Physicochemical Properties of Black Korean Goat Meat with Various Slaughter Ages
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Proximate Compositions
2.3. Shear Force
2.4. Color
2.5. pH at 24 h Post-Mortem (pH24)
2.6. Water Holding Capacity (WHC)
2.7. Cooking Yield
2.8. Mineral
2.9. Free Amino Acid
2.10. Fatty Acid Composition
2.11. Statistical Analysis
3. Results and Discussion
3.1. Proximate Composition and Shear Force
3.2. Color and pH24
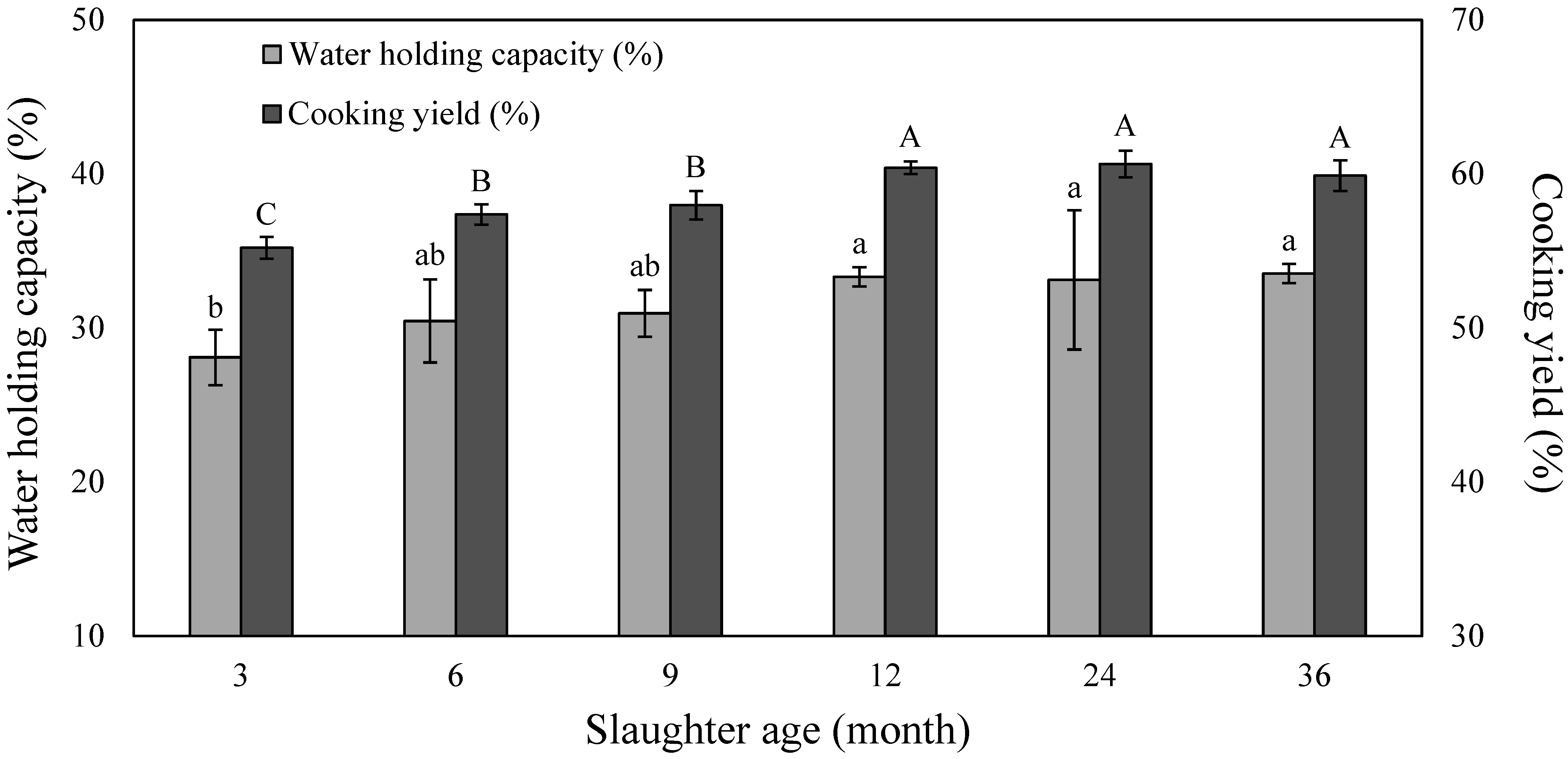
3.3. Water Holding Capacity and Cooking Yield
3.4. Minerals
3.5. Free Amino Acid
3.6. Fatty Acid Composition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dong, Y.; Zhang, X.; Xie, M.; Arefnezhad, B.; Wang, Z.; Wang, W.; Feng, S.; Huang, G.; Guan, R.; Shen, W.; et al. Reference genome of wild goat (Capra aegagrus) and sequencing of goat breeds provide insight into genic basis of goat domestication. BMC Genom. 2015, 16, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Park, J.Y.; Lee, S.Y.; Choi, Y.S.; Nam, K.C. Quality characteristics of low-fat black goat sausage using loquat leaf. J. Agric. Life Sci. 2020, 54, 59–65. [Google Scholar] [CrossRef]
- Talavera, M.; Chambers, D.H. Flavor lexicon and characteristics of artisan goat cheese from the United States. J. Sens. Stud. 2016, 31, 492–506. [Google Scholar] [CrossRef]
- Tüfekci, H.; Olfaz, M. Quality traits and fatty acid composition in meat of Hair Goat and Saanen× Hair Goat (G1) crossbred kids fattened in different systems. Arch. Anim. Breed. 2021, 64, 305–314. [Google Scholar] [CrossRef] [PubMed]
- LaRoche, E.M.; Wu, W.J.; Garcia, P.; Song, B.; Chun, C.K.Y.; Jones, C.K.; Crane, A.R.; O’Quinn, T.G.; Chao, M.D. Evaluation of skin-on goat meat processing on processing efficiency, carcass yield, meat quality, and sensory attributes. Meat Sci. 2022, 184, 108675. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, H.J.; Jang, A. Nutritional and antioxidative properties of black goat meat cuts. Asian-Australas. J. Anim. Sci. 2019, 32, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.H.; Seo, J.K.; Lee, J.G.; Yang, H.S. Physicochemical properties, volatile compounds and sensory attributes of dry-fermented Sausage manufactured with goat meat. J. Agric. Life Sci. 2021, 55, 97–107. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, H.J.; Kim, K.W.; Lee, J.; Lee, S.H.; Lee, S.S.; Choi, B.H.; Shin, D.J.; Jeon, K.H.; Choi, J.Y.; et al. Effect of Feeding Alfalfa and Concentrate on Meat Quality and Bioactive Compounds in Korean Native Black Goat Loin during Storage at 4 °C. Food Sci. Anim. Resour. 2022, 42, 517–535. [Google Scholar] [CrossRef]
- Argüello, A.; Castro, N.O.E.M.I.; Capote, J.U.A.N.; Solomon, M. Effects of diet and live weight at slaughter on kid meat quality. Meat Sci. 2005, 70, 173–179. [Google Scholar] [CrossRef]
- Abdullah, A.Y.; Obeidat, B.S.; Muwalla, M.M.; Matarneh, S.K.; Ishmais, M.A.A. Growth performance, carcass and meat characteristics of black goat kids fed sesame hulls and Prosopis juliflora pods. Asian-Australas. J. Anim. Sci. 2011, 24, 1217–1226. [Google Scholar] [CrossRef]
- Obeidat, B.S.; Gharaybeh, F.F. Effect of feeding sesame hull on growth performance, nutrient digestibility, and carcass characteristics of Black goat kids. Asian-Australas. J. Anim. Sci. 2010, 24, 206–213. [Google Scholar] [CrossRef]
- Moon, S.H.; Kim, N.Y.; Seong, H.J.; Chung, S.U.; Tang, Y.; Oh, M.; Kim, E.K. Comparative analysis of proximate composition, amino acid and fatty acid content, and antioxidant activities in fresh cuts of Korean native goat (Capra hircus coreanae) meat. Korean J. Food Preserv. 2021, 28, 303–312. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Bakhsh, A.; Lee, J.G.; Joo, S.T. Differences in muscle fiber characteristics and meat quality by muscle type and age of Korean native Black Goat. Food Sci. Anim. Resour. 2019, 39, 988–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grau, R.; Hamm, R. Eine einfache methode zur bestimmung der wasserbindung im muskel. Naturwissenschaften 1953, 40, 29–30. [Google Scholar] [CrossRef]
- Kang, K.M.; Lee, S.H.; Kim, H.Y. Effects of chicken, pork, beef, and beef crust on the Home Meal Replacement (HMR) Stock. J. Korean Soc. Food Sci. Nutr. 2020, 49, 729–734. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- David, F.; Sandra, P.; Wylie, P.L. Improving the Analysis of Fatty Acid Methyl Esters Using Retention Time Locked Methods and Retention Time Databases. In Agilent Technologies—Application; Agilent Technologies: Palo Alto, CA, USA, 2002. [Google Scholar]
- Arain, M.A.; Khaskheli, M.; Rajput, I.R.; Faraz, S.; Rao, S.; Umer, M.; Devrajani, K. Effect of slaughtering age on chemical composition of goat meat. Pak. J. Nutr. 2010, 9, 404–408. [Google Scholar] [CrossRef] [Green Version]
- Belew, J.B.; Brooks, J.C.; McKenna, D.R.; Savell, J.W. Warner–Bratzler shear evaluations of 40 bovine muscles. Meat Sci. 2003, 64, 507–512. [Google Scholar] [CrossRef]
- Liu, J.; Ellies-Oury, M.P.; Stoyanchev, T.; Hocquette, J.F. Consumer perception of beef quality and how to control, improve and predict it? Focus on eating quality. Foods 2022, 11, 1732. [Google Scholar] [CrossRef]
- Naqvi, Z.B.; Thomson, P.C.; Ha, M.; Campbell, M.A.; McGill, D.M.; Friend, M.A.; Warner, R.D. Effect of sous vide cooking and ageing on tenderness and water-holding capacity of low-value beef muscles from young and older animals. Meat Sci. 2021, 175, 108435. [Google Scholar] [CrossRef]
- Abhijith, A.; Warner, R.D.; Ha, M.; Dunshea, F.R.; Leury, B.J.; Zhang, M.; Joy, A.; Osei-Amponsah, R.; Chauhan, S.S. Effect of slaughter age and post-mortem days on meat quality of longissimus and semimembranosus muscles of Boer goats. Meat Sci. 2021, 175, 108466. [Google Scholar] [CrossRef]
- Beldarrain, L.R.; Morán, L.; Sentandreu, M.Á.; Insausti, K.; R Barron, L.J.; Aldai, N. Muscle and subcutaneous fatty acid composition and the evaluation of ageing time on meat quality parameters of Hispano-Bretón horse breed. Animals 2021, 11, 1421. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.H.; Assis, D.E.; Estrada, M.M.; Assis, G.J.; Zamudio, G.D.; Carneiro, G.B.; Filho, S.C.V.; Paulino, M.F.; Chizzotti, M.L. Carcass and meat quality traits of Nellore young bulls and steers throughout fattening. Livest. Sci. 2019, 229, 28–36. [Google Scholar] [CrossRef]
- Bakhsh, A.; Hwang, Y.H.; Joo, S.T. Effect of slaughter age on muscle fiber composition, intramuscular connective tissue, and tenderness of goat meat during post-mortem time. Foods 2019, 8, 571. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Liu, C.; Kong, Y.; Li, F.; Yue, X. Effects of intramuscular fat on meat quality and its regulation mechanism in Tan sheep. Front. Nutr. 2022, 9, 908355. [Google Scholar] [CrossRef]
- Ali, M.; Choi, Y.S.; Nam, K.C. Physicochemical attributes, free amino acids, and fatty acids of the five major cuts from Korean native black goat. Anim. Ind. Technol. 2021, 8, 23–33. [Google Scholar] [CrossRef]
- Gawat, M.; Kaur, L.; Singh, J.; Boland, M. Physicochemical and quality characteristics of New Zealand goat meat and its ultrastructural features. Food Res. Int. 2022, 161, 111736. [Google Scholar] [CrossRef]
- Kawęcka, A.; Pasternak, M. The Effect of slaughter Age on meat quality of male kids of the Polish Carpathian native goat breed. Animals 2022, 12, 702. [Google Scholar] [CrossRef]
- Park, S.Y.; Byeon, D.S.; Kim, G.W.; Kim, H.Y. Carcass and retail meat cuts quality properties of broiler chicken meat based on the slaughter age. J. Anim. Sci. Technol. 2021, 63, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Rostamani, M.; Baghaei, H.; Bolandi, M. Prediction of top round beef meat tenderness as a function of marinating time based on commonly evaluated parameters and regression equations. Food Sci. Nutr. 2021, 9, 5006–5015. [Google Scholar] [CrossRef]
- Li, J.; Liang, R.; Mao, Y.; Yang, X.; Luo, X.; Qian, Z.; Zhang, Y.; Zhu, L. Effect of dietary resveratrol supplementation on muscle fiber types and meat quality in beef cattle. Meat Sci. 2022, 194, 108986. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Liang, X.; Cao, J.; Zhang, Q.; Tan, Y.; Xu, B.; Yang, Y.; Wang, Y.; Yang, Q.; Liu, H.; et al. Denaturation manner of sarcoplasmic proteins in Pale, Soft and Exudative meat determines their positive impacts on myofibrillar water-holding capacity. Meat Sci. 2022, 185, 108723. [Google Scholar] [CrossRef]
- Saccà, E.; Corazzin, M.; Bovolenta, S.; Piasentier, E. Meat quality traits and the expression of tenderness-related genes in the loins of young goats at different ages. Animal 2019, 13, 2419–2428. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Bergamaschi, M.; Magro, L.; Petrini, A.; Bittante, G. Relationships of a detailed mineral profile of meat with animal performance and beef quality. Animals 2019, 9, 1073. [Google Scholar] [CrossRef] [Green Version]
- Higuera, J.M.D.; Santos, H.M.; Oliveira, A.F.D.; Nogueira, A.R.A. Bioaccessibility Assessment of Cu, Fe, K, Mg, P, and Zn in Thermally Treated Lamb Meat. J. Braz. Chem. Soc. 2021, 32, 2111–2119. [Google Scholar] [CrossRef]
- Babicz, M.; Kasprzyk, A. Comparative analysis of the mineral composition in the meat of wild boar and domestic pig. Ital. J. Anim. Sci. 2019, 18, 1013–1020. [Google Scholar] [CrossRef] [Green Version]
- Schönfeldt, H.C.; Naudé, R.T.; Boshoff, E. Effect of age and cut on the nutritional content of South African beef. Meat Sci. 2010, 86, 674–683. [Google Scholar] [CrossRef]
- Pogorzelska-Nowicka, E.; Atanasov, A.G.; Horbańczuk, J.; Wierzbicka, A. Bioactive compounds in functional meat products. Molecules 2018, 23, 307. [Google Scholar] [CrossRef] [Green Version]
- Mazhangara, I.R.; Chivandi, E.; Mupangwa, J.F.; Muchenje, V. The potential of goat meat in the red meat industry. Sustainability 2019, 11, 3671. [Google Scholar] [CrossRef] [Green Version]
- Church, D.D.; Hirsch, K.R.; Park, S.; Kim, I.Y.; Gwin, J.A.; Pasiakos, S.M.; Wolfe, R.R.; Ferrando, A.A. Essential amino acids and protein synthesis: Insights into maximizing the muscle and whole-body response to feeding. Nutrients 2020, 12, 3717. [Google Scholar] [CrossRef]
- Duan, W.; Liang, L.; Huang, Y.; Zhang, Y.; Sun, B.; Li, L. Effect of ginger on chemical composition, physical and sensory characteristics of chicken soup. Foods 2021, 10, 1456. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, Y.; Chen, C.; Xie, T.; Li, P. Effect of Lactobacillus plantarum and Staphylococcus xylosus on flavour development and bacterial communities in Chinese dry fermented sausages. Food Res. Int. 2020, 135, 109247. [Google Scholar] [CrossRef] [PubMed]
- Dinh, T.T.N.; Legako, J.F.; Miller, M.F.; Brooks, J.C. Effects of USDA quality grade and cooking on water-soluble precursors of beef flavor. Meat Sci. 2018, 146, 122–130. [Google Scholar] [CrossRef]
- Vannice, G.; Rasmussen, H. Position of the academy of nutrition and dietetics: Dietary fatty acids for healthy adults. J. Acad. Nutr. Diet. 2014, 114, 136–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polidori, P.; Pucciarelli, S.; Cammertoni, N.; Polzonetti, V.; Vincenzetti, S. The effects of slaughter age on carcass and meat quality of Fabrianese lambs. Small Rumin. Res. 2017, 155, 12–15. [Google Scholar] [CrossRef]
- Toplu, H.D.O.; Goksoy, E.O.; Nazligul, A.; Kahraman, T. Meat quality characteristics of Turkish indigenous Hair goat kids reared under traditional extensive production system: Effects of slaughter age and gender. Trop. Anim. Health Prod. 2013, 45, 1297–1304. [Google Scholar] [CrossRef] [PubMed]
- Budimir, K.; Trombetta, M.F.; Francioni, M.; Toderi, M.; D’Ottavio, P. Slaughter performance and carcass and meat quality of Bergamasca light lambs according to slaughter age. Small Rumin. Res. 2018, 164, 1–7. [Google Scholar] [CrossRef]
- Chen, J.; Li, J.; Liu, X.; He, Y. Effects of dietary fat saturation level on growth performance, carcass traits, blood lipid parameters, tissue fatty acid composition and meat quality of finishing pigs. Anim. Biosci. 2021, 34, 895–903. [Google Scholar] [CrossRef] [PubMed]
- McAfee, A.J.; McSorley, E.M.; Cuskelly, G.J.; Moss, B.W.; Wallace, J.M.; Bonham, M.P.; Fearon, A.M. Red meat consumption: An overview of the risks and benefits. Meat Sci. 2010, 84, 1–13. [Google Scholar] [CrossRef]
- Muzolf-Panek, M.; Kaczmarek, A. Chemometric analysis of fatty acid composition of raw chicken, beef, and pork meat with plant extract addition during refrigerated storage. Molecules 2021, 26, 4952. [Google Scholar] [CrossRef]
| Trait | Slaughter Age (Months) | SEM 1 (n = 30) | |||||
|---|---|---|---|---|---|---|---|
| 3 | 6 | 9 | 12 | 24 | 36 | ||
| Moisture (%) | 74.51 a | 73.61 b | 73.30 bc | 72.87 c | 71.02 d | 71.32 d | 0.06 |
| Crude protein (%) | 20.57 b | 21.62 a | 21.71 a | 21.28 a | 21.57 a | 21.42 a | 0.06 |
| Crude fat (%) | 3.29 b | 3.39 b | 3.51 b | 4.89 a | 5.64 a | 5.67 a | 0.08 |
| Collagen (%) | 1.15 f | 1.22 e | 1.29 d | 1.43 c | 1.52 b | 1.68 a | 0.01 |
| Shear force (N) | 25.81 d | 27.53 d | 37.70 c | 39.66 bc | 42.82 b | 47.82 a | 0.30 |
| Trait | Slaughter Age (Months) | SEM 1 (n = 30) | ||||||
|---|---|---|---|---|---|---|---|---|
| 3 | 6 | 9 | 12 | 24 | 36 | |||
| Color | CIE L* | 44.99 a | 42.47 b | 37.15 c | 35.68 d | 35.43 d | 35.47 d | 0.10 |
| CIE a* | 5.65 f | 7.44 e | 9.01 d | 11.00 c | 12.23 b | 14.03 a | 0.07 | |
| CIE b* | 5.23 e | 6.13 d | 6.50 d | 7.33 c | 9.17 b | 10.03 a | 0.07 | |
| pH24 | 5.79 c | 5.87 bc | 5.89 b | 6.06 a | 6.07 a | 6.09 a | 0.01 | |
| Trait (mg/100 g) | Slaughter Age (Months) | SEM 1 (n = 30) | |||||
|---|---|---|---|---|---|---|---|
| 3 | 6 | 9 | 12 | 24 | 36 | ||
| P | 195.06 | 193.86 | 194.91 | 203.46 | 197.11 | 198.61 | 0.89 |
| K | 14.85 c | 15.55 b | 14.99 c | 16.41 a | 16.22 a | 15.89 ab | 0.04 |
| Mg | 16.16 b | 16.43 b | 17.35 b | 22.23 a | 17.49 b | 17.34 b | 0.30 |
| Ca | 4.30 bc | 4.35 bc | 4.92 b | 5.84 a | 4.30 bc | 4.05 c | 0.06 |
| Na | 8.61 c | 9.14 c | 10.04 b | 10.86 a | 10.58 ab | 10.61 ab | 0.06 |
| Trait (μ mole/g dw) | Slaughter Age (Months) | SEM 1 (n = 30) | |||||
|---|---|---|---|---|---|---|---|
| 3 | 6 | 9 | 12 | 24 | 36 | ||
| Histidine | 0.06 c | 0.08 c | 0.14 b | 0.17 b | 0.19 b | 0.25 a | 0.01 |
| Asparagine | 0.08 d | 0.12 cd | 0.20 bc | 0.21 b | 0.26 ab | 0.32 a | 0.01 |
| Serine | 0.24 b | 0.28 b | 0.59 a | 0.63 a | 0.65 a | 0.82 a | 0.02 |
| Glutamine | 2.06 b | 2.60 b | 2.91 ab | 2.93 ab | 3.18 ab | 3.80 a | 0.09 |
| Arginine | 1.95 c | 2.69 abc | 2.42 bc | 2.43 bc | 3.08 ab | 3.38 a | 0.09 |
| Glycine | 0.77 b | 0.86 ab | 1.11 ab | 1.20 ab | 1.27 a | 1.30 a | 0.05 |
| Aspartic acid | 0.05 b | 0.08 b | 0.21 a | 0.23 a | 0.27 a | 0.30 a | 0.01 |
| Threonine | 0.36 c | 0.39 c | 0.63 bc | 0.75 b | 0.85 b | 1.17 a | 0.03 |
| Alanine | 0.16 c | 0.17 c | 0.35 b | 0.43 ab | 0.50 ab | 0.60 a | 0.02 |
| Cysteine | 0.96 c | 1.20 c | 1.79 b | 1.87 ab | 2.09 ab | 2.44 a | 0.07 |
| Tyrosine | 8.26 c | 11.30 c | 18.31 b | 20.97 ab | 21.28 ab | 24.26 a | 0.55 |
| Methionine | 0.11 c | 0.13 c | 0.25 b | 0.29 b | 0.35 ab | 0.42 a | 0.01 |
| Valine | 0.06 c | 0.12 bc | 0.16 bc | 0.18 b | 0.18 b | 0.29 a | 0.01 |
| Isoleucine | 0.08 c | 0.12 c | 0.26 b | 0.29 ab | 0.30 ab | 0.39 a | 0.01 |
| Leucine | 0.17 c | 0.22 c | 0.50 b | 0.59 ab | 0.59 ab | 0.76 a | 0.02 |
| Phenylalanine | 0.08 d | 0.12 cd | 0.19 bc | 0.24 b | 0.24 b | 0.33 a | 0.01 |
| Tryptophan | - | - | 0.01 b | 0.01 ab | 0.02 a | 0.02 a | 0.01 |
| Total | 15.22 d | 20.47 c | 30.03 b | 33.42 ab | 35.29 ab | 40.86 a | 0.50 |
| Trait (%) | Slaughter Age (Months) | SEM 1 (n = 30) | |||||
|---|---|---|---|---|---|---|---|
| 3 | 6 | 9 | 12 | 24 | 36 | ||
| Myristic acid (C14:0) | 10.50 a | 5.30 b | 3.65 c | 2.31 c | 3.019 c | 2.52 c | 0.09 |
| Palmitic acid (C16:0) | 30.82 a | 27.35 b | 24.32 bc | 21.66 c | 27.19 b | 25.93 b | 0.26 |
| Palmitoleic acid (C16:1n7) | 2.21 | 2.06 | 2.13 | 1.75 | 2.06 | 1.95 | 0.06 |
| Stearic acid (C18:0) | 12.30 b | 15.84 ab | 13.78 ab | 14.98 ab | 16.11 ab | 17.04 a | 0.33 |
| Oleic acid (C18:1n9) | 36.35 c | 42.22 b | 49.39 a | 51.68 a | 46.99 ab | 47.83 a | 0.38 |
| Vaccenic acid (C18:1n7) | 0.08 a | 0.06 ab | 0.02 c | 0.02 bc | 0.06 a | 0.04 abc | 0.01 |
| Linoleic acid (C18:2n6) | 4.70 | 4.62 | 4.21 | 4.93 | 2.94 | 2.92 | 0.16 |
| α-linolenic acid (C18:3n3) | 0.14 b | 0.13 b | 0.25 b | 0.43 a | 0.17 b | 0.13 b | 0.01 |
| Gondoic acid (C20:1n9) | 0.09 | 0.08 | 0.06 | 0.05 | 0.08 | 0.07 | 0.01 |
| Arachidonic acid (C20:4n6) | 2.42 | 2.00 | 1.94 | 1.87 | 1.15 | 1.38 | 0.09 |
| Eicosapentaenoic acid (C20:5n3) | 0.10 ab | 0.08 ab | 0.11 ab | 0.17 a | 0.09 ab | 0.05 b | 0.01 |
| Docosatetraenoate acid (C22:4 n6) | 0.21 | 0.18 | 0.09 | 0.09 | 0.11 | 0.11 | 0.01 |
| Docosahexaenoic acid (C22:6 n3) | 0.06 a | 0.06 a | 0.04 ab | 0.04 ab | 0.02 b | 0.02 ab | 0.01 |
| SFA | 53.62 a | 48.49 b | 41.75 cd | 38.98 d | 46.31 bc | 45.49 bc | 0.33 |
| UFA | 46.37 d | 51.50 c | 58.23 ab | 61.02 a | 53.67 bc | 54.50 bc | 0.33 |
| MUFA | 38.73 c | 44.42 bc | 51.60 a | 53.50 a | 49.19 ab | 49.89 ab | 0.41 |
| PUFA | 7.64 | 7.08 | 6.63 | 7.52 | 4.48 | 4.61 | 0.23 |
| n3 | 0.30 b | 0.28 b | 0.40 ab | 0.63 a | 0.28 b | 0.20 b | 0.02 |
| n6 | 7.34 | 6.81 | 6.24 | 6.89 | 4.20 | 4.41 | 0.23 |
| UFA/SFA | 0.87 d | 1.06 cd | 1.40 ab | 1.57 a | 1.17 c | 1.20 bc | 0.02 |
| MUFA/SFA | 0.73 d | 0.92 cd | 1.24 ab | 1.37 a | 1.07 bc | 1.10 bc | 0.02 |
| PUFA/SFA | 0.14 ab | 0.15 ab | 0.16 ab | 0.19 a | 0.10 b | 0.10 b | 0.01 |
| n6/n3 | 23.75 | 25.06 | 15.98 | 12.01 | 25.04 | 21.49 | 1.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, D.-M.; Kang, K.-M.; Kang, S.-M.; Kim, H.-Y. Physicochemical Properties of Black Korean Goat Meat with Various Slaughter Ages. Animals 2023, 13, 692. https://doi.org/10.3390/ani13040692
Choi D-M, Kang K-M, Kang S-M, Kim H-Y. Physicochemical Properties of Black Korean Goat Meat with Various Slaughter Ages. Animals. 2023; 13(4):692. https://doi.org/10.3390/ani13040692
Chicago/Turabian StyleChoi, Da-Mi, Kyu-Min Kang, Sun-Moon Kang, and Hack-Youn Kim. 2023. "Physicochemical Properties of Black Korean Goat Meat with Various Slaughter Ages" Animals 13, no. 4: 692. https://doi.org/10.3390/ani13040692






