A New Species of the Genus Achalinus (Squamata: Xenodermidae) from the Dabie Mountains, Anhui, China
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. DNA Sequencing
2.3. Phylogenetic Analyses
2.4. Morphometric Measurements
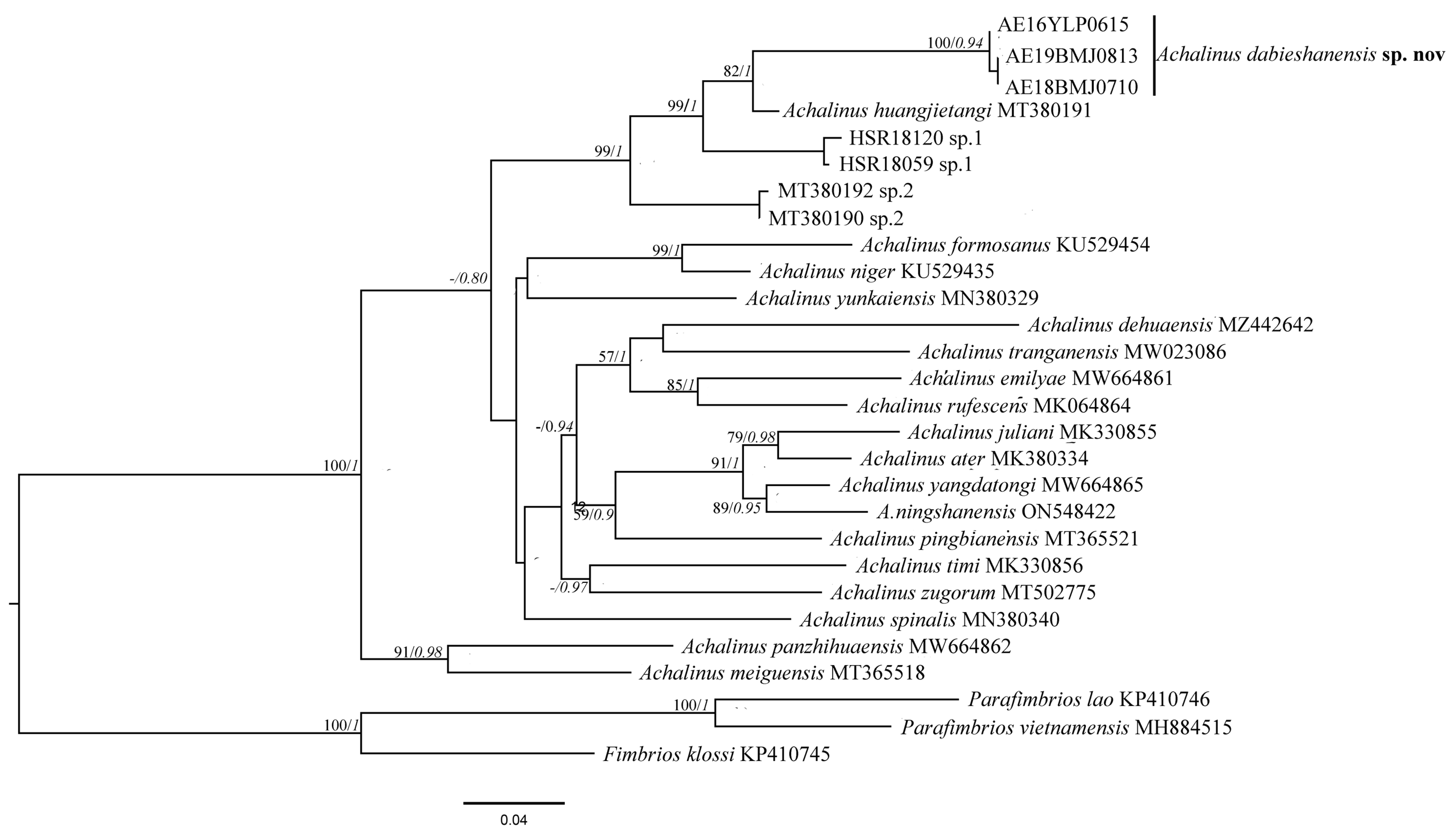
3. Results
3.1. Phylogenetic Relationship
3.2. Taxonomic Account
3.3. Diagnosis
3.4. Comparisons
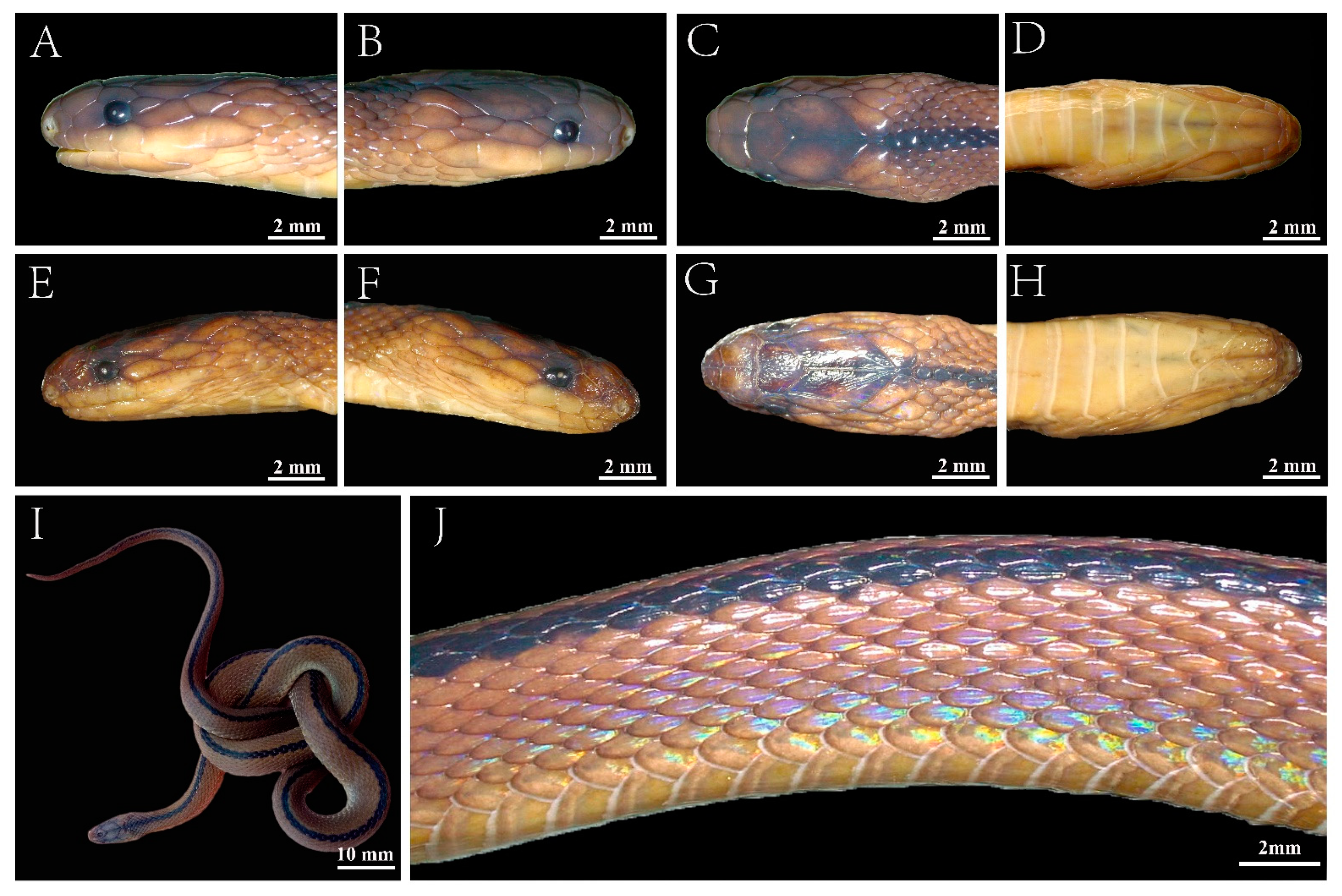
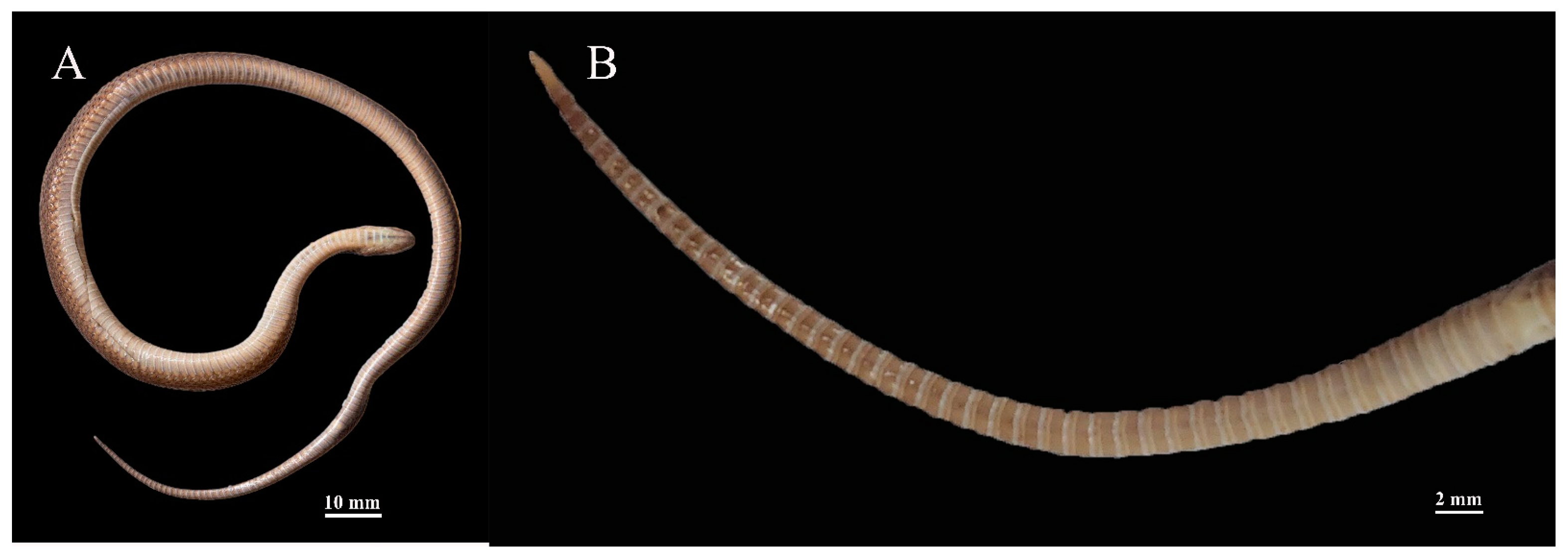
3.5. Description of Holotype
3.6. Intraspecific Morphological Variations
3.7. Etymology
3.8. Distribution and Habitat
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goris, R.C.; Maeda, N. Guide to the Amphibians and Reptiles of Japan; Krieger Publishing Company: Malabar, FL, USA, 2004. [Google Scholar]
- Karsen, S.J.; Lau, M.W.; Bogadek, A. Hong Kong Amphibians and Reptiles; Urban Council: Hong Kong, China, 1986; p. 136.
- Koshikawa, A. Three new species of reptiles from Hainan Island, Guangdong Province. Smithson. Herpetol. Inf. Serv. 1982, 53, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Ota, H.; Toyama, M. Taxonomic re-definition of Achalinus formosanus Boulenger (Xenoderminae: Colubridae: Ophidia), with description of a new subspecies. Copeia 1989, 8, 597–602. [Google Scholar] [CrossRef]
- Van, D.J. Concerning certain species of reptiles and amphibians from China, Japan, the Loo Choo Islands, and Formosa. Proc. Calif. Acad. Sci. 1912, 3, 187–258. [Google Scholar]
- Wang, Y.Y.; Chen, C.Q.; Zhao, J.; Wu, Y.; Liu, Z.T.; Yang, J.H.; Yu, W.H.; Lin, J.S.; Liu, Z.Y.; Wang, J.; et al. Color atlers of terrestrial vertebrates of the Jinggangshan region in China. Beijing Sci. Press 2017, 25, 1321–1330. [Google Scholar]
- Ziegler, T. Die Amphibien und Reptilien eines Tieflandfeuchtwald-Schutzgebietes in Vietnam; Natur & Tier Verlag: Münster, Germany, 2002. [Google Scholar]
- Zhao, E.M. Snakes of China; Anhui Science and Technology Publishing House: Hefei, China, 2006. [Google Scholar]
- Ziegler, T.; Nguyen, T.Q.; Pham, C.T.; Nguyen, T.T.; Pham, A.V.; Schingen, V.S.; Nguyen, T.T.; Le, M.D. Three new species of the snake genus Achalinus from Vietnam (Squamata: Xenodermatidae). Zootaxa 2019, 4590, 249–269. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Zeng, Z.C.; Lyu, Z.T.; Sung, Y.H.; Li, Y.Y.; Yu, C.L.; Wang, Y.Y. A new species of the genus Achalinus from southwestern Guangdong Province, China (Squamata: Xenodermatidae). Zootaxa 2019, 4674, 471–481. [Google Scholar] [CrossRef]
- Li, K.; Yu, M.; Wu, Y.Y.; Liao, L.H.; Tang, K.; Liu, Q.; Guo, P. A new species of the genus Achalinus (Squamata: Xenodermatidae) from southeastern Yunnan Province, China. Zootaxa 2020, 4860, 116–128. [Google Scholar] [CrossRef]
- Luu, Q.V.; Ziegler, T.; Ha, V.N.; Lo, O.V.; Hong, T.T.; Ngo, T.H.; Le, M.D.; Tran, D.H.; Nguyen, T.Q. A new species of Achalinus (Squamata: Xenodermidae) from Trang An Landscape Complex, Ninh Binh Province, Vietnam. Zootaxa 2020, 4877, 174–184. [Google Scholar] [CrossRef]
- Miller, A.H.; Davis, H.R.; Luong, A.M.; Do, Q.H.; Pham, C.T.; Ziegler, T.; Lee, J.L.; De-Queiroz, K.; Reynolds, R.G. Discovery of a new species of enigmatic Odd-Scaled snake (Serpentes: Xenodermidae: Achalinus) from Ha Giang Province, Vietnam. Copeia 2020, 108, 796–808. [Google Scholar] [CrossRef]
- Huang, R.Y.; Peng, L.F.; Yu, L.; Huang, T.Q.; Jiang, K.; Chang, J.K.; Yang, D.C.; Xu, Y.H.; Huang, S. A new species of the genus Achalinus from Huangshan, Anhui, China (Squamata: Xenodermidae). Asian Herpetol. Res. 2021, 12, 1–10. [Google Scholar]
- Hou, S.B.; Wang, K.; Gou, P.; Chen, J.M.; Yuan, Z.Y.; Che, J. Two new species and a new country record of the genus Achalinus (Reptilia: Squamata: Xenodermidae) from China. Zootaxa 2021, 4950, 528–546. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wu, Y.Y.; Xu, R.Y.; Zhu, F.; Ren, J.L.; Guo, P.; Dong, B.J. A new species of the Achalinus rufescens complex (Xenodermidae: Achalinus) from Fujian Province, China. Zootaxa 2021, 5026, 239–254. [Google Scholar] [CrossRef]
- Yang, D.C.; Huang, R.Y.; Jiang, K.; Burbrink, F.T.; Gong, Y.A.; Yu, J.; Zhang, Y.; Huang, T.Q.; Huang, S. A new species of the genus Achalinus (Squamata: Xenodermidae) from Ningshan County, Shaanxi Province, China. Zootaxa 2022, 5190, 127–140. [Google Scholar] [CrossRef]
- Smith, M.A. Reptilia and Amphibia; Taylor & Francis Ltd.: London, UK, 1943. [Google Scholar]
- Zhao, E.M.; Huang, M.H.; Zong, Y. Fauna Sinica: Reptilia, Vol. 3, Squamata, Serpentes; Science Press: Beijing, China, 1998. [Google Scholar]
- Guo, P.; Liu, Q.; Li, J.T.; Zhong, G.H.; Chen, Y.Y.; Wang, Y.Z. Catalogue of the type specimens of amphibians and reptiles in the herpetological museum of the Chengdu institute of biology, Chinese Academy of Sciences: III. snakes excluding Viperids (Reptilia, Serpentes). Asian Herpetol. Res. 2012, 3, 334–339. [Google Scholar]
- Peng, L.F.; Yang, D.C.; Duang, S.Q.; Huang, S. Mitochondrial genome of the common burrowing snake Achalinus spinalis (Reptilia: Xenodermatidae). Mitochondrial DNA Part B 2017, 2, 571–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.W.; Huang, X.; Pan, T.; Zhang, L.; Zhou, W.L.; Song, T.; Han, D.M. Systematics and species validity of the Dabieshan Pit Viper Protobothrops dabieshanensis. Asian Herpetol. Res. 2013, 4, 282–287. [Google Scholar]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1989. [Google Scholar]
- Nagy, Z.T.; Sonet, G.; Glaw, F.; Vences, M. First large-scale DNA barcoding assessment of reptiles in the biodiversity hotspot of Madagascar, based on newly designed COI primers. PLoS ONE 2012, 7, e34506. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef] [Green Version]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [Green Version]
- Nylander, J.A.A. MrModeltest v2. Program Distributed by the Author; Evolutionary Biology Centre: Uppsala, Sweden, 2004. [Google Scholar]
- Ronquist, F.; Teslenko, M.; Van, D.M.P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Soc. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [Green Version]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarization in bayesian phylogenetics using tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rambaut, A. FigTree version 1.4.2. 2014. Available online: http://tree.bio.ed.ac.uk/ (accessed on 22 October 2022).
- Li, J.N.; Liang, D.; Wang, Y.Y.; Guo, P.; Huang, S.; Zhang, P. A large-scale systematic framework of Chinese snakes based on a unified multilocus marker system. Mol. Phylogenetics Evol. 2020, 148, 106807. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.W.; Chen, C.; Zhang, M.H.; Wang, Z.Y.; Ma, H.H.; Sun, R.L.; Jiang, J.P.; Zhang, B.W. A New Species of the Genus Microhyla (Amphibia: Anura: Microhylidae) from the Dabie Mountains, China. Animals 2022, 12(21), 2894. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.L.; Xu, Z.; Zhang, H.; Liu, Y.X.; Liao, R.; Yang, G.D.; Sun, R.L.; Shi, J.; Ban, Q.; Li, C.L.; et al. Description of a new species of the genus Uropsilus (Eulipotyphla: Talpidae: Uropsilinae) from Dabie Mountains, Anhui, Eastern China. Zool. Res. 2021, 42, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Pan, T.; Han, D.; Zhang, L.; Hou, Y.X.; Yu, L.; Zheng, H.; Zhang, B.W. A new species of the genus Protobothrops (Squamata: Viperidae: Crotalinae) from the Dabie Mountains, Anhui, China. Asian Herpetol. Res. 2012, 3, 213–218. [Google Scholar]
- Pan, T.; Zhang, Y.N.; Wang, H.; Wu, J.; Kang, X.; Qian, L.F.; Li, K.; Zhang, Y.; Chen, J.Y.; Rao, D.Q.; et al. A new species of the genus Rhacophorus (Anura: Rhacophoridae) from Dabie Mountains in east China. Asian Herpetol. Res. 2017, 8, 1–13. [Google Scholar]
- Qian, L.F.; Sun, X.N.; Li, J.Q.; Guo, W.B.; Pan, T.; Kang, X.; Wang, H.; Jiang, J.P.; Wu, J.; Zhang, B.W. A new species of the genus Tylototriton (Amphibia: Urodela: Salamandridae) from the southern Dabie Mountains in Anhui Province. Asian Herpetol. Res. 2017, 8, 151–164. [Google Scholar]
- Wang, C.C.; Qian, L.F.; Zhang, C.L.; Guo, W.B.; Pan, T.; Wu, J.; Wang, H.; Zhang, B.W. A new species of Rana from the Dabie Mountains in eastern China (Anura, Ranidae). Zookeys 2017, 724, 135–153. [Google Scholar] [CrossRef] [Green Version]
- Tian, S.; Xu, J.L.; Wang, Y. Human infrastructure development drives decline in suitable habitat for Reeves’s pheasant in the Dabie Mountains in the last 20 years. Glob. Ecol. Conserv. 2020, 22, e00940. [Google Scholar] [CrossRef]


| ID | Species | Specimen Voucher | Locality | GenBank Number |
|---|---|---|---|---|
| 1 | Achalinus dabieshanensis sp. nov. | AHU2016EE0615 | Yaoluoping Nature Reserve, Anhui, China | MW316597 |
| 2 | Achalinus dabieshanensis sp. nov. | AHU2018EE0710 | Fuziling Provincial Reserve, Anhui, China | MW316598 |
| 3 | Achalinus dabieshanensis sp. nov. | AHU2019EE0813 | Yaoluoping Nature Reserve, Anhui, China | MW316596 |
| 4 | Achalinus ater | SYS r000852 | Anjiangping, Guangxi, China | MK064760 |
| 5 | Achalinus dehuaensis | YBU 13013 | Dehua, Fujian, China | MZ442642 |
| 6 | Achalinus emilyae | IEBR 4465 | Hoanh Bo District, Quang Ninh, Vietnam | MK330857 |
| 7 | Achalinus formosanus | RN 2004 | Taiwan Province, China | KU529454 |
| 8 | Achalinus huangjietangi | HSR18030 | Huangjialing Village, Anhui, China | MT380191 |
| 9 | Achalinus juliani | IEBR A.2018.9 | Ha Lang District, Cao Bang, Vietnam | MK330855 |
| 10 | Achalinus meiguensis | GP836 | Mianyang, Sichuan, China | MT365518 |
| 11 | Achalinus niger | RN 1159 | Taiwan Province, China | KU529435 |
| 12 | Achalinus ningshanensis | HSR19232 | Ningshan County, Shaanxi Province, China | ON548423 |
| 13 | Achalinus panzhihuaensis | KIZ 040189 | Hongbao, Yanbian, Sichuan, China | MW664862 |
| 14 | Achalinus pingbianensis | YBU 18273 | Honghe, Yunnan, China | MT365521 |
| 15 | Achalinus rufescens | SYS r001527 | Heishiding, Guangdong, China | MK064864 |
| 16 | Achalinus spinalis | SYS r001327 | Mt. Badagong, Hunan, China | MN380340 |
| 17 | Achalinus timi | IEBR A.2018.10 | Thuan Chau District, Son La, Vietnam | MK330856 |
| 18 | Achalinus tranganensis | VNUF R.2018.21 | Ninh Binh Province, Vietnam | MW023086 |
| 19 | Achalinus yangdatongi | KIZ 034327 | Wenshan Nature Reserve, Yunnan, China | MW664865 |
| 20 | Achalinus yunkaiensis | SYS r001443 | Guangdong, China | MN380329 |
| 21 | Achalinus zugorum | IEBR 4698 | Ha Giang Province, Vietnam | MT502775 |
| Out group | ||||
| 22 | Fimbrios klossi | IEBR A.2013.56 | Gia La, Vietnam | KP410745 |
| 23 | Parafimbrios lao | MNHN 2013.1002 | Louangphabang, Laos | KP410746 |
| 24 | Parafimbrios vietnamensis | IEBR A.2018.7 | Lai Chau, Vietnam | MH884515 |
| ID | Species | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | A. dabieshanensis sp. nov. | |||||||||||||||||||
| 2 | A. huangjietangi | 0.094 | ||||||||||||||||||
| 3 | A. juliani | 0.185 | 0.169 | |||||||||||||||||
| 4 | A. ater | 0.174 | 0.171 | 0.068 | ||||||||||||||||
| 5 | A. yangdatongi | 0.191 | 0.165 | 0.075 | 0.065 | |||||||||||||||
| 6 | A. rufescens | 0.185 | 0.172 | 0.129 | 0.140 | 0.127 | ||||||||||||||
| 7 | A. emilyae | 0.209 | 0.168 | 0.159 | 0.132 | 0.147 | 0.112 | |||||||||||||
| 8 | A. spinalis | 0.186 | 0.150 | 0.161 | 0.172 | 0.160 | 0.141 | 0.173 | ||||||||||||
| 9 | A. yunkaiensis | 0.168 | 0.138 | 0.149 | 0.142 | 0.132 | 0.154 | 0.147 | 0.134 | |||||||||||
| 10 | A. formosanus | 0.217 | 0.179 | 0.144 | 0.161 | 0.163 | 0.163 | 0.158 | 0.160 | 0.139 | ||||||||||
| 11 | A. niger | 0.196 | 0.156 | 0.130 | 0.131 | 0.147 | 0.145 | 0.149 | 0.143 | 0.134 | 0.080 | |||||||||
| 12 | A. pingbianensis | 0.173 | 0.144 | 0.133 | 0.127 | 0.124 | 0.143 | 0.154 | 0.148 | 0.125 | 0.164 | 0.139 | ||||||||
| 13 | A. zugorum | 0.171 | 0.162 | 0.154 | 0.154 | 0.136 | 0.160 | 0.149 | 0.152 | 0.123 | 0.151 | 0.151 | 0.117 | |||||||
| 14 | A. timi | 0.186 | 0.168 | 0.167 | 0.149 | 0.146 | 0.168 | 0.142 | 0.161 | 0.157 | 0.151 | 0.134 | 0.131 | 0.152 | ||||||
| 15 | A. tranganensis | 0.175 | 0.149 | 0.156 | 0.146 | 0.143 | 0.139 | 0.143 | 0.170 | 0.153 | 0.196 | 0.164 | 0.148 | 0.134 | 0.160 | |||||
| 16 | A. meiguensis | 0.196 | 0.173 | 0.187 | 0.167 | 0.194 | 0.208 | 0.175 | 0.175 | 0.170 | 0.173 | 0.149 | 0.191 | 0.162 | 0.174 | 0.174 | ||||
| 17 | A. panzhihuaensis | 0.191 | 0.173 | 0.183 | 0.187 | 0.178 | 0.180 | 0.197 | 0.182 | 0.178 | 0.186 | 0.173 | 0.170 | 0.174 | 0.177 | 0.187 | 0.123 | |||
| 18 | A. ningshanensis | 0.211 | 0.199 | 0.096 | 0.080 | 0.061 | 0.137 | 0.159 | 0.176 | 0.154 | 0.168 | 0.147 | 0.128 | 0.144 | 0.151 | 0.173 | 0.195 | 0.204 | ||
| 19 | A. dehuaensis | 0.211 | 0.189 | 0.165 | 0.191 | 0.158 | 0.144 | 0.178 | 0.162 | 0.168 | 0.187 | 0.175 | 0.168 | 0.164 | 0.176 | 0.152 | 0.201 | 0.174 | 0.189 |
| Voucher | AHU 2016-EE-0615 | AHU 2018-EE-0710 | AHU 2019-EE-0813 |
|---|---|---|---|
| Sex | male | female | Male |
| TL | 376 | 316 | 266 |
| SVL | 287 | 263 | 217 |
| TaL | 84 | 53 | 47 |
| TaL/TL | 22.3% | 16.8% | 17.7% |
| HW | 5.6 | 5.3 | 4.8 |
| HL | 11.2 | 9.5 | 8.7 |
| HH | 4.5 | 3.5 | 3.1 |
| HW/HL | 0.5 | 0.6 | 0.6 |
| SPL | 6 | 6 | 6 |
| SPL–Lorea | 3rd–4th | 3rd–4th | 3rd–4th |
| IFL | 5 | 5 | 5 |
| Loreal | 1 | 1 | 1 |
| HiL | 1.1 | 1.0 | 0.9 |
| LeL | 1.5 | 1.2 | 1.1 |
| HiL/LeL | 0.7 | 0.8 | 0.8 |
| LSBP | 1.2 | 0.8 | 0.9 |
| LSBI | 1.8 | 1.2 | 1.1 |
| LSBP/LSBI | 0.7 | 0.7 | 0.8 |
| LaSN | 0.3 | 0.3 | 0.3 |
| LpSN | 0.8 | 0.7 | 0.5 |
| LaSN/LpSN | 0.4 | 0.4 | 0.6 |
| SpO | 1 | 1 | 1 |
| TMP | 2 + 2 + 4/2 + 2 + 4 | 2 + 2 + 3/2 + 2 + 3 | 2 + 2 + 4/2 + 2 + 4 |
| Elogate aTMP | 2nd | 2nd | 2nd |
| Elogate mTMP | 1st | 1st | 1st |
| Elogate pTMP | 1st | 1st | 1st |
| DSR | 23 + 23 + 23 | 23 + 23 + 23 | 23 + 23 + 23 |
| V | 141 | 155 | 151 |
| SC | 55 | 45 | 46 |
| Species | TL Max. | SVL Max. | TaL Max. | TaL/TL | Int. Fus. | SPL | TMP | Loreal | IFL | aTMP [Eye Contact] | LSBI vs. LSBP | Ant DSR | Mid DSR | Post DSR | V | Post DSR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Achalinus dabieshanensissp. nov | 376 | 287 | 84 | 16.8–22.3% | no | 6 | 2 + 2 + 3 (4) | 1 | 5 | 2 | < | 23 | 23 | 23 | 141–155 | 45–55 |
| A. ater | 401 | - | 70 | 19–22% | no | 6 | 2 + 2 | 1 | 6 | 2 | > | 23 (21) | 23 (21) | 23 (21) | 160–170 | 47–70 |
| A. dehuaensis | 343 | 253 | 90 | 21–29% | no | 6 | 2 + 2 (3) + 3 (4) | 1 | 5 (6) | 1 | > | 23 | 23 | 23 | 142–153 | 63–81 |
| A.emilyae | 519.5 | 424.4 | 95.1 | 18–20.3% | no | 6 | 2 + 2 | 1 | 5 | 2 (1) | > | 23 | 23 | 23 | 157–166 | 60–65 |
| A. formosanus | 853 | 717 | 136 | 16% | no | 6 | 2 + 2 | 0 | 6–7 | 2 (1 or 2) | < | 27 (29) | 25–27 | 25 | 158–169 | 61–83 |
| A. hainanus | 310 | - | 80 | 26–27% | no | 6 | 1 + 2 + 3 | 1 | 5 | 1 | ≥ | 23 | 23 | 23 | 165–168 | 67–69 |
| A. huangjietangi | 404 | 340 | 64 | 15–23% | no | 6 | 2 + 2 + 3 | 1 | 5–6 | 2 | < | 23 | 23 | 23 | 157–170 | 47–67 |
| A. spinalis | 600 | 345 | 83 | 15–25% | no | 6–7 | 2 + 2 (3) | 1 | 6 | 1 | ≤ | 23 (25) | 23 (25) | 23 (25) | 138–175 | 39–69 |
| A. timi | 177.9 | 140 | 37.9 | 21% | no | 6 | 2 + 2 | 0 | 6 | 2 (1) | > | 25 | 25 | 25 | 170 | 72 |
| A. tranganensis | 448 | 334 | 114 | 25% | no | 6 | 2 + 3 | 1 | 6 | 2 | > | 25 | 23 | 23 | 171 | 73+ |
| A. werner | 550 | - | - | 25–30% | no | 6 | 2 + ? | 1 | 6 | 2 | = | 23 (21) | 23 (21) | 23 (21) | 157–191 | 67–98 |
| A. yangdatongi | 397 | 293 | 104 | 26.20% | no | 6 | 2 + 2 + 3 | 1 | 6 | 2 | > | 23 | 23 | 19 | 161 | 82 |
| A. yunkaiensis | 448.1 | 386.3 | 63.3 | 18.5–20.0% | no | 6 | 2 + 2 + 3 (4) | 1 | 6 | 2 | > | 23 | 23 | 23 | 151–162 | 56–79 |
| A. zugorum | 458 | 353 | 105 | 23% | no | 6 | 2 + 2 | 0 | 7 | 2 | > | 25 | 23 | 23 | 173 | 70 |
| A. jinggangensis | 460 | - | 80 | 17–22% | no | 6 | 2 (1) + 2 + 3 (4) | 0 | 6 | 2 (1) | > | 23 | 23 | 23 | 156–164 | 51–64 |
| A. juliani | 413 | 304 | 109 | 22–37% | no | 6(7) | 2 + 2 | 1 | 6 | 2 (2) | > | 25 | 23 | 23 | 173–179 | 77–91 |
| A. meiguensis | 555 | - | 81 | 15–25% | yes | 6 | 2 (3) + 2 (3) | 1 | 6(5) | 2 (3) | no suture | 21 (23) | 19–21 (23) | 19 | 146–173 | 39–60 |
| A. niger | 730 | - | 110 | 15–18% | no | 6 | 2 + 2 | 1 | 6 | 2 | ≤ | 25 | 25 | 23 | 169–185 | 52–72 |
| A. ningshanensis | 527 | 463 | 72 | 12–16% | no | 6 | 2 + 2 + 3 (4) | 1 | 5 | 2 | = | 23 | 23 | 23 (21) | 159–174 | 41–46 |
| A. panzhihuaensis | 257 | 194 | 63 | 24.60% | Yes | 6 | 2 + 2 | 1 | 6 | 2 | no suture | 23 | 23 | 19 | 160 | 73 |
| A. pingbianensis | 429 | 345 | 84 | 24% | no | 7 | 2 + 2 (+3) | 0 | 7 | 2 (1) | = | 23 | 23 | 23 | 160 | 56 |
| A. rufescens | 450 | 210 | 80 | 17–28% | no | 6 | 2 + 2 (3) | 1 | 5 | 1 | > | 23 | 23–25 | 23 | 138–165 | 48–75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Liu, K.; Huang, R.; Hu, T.; Yu, L.; Sun, R.; Zhang, Y.; Wen, J.; Zhang, B. A New Species of the Genus Achalinus (Squamata: Xenodermidae) from the Dabie Mountains, Anhui, China. Animals 2023, 13, 708. https://doi.org/10.3390/ani13040708
Zhang C, Liu K, Huang R, Hu T, Yu L, Sun R, Zhang Y, Wen J, Zhang B. A New Species of the Genus Achalinus (Squamata: Xenodermidae) from the Dabie Mountains, Anhui, China. Animals. 2023; 13(4):708. https://doi.org/10.3390/ani13040708
Chicago/Turabian StyleZhang, Caiwen, Kai Liu, Ruyi Huang, Tingli Hu, Lei Yu, Ruolei Sun, Yucai Zhang, Jing Wen, and Baowei Zhang. 2023. "A New Species of the Genus Achalinus (Squamata: Xenodermidae) from the Dabie Mountains, Anhui, China" Animals 13, no. 4: 708. https://doi.org/10.3390/ani13040708





