Simple Summary
Maternal nutrient supply during the periconceptual period has long-term effects on fetal development and tissue function. During pregnancy, the fetus relies on the dam for its nutrient supply. Our objective was to ascertain whether the periconceptual and early life plan of nutrition could affect fetal liver development and gene expression profiles. To this end, we investigated the impacts of maternal vitamin and mineral supplementation (from pre-breeding to day 83) and two rates of body-weight gain during the first 83 days of pregnancy in female fetuses of crossbred Angus beef heifers. We identified 591 unique differentially expressed genes across all six vitamin-gain contrasts. Over-represented pathways were related to energy metabolism, lipid metabolism, and mineral and amino acid transport. Our findings suggest that periconceptual maternal nutrition affects fetal hepatic function through altered expression of energy- and lipid-related genes.
Abstract
During pregnancy, the fetus relies on the dam for its nutrient supply. Nutritional stimuli during fetal organ development can program hepatic metabolism and function. Herein, we investigated the role of vitamin and mineral supplementation (VTM or NoVTM—at least 71 days pre-breeding to day 83 of gestation) and rate of weight gain (low (LG) or moderate (MG)—from breeding to day 83) on the fetal liver transcriptome and the underlying biological pathways. Crossbred Angus beef heifers (n = 35) were randomly assigned to one of four treatments in a 2 × 2 factorial design (VTM_LG, VTM_MG, NoVTM_LG, and NoVTM_MG). Gene expression was measured with RNA-Seq in fetal livers collected on day 83 ± 0.27 of gestation. Our results show that vitamin and mineral supplementation and rate of weight gain led to the differential expression of hepatic genes in all treatments. We identified 591 unique differentially expressed genes across all six VTM-gain contrasts (FDR ≤ 0.1). Over-represented pathways were related to energy metabolism, including PPAR and PI3K-Akt signaling pathways, as well as lipid metabolism, mineral transport, and amino acid transport. Our findings suggest that periconceptual maternal nutrition affects fetal hepatic function through altered expression of energy- and lipid-related genes.
1. Introduction
From humans to livestock, maternal nutrition has long been recognized as essential for pregnancy success and fetal development [1,2]. During pregnancy, a complex and coordinated array of physiological and metabolic changes occur to support the dam’s metabolism and the growing fetus [3,4]. Likewise, there are critical periods of development in which environmental stimuli lead to changes in embryonic genome expression and programming [2,3,5]. Once programmed, changes in early life may have long-lasting effects in adulthood and may even be transmitted to subsequent generations [6,7]. During this period, nutrient imbalances induce both responsive and adaptive changes in fetal development [8], which may have detrimental or beneficial effects on organ structures and functions, leading to altered metabolism later in life [5,9,10].
Several major events occur during early gestation, including organogenesis [4,10]. Compelling evidence has shown that organs are programmed in utero for postnatal growth and adult function [5,10]. The liver is formed during early gestation and plays a central role in maintaining metabolic homeostasis [11,12,13]. The developing fetal liver is modulated by several biological mechanisms, including gene expression [11]. Through nutritional cues, nutrient-sensing pathways lead to the activation or repression of genes that, in turn, regulate cellular growth and differentiation [8,11]. Although fetal nutritional requirements during early gestation are negligible compared to maternal needs [14], there are likely transitory increases in specific nutrients essential for optimal embryonic and early fetal development [10]. Thus, specific nutrient-supplementation programs during early gestation provide opportunities to offset deficiencies and improve nutrient supply to enhance the fitness of offspring [10,15].
A growing number of studies using ewes and cows have highlighted the effects of maternal nutrition during mid- to late gestation in fetal liver development and function [16,17,18,19]. Prezotto et al. reported increased liver weight in fetuses from nutrient-restricted and re-alimented beef cows compared to the control group [20]. Similarly, maternal nutrient restriction in ewes affected fetal liver morphology, gene expression, and lipid metabolism [16]. Recently, more attention has been directed toward the periconceptual period [19,21,22]. Previously, we have shown that global nutrient restriction in beef heifers from breeding to day 50 of gestation negatively affected fetal liver development and function [21,23]. Altered hepatic gene expression and organ development were reported in response to maternal periconceptual protein restriction in beef heifers [19]. These studies reinforce the roles of macronutrients and maternal diet in programming fetal development and function during gestation. Despite the importance of micronutrients, such as vitamins and minerals, in metabolism, there is still a lack of information regarding their effects on fetal development.
Vitamins and minerals are required in small quantities and are essential in key metabolic processes, such as energy metabolism, DNA synthesis, and gene-expression regulation [3,24,25]. Despite their importance in embryonic and fetal development (reviewed in [25,26,27]), cattle producers still have not widely adopted vitamin and mineral supplementation strategies [28]. Likewise, their role in fetal development during the periconceptual period and early gestation have still to be examined. Thus, our research group developed a model to investigate the role of vitamin and mineral supplementation (VTM or NoVTM—at least 60 days pre-breeding to day 83 of gestation) and two different rates of weight gain (low (LG) or moderate (MG)—from breeding to day 83) on maternal and fetal outcomes [29]. We have shown that nutritional management affects maternal and fetal development [30]. Fetuses from VTM-supplemented dams had increased levels of some trace minerals [30], while managing dams for low body-weight gain led to increased fetal liver size [29]. These changes were likely mediated by the differential expression and regulation of nutrient-transport genes in the placenta [31,32]. Furthermore, changes in nutrient availability affected the fetal liver metabolome, regarding which Crouse et al. reported that the energy metabolism superpathway was differentially affected by the main effect of VTM [33].
To further expand on the previous findings, the current study is based on the hypothesis that periconceptual maternal vitamin and mineral supplementation and moderate rates of weight gain would affect fetal metabolism through differential transcript abundance of genes associated with hepatic metabolism and function. Therefore, we aimed to identify differentially expressed genes (DEGs) in fetal livers, biological processes (BPs), and pathways underlying hepatic function and metabolism in response to periconceptual maternal nutrition. Herein, we report that maternal vitamin and mineral supplementation and rate of body-weight gain in heifers during the periconceptual and early gestation periods affected fetal liver gene expression. These genes regulate pathways such as energy and lipid metabolism and nutrient transport.
2. Materials and Methods
2.1. Ethics Statement
All animal experiments followed the relevant guidelines and regulations. The experimental design, animal management, and tissue collection were approved by the North Dakota State University Institutional Animal Care and Use Committee (IACUC #A19012).
2.2. Animal Model, Experimental Design, and Tissue Collection
The development of the experimental model and study design was previously reported by Menezes et al. [29] and McCarthy et al. [30]. In brief, crossbred Angus beef heifers with average initial body weights = 359.5 ± 7.1 kg were randomly assigned to 1 of 4 treatments in a 2 × 2 factorial arrangement. The factors examined included vitamin and mineral supplementation (VTM or NoVTM) and rate of weight gain (low gain (LG—0.28 kg/day) or moderate gain (MG—0.79 kg/day)). The nutritional management and diet composition per treatment were previously reported elsewhere [29,30]. In brief, the diet consisted of triticale hay, corn silage, modified distiller’s grains plus solubles, ground corn, and, if indicated by treatment, VTM premix. Diets were delivered once daily via a total mixed ration (TMR) in an electronic head-gate facility (American Calan; Northwood, NH, USA). Heifers on the LG treatment were maintained on the basal TMR, whereas heifers under MG were supplemented with a blend of ground corn, dried distiller’s grains plus solubles, wheat midds, fish oil, urea, and ethoxyquin top dressed on the TMR at 0.58% of BW as-fed daily. Diet compositions are described in the Supplementary Materials (Table S1). Heifers were weighed weekly, and feed intake was adjusted during the study to achieve the targeted body-weight gains.
The VTM treatment began at a minimum of 71 days before artificial insemination (AI) by supplementing the heifers with 113 g·heifer−1·d−1 of a pelleted mineral premix (Purina® Wind & Rain Storm All-Season 7.5 Complete). The VTM supplement provided macro- (calcium, magnesium, phosphorus, potassium, and sodium) and trace minerals (cobalt, copper, iodine, manganese, selenium, and zinc) and vitamins A, D, and E to meet 110% [29] of the requirements, considering the heifers’ physiological requirements [34]. Heifers in the NoVTM group received a pelleted carrier product fed at 0.45 kg/heifer/day with no added vitamins or minerals.
Heifers were estrus-synchronized [35] and bred by AI using female-sexed semen from a single sire. Pregnancy diagnosis was performed 35 days after AI, and fetal sex was determined on day 65 using transrectal ultrasonography [31,36]. At AI and within VTM groups, heifers were assigned to one of two rates of gain—LG or MG. Thus, the arrangements of treatments were as follows: (1) no vitamin and mineral supplementation and low gain (NoVTM_LG, n = 9); (2) vitamin and mineral supplementation and low gain (VTM_LG, n = 9); (3) no vitamin and mineral supplementation and moderate gain (NoVTM_MG, n = 9); and (4) vitamin and mineral supplementation and moderate gain (VTM_MG, n = 8).
The treatments continued until day 83 ± 0.27 of gestation. We focused this study on the end of the first trimester of gestation because in this period the placenta is still growing exponentially [37]. Furthermore, fetal organogenesis has already finished and secondary myogenesis is beginning [38]. The fetus was removed through ovariohysterectomy [39] and dissected as previously described [21]. The fetal liver was weighed, and an aliquot was snap-frozen on dry ice and stored at −80 °C.
2.3. RNA Isolation, Library Construction, Sequencing, and Data Processing
Hepatic total RNA was isolated using the RNeasy Plus Universal Mini Kit (Qiagen®, Germantown, MA, USA), according to the manufacturer’s protocol. The Agilent 2100 Bioanalyzer and agarose gel electrophoresis were used for sample integrity and purity analyses. Based on these, 31 samples (n = 8 per group, except VTM_MG—n = 7) met the quantity and quality parameters for library preparation. Library preparation and sequencing were carried out by Novogene Co., Ltd. (Nanjing, China). Strand-specific RNA libraries were prepared using the NEBNext® Ultra™ II Directional RNA Library Prep Kit for Illumina (New England BioLabs®, Ipswich, MA, USA). Libraries were sequenced on the Illumina® NovaSeq 6000 platform at 150 bp reads and a depth of 20 M reads/sample.
Data cleaning was based on filtering out sequencing adaptors and reads with a PhredScore lower than 30. Quality-control and read statistics were estimated with FastQC v0.11.8 [40]. The FastQC reports were aggregated by MultiQC v1.9 [41]. Cleaned reads were mapped to the Bos taurus reference genome (ARS-UCD 1.2) [42] using the STAR aligner v. 2.7.3a [43] and the gene annotation file (release 100) from the Ensembl database. MultiQC, NOISeq v.2.26.0 [44], and edgeR v.3.24.3 [45] were used for post-mapping quality control.
2.4. Differential Gene Expression, k-Means Clustering, and Functional Over-Representation Analyses
Read counts from STAR were merged and an expression dataset was created using edgeR. Based on the edgeR filterByExpr function [46], genes not expressed or lowly expressed were filtered out. Differential gene expression analysis was carried out using edgeR. This approach applies generalized linear models (GLMs) based on the negative binomial distribution while accounting for normalization factors for different library sizes [45]. To make all pairwise comparisons between the four treatment groups, six contrasts were created as follows: (1) VTM_MG vs. NoVTM_LG, (2) VTM_MG vs. VTM_LG, (3) VTM_MG vs. NoVTM_MG, (4) VTM_LG vs. NoVTM_LG, (5) VTM_LG vs. NoVTM_MG, and (6) NoVTM_MG vs. NoVTM_LG. Differentially expressed genes (DEGs) were identified after multiple-testing correction of the p-values based on the Benjamini–Hochberg methodology (FDR ≤ 0.1).
To visualize the intersections among different lists of DEGs, we used UpSet plots created using the UpSetR v.1.4.0 package [47]. To identify over-represented biological processes and pathways underlying the DEGs, we used the ShinyGo v.0.76.2 web tool [48]. Significant results were identified after p-value multiple-testing correction (FDR ≤ 0.05).
We investigated the expression patterns of genes with similar behavior to identify biological processes involved with tissue development and function. Genes with less than 1 CPM in at least 50% of the samples were filtered out. The data were normalized through the variance-stabilizing transformation (VST) and adjusted for the effect of treatment using the removebatcheffect function in Limma [49]. Normalized genes were sorted by the standard deviations (SDs). The top 2,000 genes were then selected (SD ≥ 0.2) for k-means clustering analysis using the iDEP v.96 online tool [50]. Genes were mean-centered and then hierarchically clustered using Pearson’s correlation as distance metrics. Data visualization and cluster functional analysis were performed in iDEP.
3. Results
3.1. Fetal Liver Growth and Mineral Concentration Is Affected by Maternal Diet
The effects of the diets on dam and fetal development were previously reported [29,30]. Briefly, the VTM treatment did not affect the performance or dry-matter intake of the dams. However, the rate of gain (LG vs. MG) led to increased dam body weight at day 83 [29]. Although most fetal morphometric traits were not affected by maternal diet, VTM supplementation resulted in greater fetal liver and intestine weights [29]. Likewise, fetuses from VTM-supplemented dams showed greater concentrations of Se, Cu, and Co in the liver (p ≤ 0.05) [30]. Conversely, Mo and Co had greater concentrations in the livers of fetuses from LG dams (p ≤ 0.05) [30].
3.2. Maternal Diet Affects Fetal Liver Gene Expression
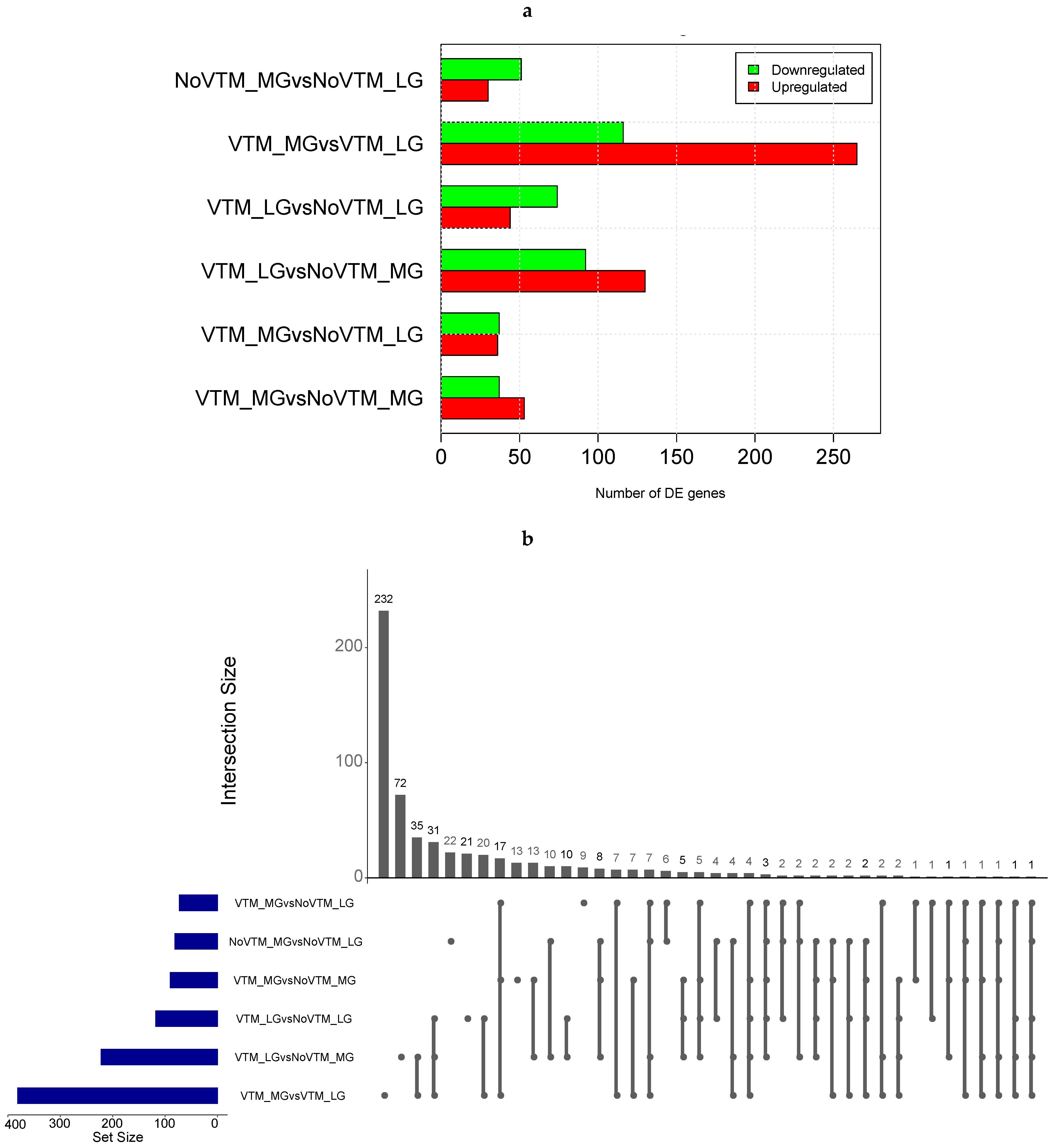
We profiled the liver transcriptomes of 31 female fetuses at day 83 of gestation from heifers receiving one of four treatments comprising the main effects of vitamin and mineral supplementation (VTM or NoVTM) and rates of weight gain (LG or MG). We generated, on average, 21.45 M of cleaned reads per sample (range from 19.9 to 27.0 M). After QC, sequencing reads were mapped to the ARS-UCD 1.2 reference genome using STAR software. On average, 94.5% of the total reads were uniquely mapped (Supplementary Table S2). After filtering for not-expressed or lowly expressed genes, 14,143 genes were used for differential expression analysis. We identified 591 DEGs across all six contrasts (FDR ≤ 0.1) (Figure 1a). Among them, we identified 4 genes from the ABC transporter family (ABCA6, ABCC2, ABCA1, and ABCB11) and 20 from the SLC transporter family, including SLC27A6, SLC2A2, and SLC2A4, most of them upregulated in the VTM-supplemented groups.
Figure 1.
Differential gene expression in the livers of fetuses from heifers receiving or not receiving vitamin–mineral supplementation (VTM or NoVTM) and fed to achieve different rates of gain (low gain (LG) or moderate gain (MG)) during early gestation. (a) Number of differentially expressed genes (DEGs) across contrasts. (b) The UpSet plot represents the intersection between the sets of DEGs from different contrasts. Each vertical bar shows the number of genes in the intersection. The dot plot reports the set participation in the intersection, and the horizontal bar graph reports the set sizes (totals of DEGs). The treatments were arranged as follows: NoVTM_LG—no vitamin and mineral supplementation and low gain; VTM_LG—vitamin and mineral supplementation and low gain; NoVTM_MG—no vitamin and mineral supplementation and moderate gain; and VTM_MG—vitamin and mineral supplementation and moderate gain.
The comparison yielding the greatest number of DEGs was for heifers receiving the vitamin and mineral supplementation but managed at different rates of gain. In this comparison, we found 381 DEGs, with 265 up- and 116 downregulated, in the VTM_MG group compared with the VTM_LG group. We identified selenium-related—SELENOI and SEPSECS—and zinc-related genes—MT1E—upregulated in the VTM_MG group. In the LG group, VTM supplementation resulted in the up- and downregulation of 44 and 74 genes, respectively. The numbers of DEGs overlapping the main effects of VTM or rate of gain (LG, MG) are shown in Figure 1b. The greatest numbers of shared genes were observed between VTM_LG and NoVTM_LG and between VTM_MG and VTM_LG (n = 65). Furthermore, we identified bta-mir-2887-1 as being downregulated in four of the contrasts (VTM_LG vs. NoVTM_LG, VTM_LG vs. NoVTM_MG, VTM_MG vs. NoVTM_LG, and VTM_MG vs. NoVTM_MG). Likewise, bta-mir-122 was upregulated in the VTM_LG vs. NoVTM_MG contrast and downregulated in VTM_MG vs. VTM_LG. The DEGs for each comparison with the fold-change values and the overlapping genes are reported in Supplementary Tables S3–S9.
3.3. Hepatic Metabolic Pathways, Mineral Absorption, and Amino Acid-Transport Genes Are Affected by Maternal Diet
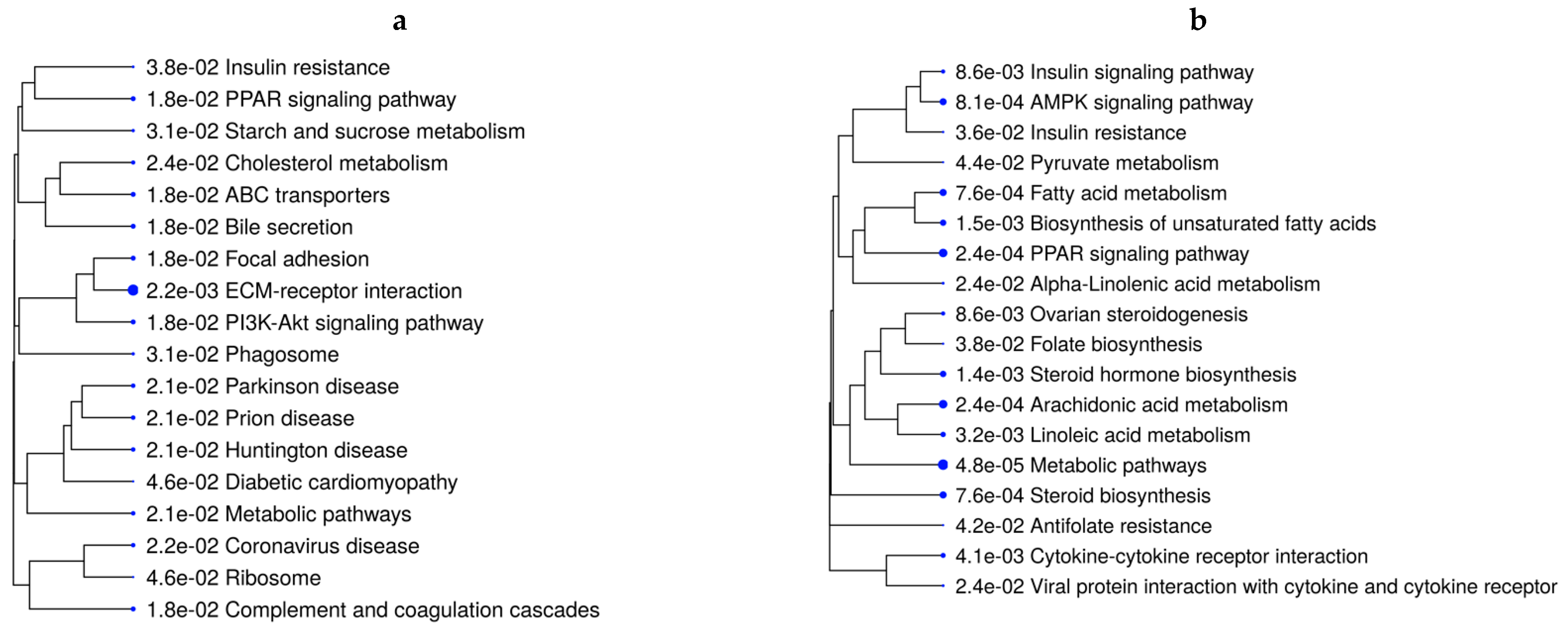
The over-representation analysis of the DEGs to identify pathways retrieved 67 significant KEGG pathways across all contrasts (44 unique pathways) (FDR ≤ 0.05). Supplementary Table S10 reports all unique and overlapping pathways across comparisons based on ShinyGo analysis. Interestingly, the mineral-absorption pathway was over-represented in all VTM-supplemented groups, regardless of the rate of gain, in comparison with no VTM supplementation. Genes in this pathway included MT1A, MT1E, and MT2A, which were upregulated in fetuses receiving VTM supplementation (Table S9). In addition to metabolic pathways over-represented in five of the comparisons, lipid- and energy-metabolism-related pathways, including PPAR signaling, PI3K-Akt signaling, AMPK signaling, and insulin resistance, were significant (Table S10). Figure 2a,b show the pathways over-represented by DEGs in response to the rate of gain for each vitamin–mineral supplement condition (VTM_MG vs. VTM_LG and NoVTM_MG vs. NoVTM_LG). Interestingly, lipid-related genes, including LPL, ACSS2, FADS2, FADS1, and FASN, were downregulated in the MG groups, regardless of the VTM treatment. Furthermore, the genes INSIG1, SLC2A4, and SREBF1 were downregulated in the NoVTM_MG vs. NoVTM_LG contrast. On the other hand, PPARG and PPARD were up- and downregulated, respectively, in VTM_MG vs. VTM_LG. Several unique pathways were over-represented by the DEGs from the NoVTM_MG vs. NoVTM_LG comparison (Figure 2a), including pyruvate metabolism, linoleic acid metabolism, and folate biosynthesis. The KEGG pathways and biological processes (BPs) identified for each of the comparisons, underlying genes, and significance values are provided in Tables S3–S9.
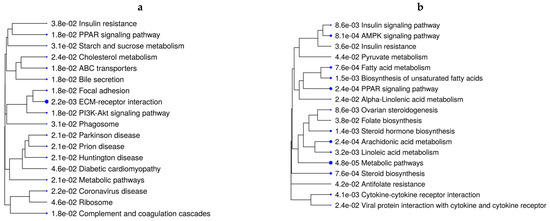
Figure 2.
Functional over-representation analysis of differentially expressed genes (DEGs) from fetal liver tissues. KEGG pathways over-represented are based on the DEGs of VTM_MG vs. VTM_LG (a) and NoVTM_MG vs. NoVTM_LG (b) comparisons. The terms are hierarchically arranged based on functional similarity. The bigger the blue dot, the more significant the term is (FDR ≤ 0.05).
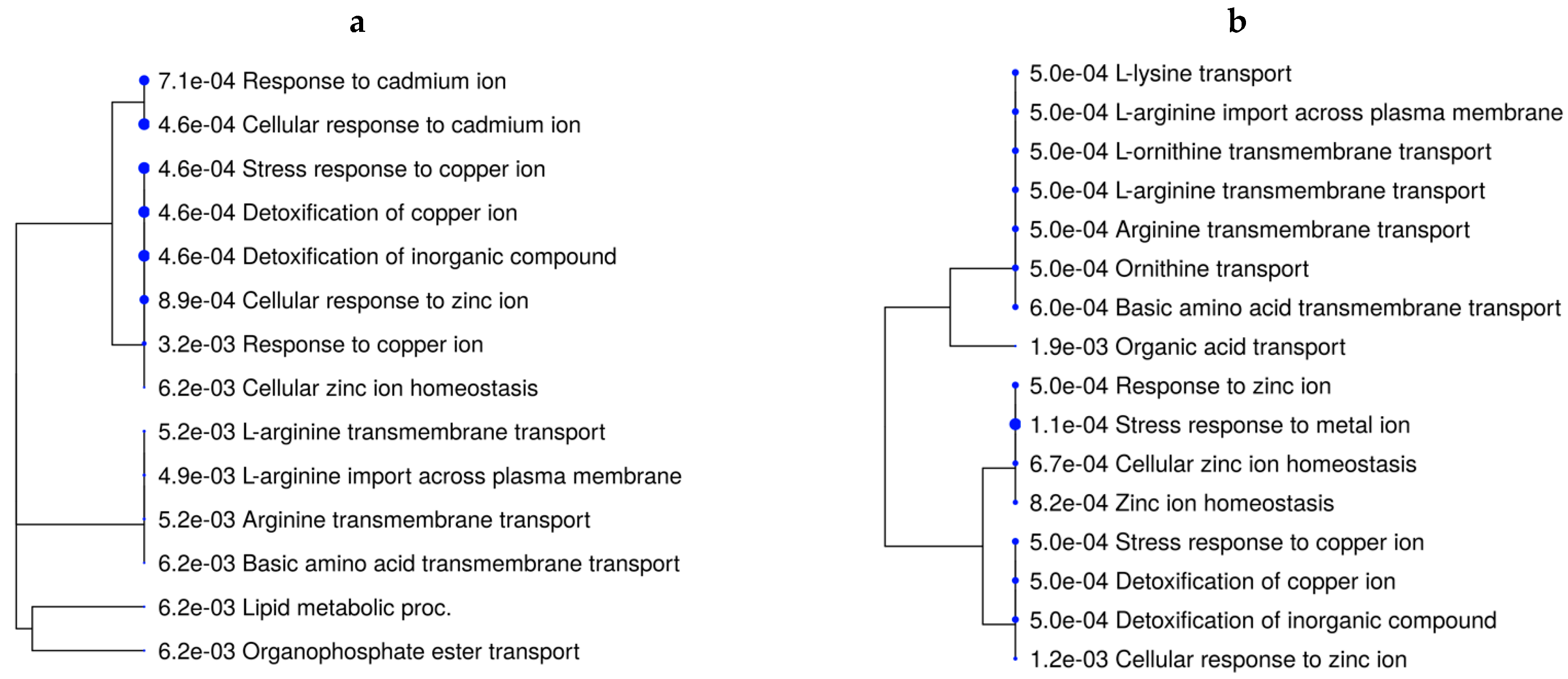
The over-represented BPs underlying the DEGs from the main effect of rate of gain were related to lipid metabolism, lipid storage, and the fatty acid metabolic process (Tables S3 and S8). When evaluating the effects of vitamin–mineral supplementation for each rate-of-gain condition (VTM_MG vs. NoVTM_MG and VTM_LG vs. NoVTM_LG—Figure 3a,b), mineral-homeostasis- and amino acid-transport-related terms were over-represented in fetuses receiving in utero VTM supplementation.
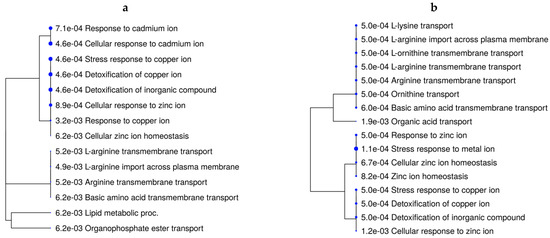
Figure 3.
Functional over-representation analysis of differentially expressed genes in fetal liver tissues. Biological processes over-represented are based on the DEGs of VTM_MG vs. NoVTM_MG (a) and VTM_LG vs. NoVTM_LG (b) comparisons. The terms are hierarchically arranged based on functional similarity. The bigger the blue dot, the more significant the term is (FDR ≤ 0.05).
The k-means clustering analysis, followed by functional over-representation, provided insights into the molecular pathways underlying gene expressions and their involvement with liver function. Based on the k-means approach, we clustered 2000 genes in four clusters (Table 1). Cluster A grouped 702 genes involved with pathways such as retinol metabolism, arachidonic acid metabolism, and the PPAR signaling pathway (FDR ≤ 0.05). Additional pathways related to metabolism included PI3K-Akt signaling and oxidative phosphorylation, which were identified in clusters B (n = 577) and D (n = 364), respectively. The genes within each cluster and the k-means values are reported in Table S11. The over-represented pathways and genes for all clusters are reported in Table S12 (FDR ≤ 0.05).

Table 1.
k-means clusters and key over-represented pathways involved with fetal liver function.
4. Discussion
As in humans, nutrition in livestock species during pregnancy has long been recognized as affecting maternal metabolism and, consequently, fetal growth and development [51]. Maternal models based on excess or restricted nutrition have shown adverse effects on the availability of nutrients and metabolites to developing fetuses [33,52,53]. During pregnancy, there are physiological changes related to maternal and fetal metabolic requirements [3,10,14]. Cumulative evidence has shown that although fetal growth during early pregnancy is minimal, there is likely increased demand for specific nutrients [10,14,15]. Thus, managing nutrient supply before and during pregnancy is critical for proper fetal development and growth [10,15]. Most studies investigating the effects of maternal nutrition on fetal outcomes have focused on macronutrients, such as proteins and energy, during the last two-thirds of gestation. Lately, we and others have extended our attention to the role of micronutrients during the periconceptual period and early pregnancy.
We have developed a beef cattle model and nutrition plan that simulates on-farm scenarios [29]. Based on this model, we have provided strong evidence that maternal supplementation programs during the periconceptual period affect both maternal and fetal development [29,30]. Changes in nutrient availability lead to maternal adaptive changes, including placental adaptations [15,54,55,56,57]. For the same heifers as those used in the current study, we have reported changes in gene expression in placental caruncle (maternal placental) and cotyledon (fetal placental) tissues [31]. Among the findings, we identified 267 unique DEGs underlying biological processes related to nutrient transport, ion homeostasis, and lipid metabolism [31]. As the placenta is a unit of nutrient transport, it is interesting to note that several of the pathways over-represented in the placenta, including PPAR signaling, insulin resistance, cholesterol metabolism, and ion and amino acid transport, were also identified in fetal livers in the current experiment. Additionally, we reported that maternal rate of gain affected the fetal intestine, liver and femur mass, and lipid and metabolite abundance. Likewise, liver mass and mineral concentration in the fetuses and amino acid abundance in the allantoic fluid [29,30] were affected by mineral and vitamin supplementation. The previous findings reported for the experimental model described here are supported by the differentially expressed genes that we identified. The same trend was also observed for pathways and biological processes.
4.1. Maternal Body-Weight Gain during Early Pregnancy Affects the Expression of Hepatic Fetal Genes Involved with Lipid and Energy Metabolism
The fetal liver develops early in gestation and is sensitive to hormonal and nutritional imbalances [11]. Tissue growth and function are programmed in utero by the coordinated function of genes [18]. By clustering the 2000 genes with the greatest expression variation, regardless of maternal diet, we identified several over-represented pathways involved with liver metabolic function. These pathways were mainly related to energy metabolism, including PPAR and PI3K-Akt signaling pathways. Pathways related to tissue development, such as ECM-receptor interaction and focal adhesion, were over-represented. Interestingly, genes acting on these pathways were differentially expressed among the treatments (MG vs. LG).
It is important to note that the maternal rate of weight gain affected both gene expression and tissue development. We have shown that fetuses from the MG group had increased fetal femur weight, suggesting a potential effect on bone growth in the MG vs. LG dams. Findings from Yin et al. [58] indicate that in utero diet influences peak bone mass in adolescents. Likewise, fetuses from heifers with LG showed a heavier total liver mass when compared to the MG group [29]. Fetal hepatic metabolites were also affected by rate of gain, including those involved with carbohydrate and energy metabolism [33]. Several other studies reported the effects of maternal nutrient restriction on fetal hepatic development [11], gene expression [18,21,23], and metabolite abundance [33]. We found bta-miR-122 to be downregulated in the VTM_MG vs. VTM_LG group but upregulated in the VTM_LG vs. NoVTM_MG group. This miRNA acts to regulate cholesterol metabolism and terminal differentiation of hepatocytes [59].
We identified 591 unique DEGs across all six contrasts. However, the effect of rate of gain (LG or MG) combined with VTM supplementation seems to be stronger, as more genes were differentially expressed in this comparison (i.e., VTM_MG vs. VTM_LG). Several genes involved with energy homeostasis and lipid metabolism pathways were downregulated in the VTM_MG compared to the VTM_LG group. Although with a less pronounced effect on the number of DEGs, similar energy-related pathways were over-represented when there was no vitamin and mineral supplementation. Interestingly, 22 genes were shared between both contrasts—VTM_MG vs. VTM_LG and NoVTM_MG vs. NoVTM_LG. Among the genes, those related to lipid metabolism, including ACSS2, FADS1, FADS2, FASN, and LPL, were downregulated in the MG group regardless of the VTM treatment. Supporting our findings, decreased abundance of hepatic metabolites was reported by Crouse et al. in the livers of fetuses from MG compared to LG and from VTM compared with NoVTM groups [33]. Similarly, in a lipidomic study using the same fetuses as those evaluated here, Menezes et al. demonstrated that moderate rates of gain resulted in greater concentrations of hepatic polyunsaturated fatty acids and diacylglycerols and lower concentrations of monoacylglycerols [60]. Furthermore, they reported that 152 metabolites were significantly affected by the main effect of rate of gain, and, interestingly, greater abundances were observed in the livers of fetuses from cows under LG compared to MG [60]. In sheep, nutrient-restricted fetuses during late gestation were found to have decreased liver size and lipid metabolism [16].
The liver is a key organ controlling body energy metabolism [12]. Here we identified the PI3K-Akt signaling pathway as being over-represented in our gene-clustering approach and DEGs from the VTM_MG vs. VTM_LG contrast. PI3K-Akt is a nutrient-sensing pathway critical to biological processes, such as nutrient uptake, energy metabolism, and cell growth and differentiation [61,62]. We previously reported that this pathway was affected in 50-day-old fetuses from nutrient-restricted heifers [23]. Furthermore, placental cotyledons of nutrient-restricted ewes during the periconceptual period had increased activity of the PI3K/Akt pathway [63]. Many mineral-dependent enzymes are involved with energy and lipid metabolism [24,64,65]. Selenium is among the minerals modulating PI3K/Akt and energy metabolism [66]. Over-represented pathways affected by VTM supplementation included biosynthesis of unsaturated fatty acids, the PPAR signaling pathway, and fatty acid metabolism. The PPAR protein is sensitive to nutrient availability and plays a key role in tissue development [8]. Likewise, FASN encodes rate-limiting enzymes for long-chain fatty acid synthesis [67].
The upregulation of energy-metabolism-related genes in fetuses from heifers in the LG group combined with increased liver size may suggest compensatory adaptive mechanisms to uptake more nutrients [20]. The glucose-transport gene SLC2A2 (GLUT2) was upregulated in the VTM_MG vs. VTM_LG comparison, whereas SLC2A4 (GLUT4) was downregulated in NoVTM_MG vs. NoVTM_LG. Changes in response to reduced nutrient availability and compensatory growth are related to enhanced insulin secretion [68], likely explaining the over-representation of pathways such as insulin resistance and the insulin signaling pathway by the DEGs. Although these findings warrant further investigation of the mechanisms and long-term consequences for offspring performance, it has been shown that suboptimal maternal nutrition in ewes during early development of the fetal liver led to greater lipid accumulation within the liver following obesity during adulthood [17].
4.2. Periconceptual Maternal Mineral and Vitamin Supplementation Affect the Expression of Hepatic Fetal Genes Involved with Mineral Homeostasis and Amino Acid Transport
In addition to the effects of rate of weight gain, it is important to highlight the role of maternal vitamin and mineral supplementation on early fetal development. Although vitamins and minerals are required in small amounts, they are essential for proper fetal development, as they serve in structural, physiological, catalytic, and regulatory functions [54,65,69]. Furthermore, a growing number of studies has shown their role in modulating gene expression [65,70,71,72]. We previously reported that fetal liver mass was increased in VTM compared to NoVTM dams [29]. Furthermore, maternal vitamin and mineral supplementation (VTM) led to increased concentrations of Se, Cu, Mn, and Co in the livers of beef heifers and their fetuses [29]. Likewise, there was an increased abundance of Co, Zn, and Mo in allantoic and amniotic fluids at day 83 [30]. We also reported increased concentrations of histidine, aspartate, and 12 out of 14 neutral amino acids in the allantoic fluid of VTM-supplemented dams (p ≤ 0.05) [73]. Finally, fetal hepatic metabolites underlying amino acid metabolism were affected by maternal VTM supplementation [33].
The differentially expressed genes affected by the main effect of VTM supplementation included the metallothionein-coding genes MT1A, MT1E, and MT2A. Metallothioneins (MTs) are small, cysteine-rich, heavy-metal-binding proteins involved with protective stress responses [74]. The MT genes were upregulated in all contrasts, except VTM_MG vs. VTM_LG. The expression of MT genes is influenced by changes in trace-element availability [75]. Among the metal-transporter-coding genes, SLC30A10 plays a role in body Mn levels [76] and was downregulated in the VTM_LG vs. NoVTM_LG comparison. Likewise, the selenoprotein-coding genes SELENOI and SEPSECS were upregulated in the VTM_MG vs. VTM_LG comparison. The mineral absorption pathway was over-represented by the DEGs from the VTM-supplemented groups. The MT coding genes underlie BP-related terms, such as the response to copper ions and detoxification of copper ions. Although there were no significant treatment effects on hepatic Zn levels, we found BP-related terms that were over-represented, such as Zn ion homeostasis and cellular response to Zn ions. Collectively, these findings show increased storage of specific trace minerals in the fetal liver, with consequences for gene expression and mineral transport. However, the interplay between specific trace minerals, their mechanisms of transport to the fetus, and their role in modulating gene expression and fetal programming warrant further studies.
We found over-represented BPs involved with amino acid (AA) transport of L-arginine, L-lysine, and ornithine. Essential AAs play key roles as regulators of metabolic and nutrient-sensing pathways, such as PPARs and AMPK signaling pathways [77]. According to Hussain et al., arginine is essential for growth and development, whereas ornithine plays a role in gene-expression regulation and protein synthesis [77]. Our research group has previously shown that vitamin and mineral supplements lead to increased levels of neutral AAs in the allantoic fluid of beef heifers [73]. Furthermore, we reported differential expression of nutrient-transport-coding genes in the placenta [31]. Lastly, Crouse et al. reported that fetal liver metabolites involved with the glycine, serine, and threonine pathway and the leucine, isoleucine, and valine pathway had greater abundances in the NoVTM_LG group [33]. Our results show that vitamin and mineral supplementation and rate of weight gain led to fetal metabolic adaptations and likely increased transport and use of AAs. However, questions related to maternal–fetal crosstalk and nutrient balance still remain.
5. Conclusions
This is the first study to investigate fetal hepatic gene expression in response to maternal vitamin and mineral supplementation and/or rate of weight gain during the periconceptual period. We have provided evidence that the maternal rate of gain during the first 83 days of pregnancy differentially regulated genes involved with metabolic-related pathways, including the PPAR, PI3K-Akt, AMPK, and insulin signaling pathways. These changes were accompanied by increased expression of genes involved with lipid and energy metabolism in fetuses from cows under a low rate of body-weight gain. Furthermore, mineral and vitamin supplementation led to the increased expression of genes involved with mineral homeostasis and the transport of amino acids. Collectively, our findings provide evidence of a potential compensatory attempt by the fetus to enhance energy metabolism under a low rate of weight gain. However, further research is needed to determine whether, and the extent to which, the changes in pathways affected during early gestation persist in the perinatal period and are translated into altered metabolic phenotypes in offspring.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani13040600/s1, Table S1: Nutrient compositions of total mixed rations and supplements provided to beef heifers. Table S2: Summary of RNA sequencing, mapping statistics, and treatments. Table S3: Differentially expressed genes, KEGG pathways, and biological process between NoVTM_MG and NoVTM_LG. Table S4: Differentially expressed genes, KEGG pathways, and biological process between VTM_LG and NoVTM_LG. Table S5: Differentially expressed genes, KEGG pathways, and biological process between VTM_LG and NoVTM_MG. Table S6: Differentially expressed genes, KEGG pathways, and biological process between VTM_MG and NoVTM_LG. Table S7: Differentially expressed genes, KEGG pathways, and biological process between VTM_MG and NoVTM_MG. Table S8: Differentially expressed genes, KEGG pathways, and biological process between VTM_MG and VTM_LG. Table S9: Overlapping differentially expressed genes among contrasts. Table S10: Overlapping KEGG pathways among contrasts. Table S11: k-means clustering analysis of fetal liver genes at day 83 of pregnancy. Table S12: Over-represented KEGG pathways from k-means clustering analysis of fetal liver genes at day 83 of pregnancy (FDR < 0.05).
Author Contributions
Conceptualization, K.L.M., J.C.F., R.S., J.S.C. and C.R.D.; Formal analysis, W.J.S.D.; Funding acquisition, K.L.M., J.S.C. and C.R.D.; Investigation, A.K.W., K.L.M., C.J.K., F.B., L.P.R., P.P.B., K.K.S., J.D.K., S.T.D. and T.L.N.; Methodology, W.J.S.D., A.K.W., K.L.M., L.P.R., P.P.B., K.K.S., J.D.K., S.T.D., T.L.N., J.C.F., R.S., J.S.C. and C.R.D.; Project administration, C.R.D.; Software, W.J.S.D.; Supervision, A.K.W. and C.R.D.; Writing—original draft, W.J.S.D.; Writing—review and editing, W.J.S.D., A.K.W., K.L.M., C.J.K., F.B., L.P.R., P.P.B., K.K.S., J.D.K., S.T.D., T.L.N., J.C.F., R.S., J.S.C. and C.R.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the North Dakota Agricultural Experiment Station (NDAES), the North Dakota State Board of Agricultural Research and Education (SBARE)—grant number 19-23-0155—and by Purina Animal Nutrition LLC, Gray Summit, MO, USA. The first author (W.J.S.D.) was financially supported by the Agricultural Research Service, U.S. Department of Agriculture, under Agreement No. 58-6010-1-005, by the Alabama Agricultural Experiment Station—Hatch program of the National Institute of Food and Agriculture, U.S. Department of Agriculture.
Institutional Review Board Statement
All experiments and methods were performed following the relevant guidelines and regulations. The experimental design, animal management, and tissue collection were approved by the North Dakota State University Institutional Animal Care and Use Committee (IACUC A19012).
Informed Consent Statement
Not applicable.
Data Availability Statement
All relevant data are included in the paper and in the Supplementary Materials. All sequencing data will be made publicly available in NCBI’s Gene Expression Omnibus upon acceptance of the paper.
Acknowledgments
The authors would like to thank Purina Animal Nutrition LLC (Land O’Lakes, Inc., Arden Hills, MN, USA) for providing financial support for this research. The authors would also like to thank the North Dakota State Board of Agricultural Research and Education, Graduate Research Assistantship Program, and the North Dakota Agricultural Experiment Station for their support of this effort and additional product support from Zoetis Animal Health (Parsnippany, NJ, USA) and ST Genetics (Navasota, TX, USA). Appreciation is expressed to personnel at the Central Grasslands Research Extension Center and the Animal Nutrition and Physiology Center for assistance with animal handling and feeding and to the NDSU Animal Science Nutrition Laboratory. This work used resources of the Center for Computationally Assisted Science and Technology (CCAST) at North Dakota State University and the Auburn University Easley Cluster.
Conflicts of Interest
Authors J. C. Forcherio and R. Scott are employees of Purina Animal Nutrition LLC (Land O’Lakes, Inc., Arden Hills, MN, USA), which sponsored the sample analysis for this experiment. Purina Animal Nutrition LLC manufactured the Purina® Wind & Rain® Storm® AllSeason 7.5 Complete mineral, the VTM and NoVTM pellets, and the protein–energy supplement used in this study. The funders had no role in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results which were obtained entirely independently by North Dakota State University and Auburn University personnel. The first author led the writing of the paper with inputs from the co-authors, who have declared no conflicts of interest.
References
- Barker, D.J.P. Fetal origins of coronary heart disease. BMJ 1995, 311, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, L.P.; Borowicz, P.P.; Caton, J.S.; Vonnahme, K.A.; Luther, J.S.; Hammer, C.J.; Maddock Carlin, K.R.; Grazul-Bilska, A.T.; Redmer, D.A. Developmental programming: The concept, large animal models, and the key role of uteroplacental vascular development. J. Anim. Sci. 2010, 88, 61–72. [Google Scholar] [CrossRef]
- Khayat, S.; Fanaei, H.; Ghanbarzehi, A. Minerals in pregnancy and lactation: A review article. J. Clin. Diagnostic Res. 2017, 11, QE01–QE05. [Google Scholar] [CrossRef]
- King, J.C. Physiology of pregnancy and nutrient metabolism. Am. J. Clin. Nutr. 2000, 71, 1218S–1225S. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, K.M.; Barker, D.J.P. Fetal nutrition and adult disease. Am. J. Clin. Nutr. 2000, 71, 1344–1352. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.A.; Buklijas, T.; Low, F.M.; Beedle, A.S. Epigenetic mechanisms that underpin metabolic and cardiovascular diseases. Nat. Rev. Endocrinol. 2009, 5, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Skinner, M.K.; Haque, C.G.B.M.; Nilsson, E.; Bhandari, R.; McCarrey, J.R. Environmentally induced transgenerational epigenetic reprogramming of primordial germ cells and the subsequent germ line. PLoS ONE 2013, 8, e66318. [Google Scholar] [CrossRef]
- Maloney, C.A.; Rees, W.D. Gene-nutrient interactions during fetal development. Reproduction 2005, 130, 401–410. [Google Scholar] [CrossRef]
- Reynolds, L.P.; Ward, A.K.; Caton, J.S. Epigenetics and developmental programming in ruminants: Long-term impacts on growth and development. In Biology of Domestic Animals; Scanes, C.G., Hill, R.A., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 85–120. ISBN 9781315152080. [Google Scholar]
- Caton, J.S.; Crouse, M.S.; McLean, K.J.; Dahlen, C.R.; Ward, A.K.; Cushman, R.A.; Grazul-Bilska, A.T.; Neville, B.W.; Borowicz, P.P.; Reynolds, L.P. Maternal periconceptual nutrition, early pregnancy, and developmental outcomes in beef cattle. J. Anim. Sci. 2020, 98, skaa358. [Google Scholar] [CrossRef]
- Hyatt, M.A.; Budge, H.; Symonds, M.E. Early developmental influences on hepatic organogenesis. Organogenesis 2008, 4, 170–175. [Google Scholar] [CrossRef]
- Rui, L. Energy metabolism in the liver. Compr. Physiol. 2014, 176, 177–197. [Google Scholar] [CrossRef]
- Sookoian, S.; Gianotti, T.F.; Burgueño, A.L.; Pirola, C.J. Fetal metabolic programming and epigenetic modifications: A systems biology approach. Pediatr. Res. 2013, 73, 531–542. [Google Scholar] [CrossRef]
- Caton, J.S.; Crouse, M.S.; Reynolds, L.P.; Neville, T.L.; Dahlen, C.R.; Ward, A.K.; Swanson, K.C. Maternal nutrition and programming of offspring energy requirements. Transl. Anim. Sci. 2019, 3, 976–990. [Google Scholar] [CrossRef] [PubMed]
- Vonnahme, K.A.; Lemley, C.O.; Caton, J.S.; Meyer, A.M. Impacts of maternal nutrition on vascularity of nutrient transferring tissues during gestation and lactation. Nutrients 2015, 7, 3497–3523. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.I.; Liefeld, A.; Vásquez-Hidalgo, M.A.; Vonnahme, K.A.; Grazul-Bilska, A.T.; Swanson, K.C.; Mishra, N.; Reed, S.A.; Zinn, S.A.; Govoni, K.E. Mid- to late-gestational maternal nutrient restriction followed by realimentation alters development and lipid composition of liver and skeletal muscles in ovine fetuses. J. Anim. Sci. 2021, 99, skab299. [Google Scholar] [CrossRef]
- Hyatt, M.A.; Gardner, D.S.; Sebert, S.; Wilson, V.; Davidson, N.; Nigmatullina, Y.; Chan, L.L.Y.; Budge, H.; Symonds, M.E. Suboptimal maternal nutrition, during early fetal liver development, promotes lipid accumulation in the liver of obese offspring. Reproduction 2011, 141, 119–126. [Google Scholar] [CrossRef]
- Hyatt, M.A.; Gopalakrishnan, G.S.; Bispham, J.; Gentili, S.; McMillen, I.C.; Rhind, S.M.; Rae, M.T.; Kyle, C.E.; Brooks, A.N.; Jones, C.; et al. Maternal nutrient restriction in early pregnancy programs hepatic mRNA expression of growth-related genes and liver size in adult male sheep. J. Endocrinol. 2007, 192, 87–97. [Google Scholar] [CrossRef]
- Copping, K.J.; Hoare, A.; McMillen, I.C.; Rodgers, R.J.; Wallace, C.R.; Perry, V.E.A. Maternal periconceptional and first trimester protein restriction in beef heifers: Effects on maternal performance and early fetal growth. Reprod. Fertil. Dev. 2020, 32, 835–850. [Google Scholar] [CrossRef]
- Prezotto, L.D.; Camacho, L.E.; Lemley, C.O.; Keomanivong, F.E.; Caton, J.S.; Vonnahme, K.A.; Swanson, K.C. Nutrient restriction and realimentation in beef cows during early and mid-gestation and maternal and fetal hepatic and small intestinal in vitro oxygen consumption. Animal 2016, 10, 829–837. [Google Scholar] [CrossRef]
- Crouse, M.S.; Caton, J.S.; Cushman, R.A.; McLean, K.J.; Dahlen, C.R.; Borowicz, P.P.; Reynolds, L.P.; Ward, A.K. Moderate nutrient restriction of beef heifers alters expression of genes associated with tissue metabolism, accretion, and function in fetal liver, muscle, and cerebrum by day 50 of gestation. Transl. Anim. Sci. 2019, 3, 855–866. [Google Scholar] [CrossRef]
- Diniz, W.J.S.; Bobe, G.; Klopfenstein, J.J.; Gultekin, Y.; Davis, T.Z.; Ward, A.K.; Hall, J.A. Supranutritional maternal organic selenium supplementation during different trimesters of pregnancy affects the muscle gene transcriptome of newborn beef calves in a time-dependent manner. Genes 2021, 12, 1884. [Google Scholar] [CrossRef] [PubMed]
- Diniz, W.J.S.; Crouse, M.S.; Cushman, R.A.; McLean, K.J.; Caton, J.S.; Dahlen, C.R.; Reynolds, L.P.; Ward, A.K. Cerebrum, liver, and muscle regulatory networks uncover maternal nutrition effects in developmental programming of beef cattle during early pregnancy. Sci. Rep. 2021, 11, 2771. [Google Scholar] [CrossRef]
- Cammack, R.; Wrigglesworth, J.M.; Baum, H. Iron-dependent enzymes in mammalian systems. In Iron: Transport and Storage; Ponka, P., Schulman, H.M., Woodworth, R.C., Richter, G.W., Eds.; CRC Press: Boca Raton, FL, USA, 1990; pp. 17–39. [Google Scholar]
- Hostetler, C.E.; Kincaid, R.L.; Mirando, M.A. The role of essential trace elements in embryonic and fetal development in livestock. Vet. J. 2003, 166, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Harvey, K.M.; Cooke, R.F.; Marques, R.D.S. Supplementing trace minerals to beef cows during gestation to enhance productive and health responses of the offspring. Animals 2021, 11, 1159. [Google Scholar] [CrossRef] [PubMed]
- Dahlen, C.R.; Reynolds, L.P.; Caton, J.S. Selenium supplementation and pregnancy outcomes. Front. Nutr. 2022, 9, 2576. [Google Scholar] [CrossRef]
- USDA Beef 2017. Beef Cow-Calf Management Practices in the United States, 2017, Report 1. Available online: https://www.aphis.usda.gov/aphis/ourfocus/animalhealth/monitoring-and-surveillance/nahms/NAHMS_Beef_CowCalf_Studies (accessed on 1 February 2021).
- Menezes, A.C.B.; McCarthy, K.L.; Kassetas, C.J.; Baumgaertner, F.; Kirsch, J.D.; Dorsam, S.T.; Neville, T.L.; Ward, A.K.; Borowicz, P.P.; Reynolds, L.P.; et al. Vitamin and mineral supplementation and rate of gain in beef heifers I: Effects on dam hormonal and metabolic status, fetal tissue and organ mass, and concentration of glucose and fructose in fetal fluids at d 83 of gestation. Animals 2022, 12, 1757. [Google Scholar] [CrossRef]
- McCarthy, K.L.; Menezes, A.C.B.; Kassetas, C.J.; Baumgaertner, F.; Kirsch, J.D.; Dorsam, S.T.; Neville, T.L.; Ward, A.K.; Borowicz, P.P.; Reynolds, L.P.; et al. Vitamin and mineral supplementation and rate of gain in beef heifers II: Effects on concentration of trace minerals in maternal liver and fetal liver, muscle, allantoic, and amniotic fluids at day 83 of gestation. Animals 2022, 12, 1925. [Google Scholar] [CrossRef]
- Diniz, W.J.S.; Reynolds, L.P.; Borowicz, P.P.; Ward, A.K.; Sedivec, K.K.; McCarthy, K.L.; Kassetas, C.J.; Baumgaertner, F.; Kirsch, J.D.; Dorsam, S.T.; et al. Maternal vitamin and mineral supplementation and rate of maternal weight gain affects placental expression of energy metabolism and transport-related genes. Genes 2021, 12, 385. [Google Scholar] [CrossRef]
- Diniz, W.J.S.; Reynolds, L.P.; Ward, A.K.; Borowicz, P.P.; Sedivec, K.K.; McCarthy, K.L.; Kassetas, C.J.; Baumgaertner, F.; Kirsch, J.D.; Dorsam, S.T.; et al. Untangling the placentome gene network of beef heifers in early gestation. Genomics 2022, 114, 110274. [Google Scholar] [CrossRef]
- Crouse, M.S.; McCarthy, K.L.; Menezes, A.C.B.; Kassetas, C.J.; Baumgaertner, F.; Kirsch, J.D.; Dorsam, S.; Neville, T.L.; Ward, A.K.; Borowicz, P.P.; et al. Vitamin and mineral supplementation and rate of weight gain during the first trimester of gestation in beef heifers alters the fetal liver amino acid, carbohydrate, and energy profile at day 83 of gestation. Metabolites 2022, 12, 696. [Google Scholar] [CrossRef] [PubMed]
- NRC. Nutrient Requirements of Beef Cattle: Eighth Revised Edition; The National Academies Press: Washington, DC, USA, 2016; ISBN 978-0-309-31702-3. [Google Scholar]
- Lamb, G.C.; Dahlen, C.R.; Larson, J.E.; Marquezini, G.; Stevenson, J.S. Control of the estrous cycle to improve fertility for fixed-time artificial insemination in beef cattle: A review. J. Anim. Sci. 2010, 88, E181–E192. [Google Scholar] [CrossRef] [PubMed]
- Lamb, G.C.; Dahlen, C.R.; Brown, D.R. Reproductive Ultrasonography for Monitoring Ovarian Structure Development, Fetal Development, Embryo Survival, and Twins in Beef Cows. Prof. Anim. Sci. 2003, 19, 135–143. [Google Scholar] [CrossRef]
- Reynolds, L.P.; Redmer, D.A. Utero-placental vascular development and placental function. J. Anim. Sci. 1995, 73, 1839–1851. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Tong, J.; Zhao, J.; Underwood, K.R.; Zhu, M.; Ford, S.P.; Nathanielsz, P.W. Fetal programming of skeletal muscle development in ruminant animals. J. Anim. Sci. 2010, 88 (Suppl. 13), E51–E60. [Google Scholar] [CrossRef] [PubMed]
- McLean, K.J.; Dahlen, C.R.; Borowicz, P.P.; Reynolds, L.P.; Crosswhite, M.R.; Neville, B.W.; Walden, S.D.; Caton, J.S. Technical note: A new surgical technique for ovariohysterectomy during early pregnancy in beef heifers. J. Anim. Sci. 2016, 94, 5089–5096. [Google Scholar] [CrossRef]
- Andrews, S.; FASTQC. A Quality Control Tool for High Throughput Sequence Data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 1 February 2021).
- Ewels, P.; Magnusson, M.; Lundin, S.; Käller, M. MultiQC: Summarize analysis results for multiple tools and samples in a single report. Bioinformatics 2016, 32, 3047–3048. [Google Scholar] [CrossRef] [PubMed]
- Rosen, B.D.; Bickhart, D.M.; Schnabel, R.D.; Koren, S.; Elsik, C.G.; Tseng, E.; Rowan, T.N.; Low, W.Y.; Zimin, A.; Couldrey, C.; et al. De novo assembly of the cattle reference genome with single-molecule sequencing. Gigascience 2020, 9, giaa021. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Tarazona, S.; Furió-Tarí, P.; Turrà, D.; Di Pietro, A.; Nueda, M.J.; Ferrer, A.; Conesa, A. Data quality aware analysis of differential expression in RNA-seq with NOISeq R/Bioc package. Nucleic Acids Res. 2015, 43, e140. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Chen, Y.; Lun, A.T.L.; Smyth, G.K. From reads to genes to pathways: Differential expression analysis of RNA-Seq experiments using Rsubread and the edgeR quasi-likelihood pipeline. F1000Research 2016, 5, 1438. [Google Scholar] [CrossRef] [PubMed]
- Conway, J.R.; Lex, A.; Gehlenborg, N. UpSetR: An R package for the visualization of intersecting sets and their properties. Bioinformatics 2017, 33, 2938–2940. [Google Scholar] [CrossRef]
- Ge, S.X.; Jung, D.; Yao, R. ShinyGO: A graphical gene-set enrichment tool for animals and plants. Bioinformatics 2020, 36, 2628–2629. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Ge, S.X.; Son, E.W.; Yao, R. iDEP: An integrated web application for differential expression and pathway analysis of RNA-Seq data. BMC Bioinform. 2018, 19, 534. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.M. 147 Nutritional advances in fetal and neonatal development: Mineral nutrition. J. Anim. Sci. 2020, 98, 121. [Google Scholar] [CrossRef]
- Harvey, K.M.; Cooke, R.F.; Moriel, P. Impacts of nutritional management during early postnatal life on long-term physiological and productive responses of beef cattle. Front. Anim. Sci. 2021, 2, 44. [Google Scholar] [CrossRef]
- Reed, S.A.; Raja, J.S.; Hoffman, M.L.; Zinn, S.A.; Govoni, K.E. Poor maternal nutrition inhibits muscle development in ovine offspring. J. Anim. Sci. Biotechnol. 2014, 5, 43. [Google Scholar] [CrossRef]
- Thayer, Z.M.; Rutherford, J.; Kuzawa, C.W. Maternal nutritional buffering model: An evolutionary framework for pregnancy nutritional intervention. Evol. Med. Public Health 2020, 2020, 14–27. [Google Scholar] [CrossRef]
- Grazul-Bilska, A.T.; Borowicz, P.P.; Johnson, M.L.; Minten, M.A.; Bilski, J.J.; Wroblewski, R.; Redmer, D.A.; Reynolds, L.P. Placental development during early pregnancy in sheep: Vascular growth and expression of angiogenic factors in maternal placenta. Reproduction 2010, 140, 165–174. [Google Scholar] [CrossRef]
- Trollmann, R.; Gassmann, M. The role of hypoxia-inducible transcription factors in the hypoxic neonatal brain. Brain Dev. 2009, 31, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Sandovici, I.; Hoelle, K.; Angiolini, E.; Constância, M. Placental adaptations to the maternal-fetal environment: Implications for fetal growth and developmental programming. Reprod. Biomed. Online 2012, 25, 68–89. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Dwyer, T.; Riley, M.; Cochrane, J.; Jones, G. The association between maternal diet during pregnancy and bone mass of the children at age 16. Eur. J. Clin. Nutr. 2010, 64, 131–137. [Google Scholar] [CrossRef]
- Valdmanis, P.N.; Kim, H.K.; Chu, K.; Zhang, F.; Xu, J.; Munding, E.M.; Shen, J.; Kay, M.A. miR-122 removal in the liver activates imprinted microRNAs and enables more effective microRNA-mediated gene repression. Nat. Commun. 2018, 9, 5321. [Google Scholar] [CrossRef]
- Menezes, A.C.B.; Dahlen, C.R.; McCarthy, K.L.; Kassetas, C.J.; Baumgaertner, F.; Kirsch, J.D.; Dorsam, S.T.; Neville, T.L.; Ward, A.K.; Borowicz, P.P.; et al. Fetal Hepatic Lipidome Is More Greatly Affected by Maternal Rate of Gain Compared with Vitamin and Mineral Supplementation at Day 83 of Gestation. Metabolites 2023, 13, 175. [Google Scholar] [CrossRef]
- Fresno Vara, J.Á.; Casado, E.; de Castro, J.; Cejas, P.; Belda-Iniesta, C.; González-Barón, M. P13K/Akt signalling pathway and cancer. Cancer Treat. Rev. 2004, 30, 193–204. [Google Scholar] [CrossRef]
- Yu, J.S.L.; Cui, W. Proliferation, survival and metabolism: The role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development 2016, 143, 3050–3060. [Google Scholar] [CrossRef]
- Zhu, M.J.; Du, M.; Hess, B.W.; Nathanielsz, P.W.; Ford, S.P. Periconceptional nutrient restriction in the ewe alters MAPK/ERK1/2 and PI3K/Akt growth signaling pathways and vascularity in the placentome. Placenta 2007, 28, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Stangl, G.I.; Schwarz, F.J.; Kirchgessner, M. Cobalt deficiency effects on trace elements, hormones and enzymes involved in energy metabolism of cattle. Int. J. Vitam. Nutr. Res. 1999, 69, 120–126. [Google Scholar] [CrossRef]
- Da Silva Diniz, W.J.; Banerjee, P.; Regitano, L.C.A. Cross talk between mineral metabolism and meat quality: A systems biology overview. Physiol. Genomics 2019, 51, 529–538. [Google Scholar] [CrossRef]
- Liu, X.; Yao, Z. Chronic over-nutrition and dysregulation of GSK3 in diseases. Nutr. Metab. 2016, 13, 49. [Google Scholar] [CrossRef]
- Mihaylova, M.M.; Shaw, R.J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 2011, 13, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Hornick, J.L.; Van Eenaeme, C.; Gérard, O.; Dufrasne, I.; Istasse, L. Mechanisms of reduced and compensatory growth. Domest. Anim. Endocrinol. 2000, 19, 121–132. [Google Scholar] [CrossRef] [PubMed]
- McDowell, L.R. Minerals in Animal and Human Nutrition, 2nd ed.; Elsevier: Wallingford, UK, 2003; ISBN 9780444513670. [Google Scholar]
- Beckett, E.L.; Yates, Z.; Veysey, M.; Duesing, K.; Lucock, M. The role of vitamins and minerals in modulating the expression of microRNA. Nutr. Res. Rev. 2014, 27, 94–106. [Google Scholar] [CrossRef] [PubMed]
- da Silva Diniz, W.J.; Coutinho, L.L.; Tizioto, P.C.; Cesar, A.S.M.; Gromboni, C.F.; Nogueira, A.R.A.; de Oliveira, P.S.N.; de Souza, M.M.; Regitano, L.C.D.A. Iron content affects lipogenic gene expression in the muscle of Nelore beef cattle. PLoS ONE 2016, 11, e0161160. [Google Scholar] [CrossRef]
- Afonso, J.; Fortes, M.R.S.; Reverter, A.; da Silva Diniz, W.J.; Cesar, A.S.M.; de Lima, A.O.; Petrini, J.; de Souza, M.M.; Coutinho, L.L.; Mourão, G.B.; et al. Genetic regulators of mineral amount in Nelore cattle muscle predicted by a new co-expression and regulatory impact factor approach. Sci. Rep. 2020, 10, 8436. [Google Scholar] [CrossRef]
- Menezes, A.C.B.; McCarthy, K.L.; Kassetas, C.J.; Baumgaertner, F.; Kirsch, J.D.; Dorsam, S.; Neville, T.L.; Ward, A.K.; Borowicz, P.P.; Reynolds, L.P.; et al. Vitamin and mineral supplementation and rate of gain during the first trimester of gestation affect concentrations of amino acids in maternal serum and allantoic fluid of beef heifers. J. Anim. Sci. 2021, 99, skab024. [Google Scholar] [CrossRef] [PubMed]
- Ruttkay-Nedecky, B.; Nejdl, L.; Gumulec, J.; Zitka, O.; Masarik, M.; Eckschlager, T.; Stiborova, M.; Adam, V.; Kizek, R. The role of metallothionein in oxidative stress. Int. J. Mol. Sci. 2013, 14, 6044–6066. [Google Scholar] [CrossRef] [PubMed]
- Bremner, I.; Beattie, J.H. Metallothionein and the trace minerals. Annu. Rev. Nutr. 1990, 10, 63–83. [Google Scholar] [CrossRef]
- Mercadante, C.J.; Prajapati, M.; Conboy, H.L.; Dash, M.E.; Herrera, C.; Pettiglio, M.A.; Cintron-Rivera, L.; Salesky, M.A.; Rao, D.B.; Bartnikas, T.B. Manganese transporter Slc30a10 controls physiological manganese excretion and toxicity. J. Clin. Investig. 2019, 129, 5442. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Murtaza, G.; Metwally, E.; Yang, H.; Kalhoro, M.S.; Kalhoro, D.H.; Chughtai, M.I.; Yin, Y. Role of Dietary Amino Acids and Nutrient Sensing System in Pregnancy Associated Disorders. Front. Pharmacol. 2020, 11, 2153. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).