Evaluation of Environmental Sampling for Detection of Mycobacterium avium subspecies paratuberculosis in the Pre-Weaned Calf Area and Calving Area of Infected Dairy Farms Enrolled in a Voluntary Johne’s Disease Control Programme
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Herd Selection
2.2. RAMP Scores
2.3. Apparent Within-Herd Prevalence
2.4. Sampling
2.5. Boot Swab Sample Processing
2.6. DNA Extraction and Amplification
2.7. Data Analysis
3. Results
3.1. Apparent Within-Herd Prevalence
3.2. Environmental Samples
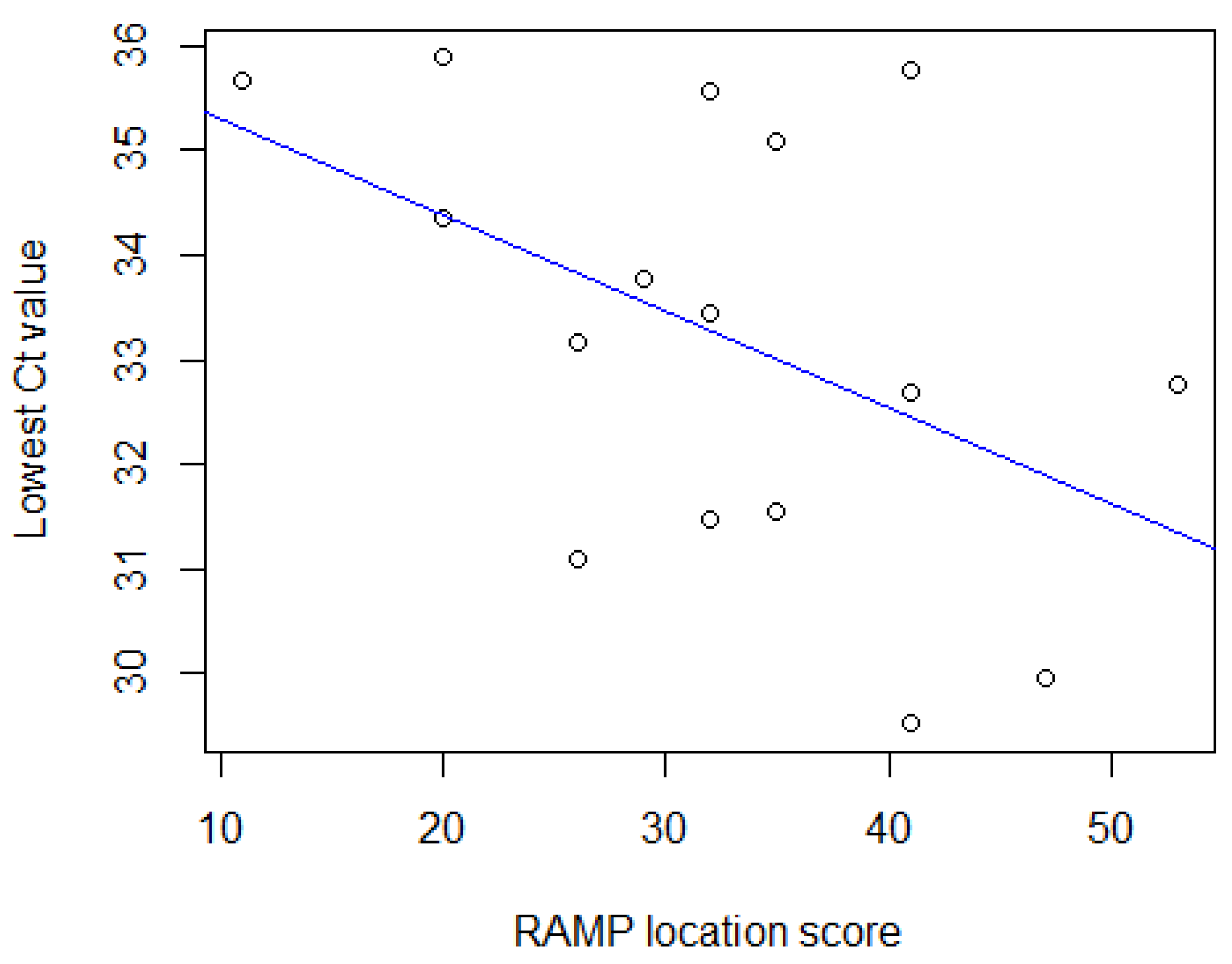
3.3. RAMP Scores
3.4. Logistic Regression Models
3.5. Correlation Tests
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whittington, R.J.; Marshall, D.J.; Nicholls, P.J.; Marsh, I.B.; Reddacliff, L.A. Survival and dormancy of Mycobacterium avium subsp. paratuberculosis in the environment. Appl. Environ. Microbiol. 2004, 70, 2989–3004. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, R.W. Pathogenesis of Paratuberculosis. Vet. Clin. N. Am. Food Anim. Pract. 2011, 27, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.B.; Shalloo, L. Invited review: The economic impact and control of paratuberculosis in cattle. J. Dairy Sci. 2015, 98, 5019–5039. [Google Scholar] [CrossRef] [PubMed]
- McAloon, C.G.; Whyte, P.; More, S.J.; Green, M.J.; O’Grady, L.; Garcia, A.; Doherty, M.L. The effect of paratuberculosis on milk yield—A systematic review and meta-analysis. J. Dairy Sci. 2016, 99, 1449–1460. [Google Scholar] [CrossRef]
- Ott, S.L.; Wells, S.J.; Wagner, B.A. Herd-level economic losses associated with Johne’s disease on US dairy operations. Prev. Vet. Med. 1999, 40, 179–192. [Google Scholar] [CrossRef]
- Waddell, L.A.; RajiĆ, A.; StÄRk, K.D.C.; McEwen, S.A. The zoonotic potential of Mycobacterium avium ssp. paratuberculosis: A systematic review and meta-analyses of the evidence. Epidemiol. Infect. 2015, 143, 3135–3157. [Google Scholar] [CrossRef]
- Field, N.L.; McAloon, C.G.; Gavey, L.; Mee, J.F. Mycobacterium avium subspecies paratuberculosis infection in cattle—A review in the context of seasonal pasture-based dairy herds. Ir. Vet. J. 2022, 75, 12. [Google Scholar] [CrossRef]
- Geraghty, T.; Graham, D.A.; Mullowney, P.; More, S.J. A review of bovine Johne’s disease control activities in 6 endemically infected countries. Prev. Vet. Med. 2014, 116, 1–11. [Google Scholar] [CrossRef]
- Groenendaal, H.; Nielen, M.; Jalvingh, A.W.; Horst, S.H.; Galligan, D.T.; Hesselink, J.W. A simulation of Johne’s disease control. Prev. Vet. Med. 2002, 54, 225–245. [Google Scholar] [CrossRef]
- Kudahl, A.B.; Østergaard, S.; Sørensen, J.T.; Nielsen, S.S. A stochastic model simulating paratuberculosis in a dairy herd. Prev. Vet. Med. 2007, 78, 97–117. [Google Scholar] [CrossRef]
- Doré, E.; Paré, J.; Côté, G.; Buczinski, S.; Labrecque, O.; Roy, J.P.; Fecteau, G. Risk factors associated with transmission of Mycobacterium avium subsp. paratuberculosis to calves within dairy herd: A systematic review. J. Vet. Intern. Med. 2012, 26, 32–45. [Google Scholar] [CrossRef] [PubMed]
- McAloon, C.G.; Roche, S.; Ritter, C.; Barkema, H.W.; Whyte, P.; More, S.J.; O’Grady, L.; Green, M.J.; Doherty, M.L. A review of paratuberculosis in dairy herds—Part 1: Epidemiology. Vet. J. 2019, 246, 59–65. [Google Scholar] [CrossRef]
- Khol, J.L.; Wassertheurer, M.; Sodoma, E.; Revilla-Fernandez, S.; Damoser, J.; Oserreicher, E.; Dunser, M.; Kleb, U.; Baumgartner, W. Long-term detection of Mycobacterium avium subspecies paratuberculosis in individual and bulk tank milk from a dairy herd with a low prevalence of Johne’s disease. J. Dairy Sci. 2013, 96, 3517–3524. [Google Scholar] [CrossRef] [PubMed]
- Stabel, J.R.; Bradner, L.; Robbe-Austerman, S.; Beitz, D.C. Clinical disease and stage of lactation influence shedding of Mycobacterium avium subspecies paratuberculosis into milk and colostrum of naturally infected dairy cows. J. Dairy Sci. 2014, 97, 6296–6304. [Google Scholar] [CrossRef] [PubMed]
- Whittington, R.; Donat, K.; Weber, M.F.; Kelton, D.; Nielsen, S.S.; Eisenberg, S.; Arrigoni, N.; Juste, R.; Saez, J.L.; Dhand, N.; et al. Control of paratuberculosis: Who, why and how. A review of 48 countries. BMC Vet. Res. 2019, 15, 1–29. [Google Scholar] [CrossRef]
- Ridge, S.; Baker, I.; Hannah, M. Effect of compliance with recommended calf-rearing practices on control of bovine Johne’s disease. Aust. Vet. J. 2005, 83, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Sorge, U.; Kelton, D.; Lissemore, K.; Godkin, A.; Hendrick, S.; Wells, S. Attitudes of Canadian dairy farmers toward a voluntary Johne’s disease control program. J. Dairy Sci. 2010, 93, 1491–1499. [Google Scholar] [CrossRef]
- Gavey, L.; Citer, L.; More, S.J.; Graham, D. The Irish Johne’s Control Programme. Front. Vet. Sci. 2021, 8, 703843. [Google Scholar] [CrossRef]
- Raizman, E.A.; Wells, S.J.; Godden, S.M.; Fetrow, J.; Friendshuh, K.; Michael Oakes, J. Characterization of Minnesota dairy herds participating in a Johne’s disease control program and evaluation of the program risk assessment tool. Prev. Vet. Med. 2006, 75, 22–33. [Google Scholar] [CrossRef]
- Donat, K.; Hahn, N.; Eisenberg, T.; Schlez, K.; Köhler, H.; Wolter, W.; Rohde, M.; Pützschel, R.; Rösler, U.; Failing, K.; et al. Within-herd prevalence thresholds for the detection of Mycobacterium avium subspecies paratuberculosis-positive dairy herds using boot swabs and liquid manure samples. Epidemiol. Infect. 2016, 144, 413–424. [Google Scholar] [CrossRef]
- Eisenberg, T.; Wolter, W.; Lenz, M.; Schlez, K.; Zschock, M. Boot swabs to collect environmental samples from common locations in dairy herds for Mycobacterium avium ssp paratuberculosis (MAP) detection. J. Dairy Res. 2013, 80, 485–489. [Google Scholar] [CrossRef]
- Caldwell, D.J.; Hargis, B.M.; Corrier, D.E.; DeLoach, J.R. Frequency of Isolation of Salmonella from Protective Foot Covers Worn in Broiler Houses as Compared to Drag-Swab Sampling. Avian Dis. 1998, 42, 381–384. [Google Scholar] [CrossRef]
- Talorico, A.A.; Bailey, M.A.; Munoz, L.R.; Chasteen, K.S.; Pal, A.; Krehling, J.T.; Bourassa, D.V.; Buhr, R.J.; Macklin, K.S. The use of roller swabs for Salmonella detection in poultry litter. J. Appl. Poult. Res. 2021, 30, 100163. [Google Scholar] [CrossRef]
- Wolf, R.; Barkema, H.W.; De Buck, J.; Slomp, M.; Flaig, J.; Haupstein, D.; Pickel, C.; Orsel, K. High herd-level prevalence of Mycobacterium avium subspecies paratuberculosis in Western Canadian dairy farms, based on environmental sampling. J. Dairy Sci. 2014, 97, 6250–6259. [Google Scholar] [CrossRef]
- Nielsen, S.S.; Toft, N. Ante mortem diagnosis of paratuberculosis: A review of accuracies of ELISA, interferon-γ assay and faecal culture techniques. Vet. Microbiol. 2008, 129, 217–235. [Google Scholar] [CrossRef] [PubMed]
- Pillars, R.B.; Grooms, D.L.; Kaneene, J.B. Longitudinal study of the distribution of Mycobacterium avium subsp paratuberculosis in the environment of dairy herds in the Michigan Johne’s disease control demonstration herd project. Can. Vet. J. Rev. Vet. Can. 2009, 50, 1039–1046. [Google Scholar]
- Raizman, E.A.; Wells, S.J.; Godden, S.M.; Bey, R.F.; Oakes, M.J.; Bentley, D.C.; Olsen, K.E. The distribution of Mycobacterium avium ssp paratuberculosis in the environment surrounding Minnesota dairy farms. J. Dairy Sci. 2004, 87, 2959–2966. [Google Scholar] [CrossRef] [PubMed]
- McAloon, C.G.; Whyte, P.; More, S.J.; O’Grady, L.; Doherty, M.L. Development of a HACCP-based approach to control paratuberculosis in infected Irish dairy herds. Prev. Vet. Med. 2015, 120, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Pieper, L.; DeVries, T.J.; Sorge, U.S.; Godkin, A.; Hand, K.J.; Perkins, N.R.; Imada, J.; Kelton, D.F. Variability in Risk Assessment and Management Plan (RAMP) scores completed as part of the Ontario Johne’s Education and Management Assistance Program (2010–2013). J. Dairy Sci. 2015, 98, 2419–2426. [Google Scholar] [CrossRef] [PubMed]
- Elguezabal, N.; Bastida, F.; Sevilla, I.A.; González, N.; Molina, E.; Garrido, J.M.; Juste, R.A. Estimation of Mycobacterium avium subsp. paratuberculosis growth parameters: Strain characterization and comparison of methods. Appl. Env. Microbiol. 2011, 77, 8615–8624. [Google Scholar] [CrossRef] [PubMed]

| Farm | Pre-Weaned Calf Area PCR Result | Pre-Weaned Calf Area Ct Value * | Pre-Weaned Calf Area RAMP SCORE /80 | Calving Area PCR Result | Calving Area Ct Value * | Calving Area RAMP Score /80 | Total FarmRAMP Score /227 | aWHP (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | Positive | 35.76 | 41 | Negative | 0 | 50 | 131 | 8 |
| 2 | Negative | 0 | 44 | Negative | 0 | 41 | 125 | 21 |
| 3 | Positive | 31.48 | 32 | Positive | 31.56 | 35 | 104 | 8 |
| 4 | Negative | 0 | 29 | Inconclusive | 38.27 | 29 | 119 | 5 |
| 5 | Negative | 0 | 23 | Negative | 0 | 23 | 68 | 1 |
| 6 | Inconclusive | 40.51 | 17 | Positive | 31.1 | 26 | 74 | 2 |
| 7 | Negative | 0 | 20 | Negative | 0 | 29 | 83 | 3 |
| 8 | Inconclusive | 37.15 | 14 | Positive | 33.43 | 32 | 83 | 6 |
| 9 | Negative | 0 | 11 | Negative | 0 | 38 | 80 | 1 |
| 10 | Inconclusive | 39.41 | 26 | Positive | 33.17 | 26 | 104 | 1 |
| 11 | Inconclusive | 37.65 | 32 | Positive | 29.95 | 47 | 134 | 15 |
| 12 | Negative | 0 | 23 | Positive | 35.08 | 35 | 83 | 2 |
| 13 | Positive | 32.77 | 53 | Positive | 29.52 | 41 | 155 | 4 |
| 14 | Negative | 0 | 20 | Negative | 0 | 23 | 68 | 2 |
| 15 | Negative | 0 | 32 | Inconclusive | 39.34 | 32 | 98 | 8 |
| 16 | Positive | 34.34 | 20 | Positive | 32.68 | 41 | 107 | 2 |
| 17 | Negative | 0 | - | Negative | 0 | - | - | - |
| 18 | Negative | 0 | 29 | Negative | 0 | 17 | 71 | 3 |
| 19 | Positive | 35.66 | 11 | Inconclusive | 36.15 | 38 | 80 | 5 |
| 20 | Negative | 0 | 35 | Negative | 0 | 38 | 125 | 0 |
| 21 | Negative | 0 | 20 | Negative | 0 | 23 | 59 | 4 |
| 22 | Negative | 0 | 8 | Positive | 33.78 | 29 | 59 | 6 |
| 23 | Inconclusive | 36.69 | 23 | Negative | 0 | 35 | 92 | 10 |
| 24 | Negative | 0 | 23 | Negative | 38.09 | 44 | 98 | 3 |
| 25 | Positive | 35.55 | 32 | Inconclusive | 41.1 | 23 | 98 | 5 |
| 26 | Negative | 0 | 35 | Inconclusive | 40.43 | 56 | 155 | 2 |
| 27 | Negative | 0 | 23 | Negative | 0 | 38 | 101 | - |
| 28 | Inconclusive | 41.05 | 11 | Positive | 35.88 | 20 | 47 | 2 |
| Positive | Inconclusive | Negative | Total | |
|---|---|---|---|---|
| Calf pens | 6 | 6 | 16 | 28 |
| Calving pens | 10 | 5 | 13 | 28 |
| Total | 16 | 11 | 29 | 56 |
| (1) Farm-Level | ||||
| Estimate | Odds Ratio | 95% CI | P-Value | |
| (Intercept) | −0.60395 | 0.54665 | 0.024, 11.11 | 0.692975 |
| log(aWHP + 1) | 0.422476 | 1.525734 | 0.477, 5.508 | 0.483722 |
| Overall RAMP | −0.00051 | 0.999494 | 0.971, 1.029 | 0.971782 |
| (2)Pre-weaned calf area only | ||||
| Estimate | Odds Ratio | 95% CI | P-value | |
| (Intercept) | −3.42849 | 0.032436 | 0.001, 0.584 | 0.034072 |
| Calf pen RAMP | 0.06061 | 1.062484 | 0.97, 1.185 | 0.211207 |
| log(aWHP + 1) | 0.348346 | 1.416723 | 0.36, 6.733 | 0.626936 |
| (3) Calving area only | ||||
| Estimate | Odds Ratio | 95% CI | P-value | |
| (Intercept) | −0.34837 | 0.705836 | 0.029, 16.388 | 0.825372 |
| Calving pen RAMP | −0.00639 | 0.993632 | 0.907, 1.083 | 0.883681 |
| log(aWHP + 1) | 0.059468 | 1.061271 | 0.314, 3.697 | 0.922076 |
| (4) Sample level | ||||
| Estimate | Odds Ratio | 95% CI | P-value | |
| (Intercept) | −2.07968 | 0.12497 | 0.004, 1.512 | 0.118357 |
| Overall RAMP | 0.008885 | 1.008925 | 0.984, 1.038 | 0.450377 |
| log(aWHP + 1) | 0.226056 | 1.253646 | 0.447, 4.114 | 0.641828 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Field, N.L.; Mee, J.F.; McAloon, C.G. Evaluation of Environmental Sampling for Detection of Mycobacterium avium subspecies paratuberculosis in the Pre-Weaned Calf Area and Calving Area of Infected Dairy Farms Enrolled in a Voluntary Johne’s Disease Control Programme. Animals 2023, 13, 669. https://doi.org/10.3390/ani13040669
Field NL, Mee JF, McAloon CG. Evaluation of Environmental Sampling for Detection of Mycobacterium avium subspecies paratuberculosis in the Pre-Weaned Calf Area and Calving Area of Infected Dairy Farms Enrolled in a Voluntary Johne’s Disease Control Programme. Animals. 2023; 13(4):669. https://doi.org/10.3390/ani13040669
Chicago/Turabian StyleField, Niamh L., John F. Mee, and Conor G. McAloon. 2023. "Evaluation of Environmental Sampling for Detection of Mycobacterium avium subspecies paratuberculosis in the Pre-Weaned Calf Area and Calving Area of Infected Dairy Farms Enrolled in a Voluntary Johne’s Disease Control Programme" Animals 13, no. 4: 669. https://doi.org/10.3390/ani13040669
APA StyleField, N. L., Mee, J. F., & McAloon, C. G. (2023). Evaluation of Environmental Sampling for Detection of Mycobacterium avium subspecies paratuberculosis in the Pre-Weaned Calf Area and Calving Area of Infected Dairy Farms Enrolled in a Voluntary Johne’s Disease Control Programme. Animals, 13(4), 669. https://doi.org/10.3390/ani13040669





