 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boiten, G.; van Iersel, R.; Pinxten, R.; Eens, M. Females Are More Aggressive Than Males towards Same- and Opposite-Sex Intruders in the Blue Tit (Cyanistes caeruleus). Animals 2023, 13, 585. https://doi.org/10.3390/ani13040585
Boiten G, van Iersel R, Pinxten R, Eens M. Females Are More Aggressive Than Males towards Same- and Opposite-Sex Intruders in the Blue Tit (Cyanistes caeruleus). Animals. 2023; 13(4):585. https://doi.org/10.3390/ani13040585
Chicago/Turabian StyleBoiten, Gust, Robin van Iersel, Rianne Pinxten, and Marcel Eens. 2023. "Females Are More Aggressive Than Males towards Same- and Opposite-Sex Intruders in the Blue Tit (Cyanistes caeruleus)" Animals 13, no. 4: 585. https://doi.org/10.3390/ani13040585





