Species-Specific Spatial Patterns of Variation in Sexual Dimorphism by Two Lizards Settled in the Same Geographic Context
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
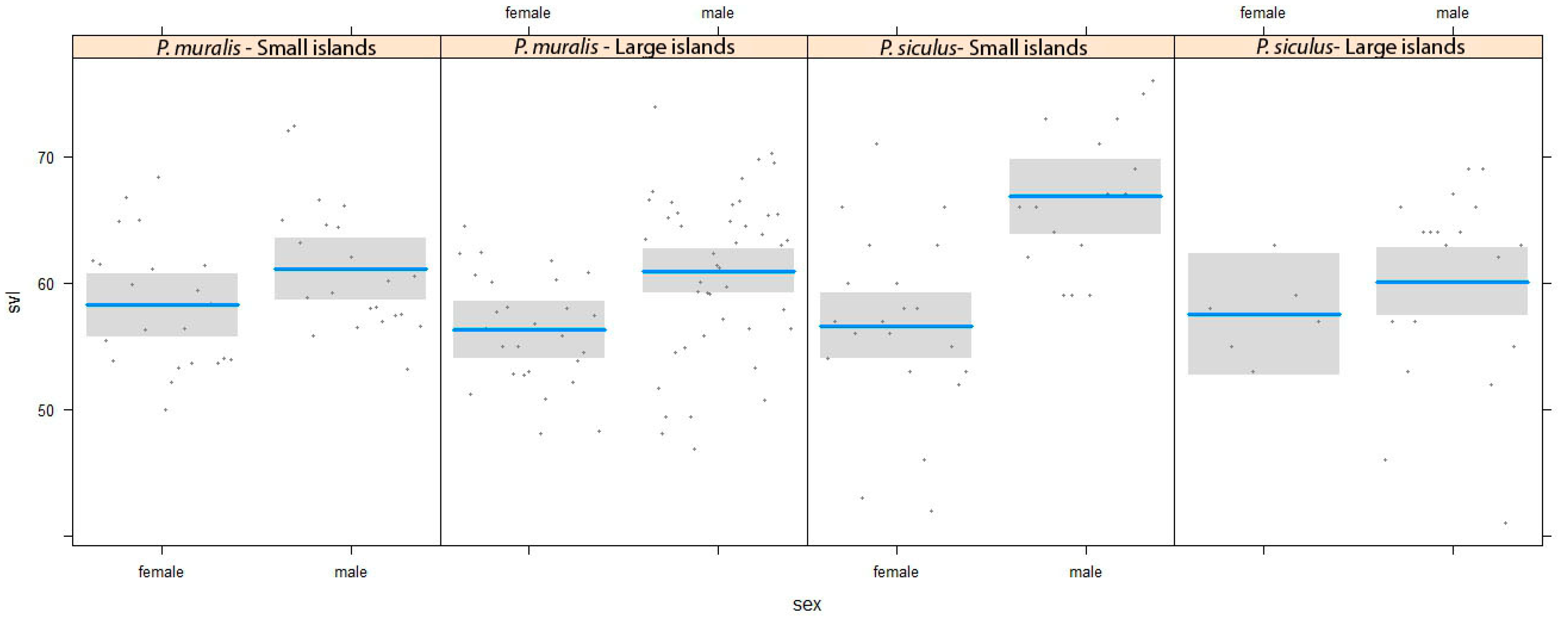
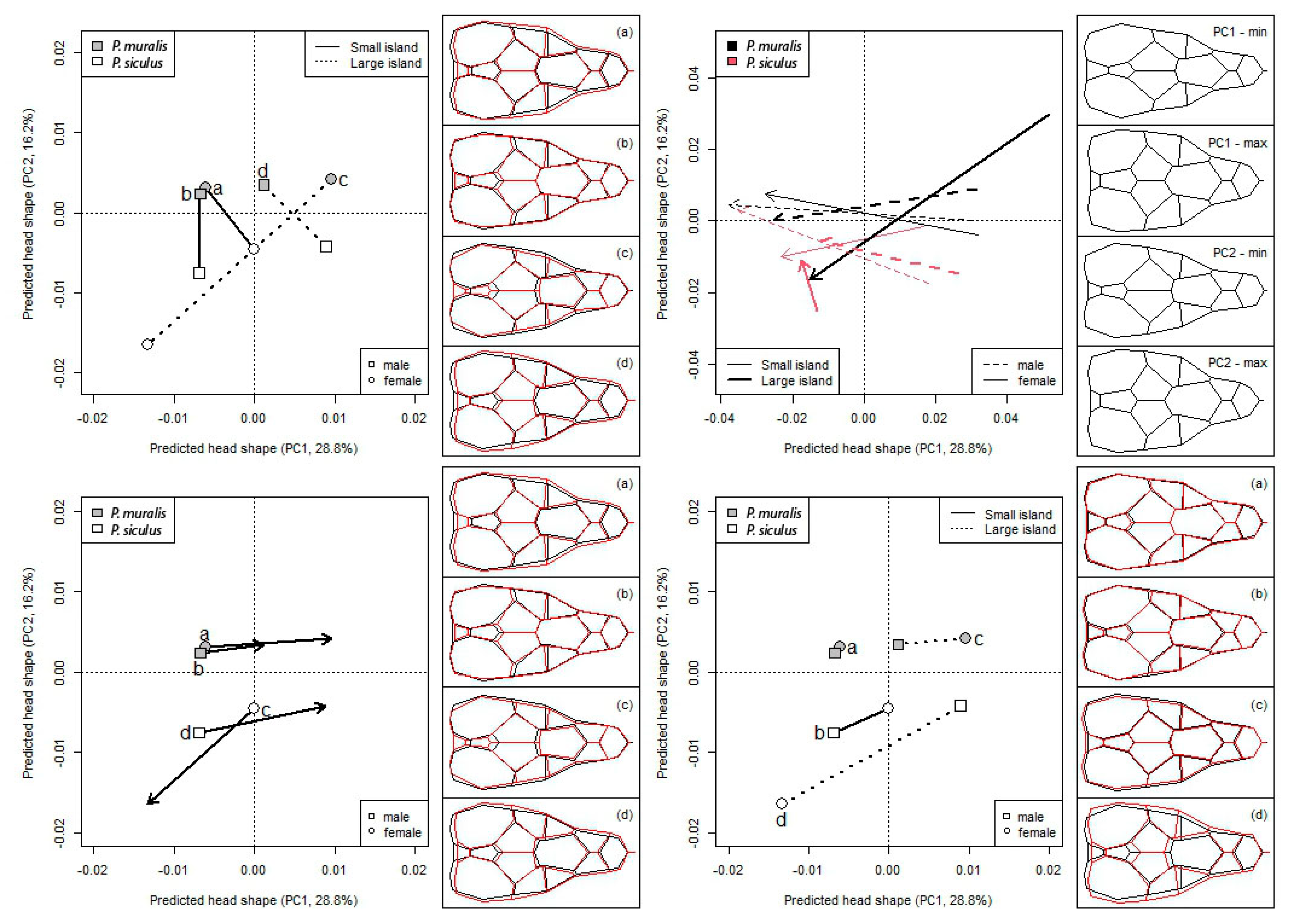
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Slatkin, M. Ecological Causes of Sexual Dimorphism. Evolution 1984, 38, 622–630. [Google Scholar] [CrossRef]
- Bulté, G.; Irschick, D.J.; Blouin-Demers, G. The Reproductive Role Hypothesis Explains Trophic Morphology Dimorphism in the Northern Map Turtle. Funct. Ecol. 2008, 22, 824–830. [Google Scholar] [CrossRef]
- Darwin, C.R. The Descent of Man and Selection in Relation to Sex; John Murray: London, UK, 1871; Available online: http://darwin-online.org.uk/content/frameset?pageseq=1&itemID=F937.1&viewtype=text (accessed on 6 January 2023).
- Andersson, M. Sexual Selection; Princeton University Press: New York, NY, USA, 1994. [Google Scholar]
- Stuart-Fox, D.; Moussalli, A. Sex-Specific Ecomorphological Variation and the Evolution of Sexual Dimorphism in Dwarf Chameleons (Bradypodion Spp.). J. Evol. Biol. 2007, 20, 1073–1081. [Google Scholar] [CrossRef]
- Kaliontzopoulou, A.; Carretero, M.A.; Llorente, G.A. Intraspecific Ecomorphological Variation: Linear and Geometric Morphometrics Reveal Habitat-Related Patterns within Podarcis Bocagei Wall Lizards. J. Evol. Biol. 2010, 23, 1234–1244. [Google Scholar] [CrossRef]
- Bonduriansky, R. The Evolution of Condition-Dependent Sexual Dimorphism. Am. Nat. 2007, 169, 9–19. [Google Scholar] [CrossRef]
- Andersson, M.; Lwasa, Y. Sexual Selection and Mate Choice. Trends Ecol. Evol. 1996, 11, 6. [Google Scholar] [CrossRef]
- Casselman, S.J.; Schulte-Hostedde, A.I. Reproductive Roles Predict Sexual Dimorphism in Internal and External Morphology of Lake Whitefish, Coregonus Clupeaformis. Ecol. Freshw. Fish 2004, 13, 217–222. [Google Scholar] [CrossRef]
- Muraro, M.; Sherpa, S.; Barzaghi, B.; Bombi, P.; Borgatti, D.; Di Canio, V.; Dalpasso, A.; Falaschi, M.; Gambioli, B.; Manenti, R.; et al. Condition - and Context - Dependent Variation of Sexual Dimorphism across Lizard Populations at Different Spatial Scales. Sci. Rep. 2022, 1–10. [Google Scholar] [CrossRef]
- Cothran, R.D.; Jeyasingh, P.D. Condition Dependence of a Sexually Selected Trait in a Crustacean Species Complex: Importance of the Ecological Context. Evolution 2010, 64, 2535–2546. [Google Scholar] [CrossRef]
- Kemp, D.J. Resource-Mediated Condition Dependence in Sexually Dichromatic Butterfly Wing Coloration. Evolution 2008, 62, 2346–2358. [Google Scholar] [CrossRef]
- Okada, K.; Miyatake, T. Plasticity of Size and Allometry in Multiple Sexually Selected Traits in an Armed Beetle Gnatocerus Cornutus. Evol. Ecol. 2010, 24, 1339–1351. [Google Scholar] [CrossRef]
- Bonduriansky, R.; Mallet, M.A.; Arbuthnott, D.; Pawlowsky-Glahn, V.; Jos Egozcue, J.; Rundle, H.D. Differential Effects of Genetic vs. Environmental Quality in Drosophila Melanogaster Suggest Multiple Forms of Condition Dependence. Ecol. Lett. 2015, 18, 317–326. [Google Scholar] [CrossRef]
- Sacchi, R.; Mangiacotti, M.; Scali, S.; Sannolo, M.; Zuffi, M.A.L.; Pellitteri-Rosa, D.; Bellati, A.; Galeotti, P.; Fasola, M. Context-Dependent Expression of Sexual Dimorphism in Island Populations of the Common Wall Lizard (Podarcis Muralis). Biol. J. Linn. Soc. 2015, 114, 552–565. [Google Scholar] [CrossRef]
- Post, E.; Langvatn, R.; Forchhammer, M.C.; Stenseth, N.C. Environmental Variation Shapes Sexual Dimorphism in Red Deer. Proc. Natl. Acad. Sci. USA 1999, 96, 4467–4471. [Google Scholar] [CrossRef]
- Weladji, R.B.; Holand, Ø.; Steinheim, G.; Colman, J.E.; Gjøstein, H.; Kosmo, A. Sexual Dimorphism and Intercorhort Variation in Reindeer Calf Antler Length Is Associated with Density and Weather. Oecologia 2005, 145, 549–555. [Google Scholar] [CrossRef]
- Schoener, T.W. Competition and the Niche in Reptiles. In Biology of the Reptilia; Gans, C., Tinkel, D., Eds.; Academic Press: New York, NY, USA, 1977; pp. 35–136. [Google Scholar]
- Cox, R.M.; Skelly, S.L.; John-Alder, H.B. A Comparative Test of Adaptive Hypotheses for Sexual Size Dimorphism in Lizards. Evolution 2003, 57, 1653–1669. [Google Scholar] [CrossRef]
- Kaliontzopoulou, A.; Carretero, M.Á.; Llorente, G.A. Multivariate and Geometric Morphometrics in the Analysis of Sexual Dimorphism Variation in Podarcis Lizards. J. Morphol. 2007, 268, 254–274. [Google Scholar] [CrossRef]
- Olsson, M.; Shine, R.; Wapstra, E.; Ujvari, B.; Madsen, T. Sexual Dimorphism in Lizard Body Shape: The Roles of Sexual Selection and Fecundity Selection. Evolution 2002, 56, 1538–1542. [Google Scholar] [CrossRef]
- Braña, F. Sexual Dimorphism in Lacertid Lizards: Male Head Increase vs Female Abdomen Increase? OIKOS 1996, 75, 511–523. [Google Scholar] [CrossRef]
- Herrel, A.; Van Damme, R.; de Vree, F. Sexual Dimorphism of Head Size in Podarcis Hispanica Atrata: Testing the Diatary Divergence Hypothesis by Bite Force Analysis. Netherlands J. Zool. 1996, 46, 253–262. [Google Scholar]
- Herrel, A.; Van Damme, R.; Vanhooydonck, B.; De Vree, F. The Implications of Bite Performance for Diet in Two Species of Lacertid Lizards. Can. J. Zool. 2001, 79, 662–670. [Google Scholar] [CrossRef]
- Lailvaux, S.P.; Irschick, D.J. The Evolution of Performance-Based Male Fighting Ability in Caribbean Anolis Lizards. Am. Nat. 2007, 170, 573–586. [Google Scholar] [CrossRef]
- Kaliontzopoulou, A.; Adams, D.C.; van der Meijden, A.; Perera, A.; Carretero, M.A. Relationships between Head Morphology, Bite Performance and Ecology in Two Species of Podarcis Wall Lizards. Evol. Ecol. 2012, 26, 825–845. [Google Scholar] [CrossRef]
- Sillero, N.; Campos, J.; Bonardi, A.; Corti, C.; Creemers, R.; Crochet, P.A.; Isailović, J.C.; Denoël, M.; Ficetola, G.F.; Gonçalves, J.; et al. Updated Distribution and Biogeography of Amphibians and Reptiles of Europe. Amphib. Reptil. 2014, 35, 1–31. [Google Scholar] [CrossRef]
- Corti, C.; Bologna, M.A.; Capula, M. Podarcis Siculus (Rafinesque-Schmaltz, 1810). In Fauna d’Italia, Reptilia; Corti, C., Capula, M., Luiselli, L., Razzetti, E., Sindaco, R., Eds.; Calderini: Bologna, Italy, 2011; pp. 406–417. [Google Scholar]
- Biaggini, M.; Bombi, P.; Capula, M.; Corti, C. Podarcis Muralis (Laurenti, 1768). In Fauna d’Italia; Corti, C., Capula, M., Luiselli, L., Razzetti, E., Eds.; Calderini: Bologna, Italy, 2011; pp. 391–401. [Google Scholar]
- Barbault, R.; Mou, Y.-P. Population Dynamics of the Common Wall Lizard, Podarcis Muralis, in Southwestern France. Herpetologica 1988, 44, 38–47. [Google Scholar]
- Scali, S.; Sacchi, R.; Mangiacotti, M.; Pupin, F.; Gentilli, A.; Zucchi, C.; Sannolo, M.; Pavesi, M.; Zuffi, M.A.L. Does a Polymorphic Species Have a ‘ Polymorphic ’ Diet ? A Case Study from a Lacertid Lizard. Biol. J. Linn. Soc. 2015, 117, 492–502. [Google Scholar] [CrossRef]
- Avery, R.A. Activity Patterns, Thermoregulation and Food Consumption in Two Sympatric Lizard Species (Podarcis Muralis and P. Sicula) from Central Italy. J. Anim. Ecol. 1978, 47, 143–158. [Google Scholar] [CrossRef]
- Verwaijen, D.; Van Damme, R. Foraging Mode and Its Flexibility in Lacertid Lizards from Europe. J. Herpetol. 2008, 42, 124–133. [Google Scholar] [CrossRef]
- Fornasiero, S.; Zuffi, M.A.L. Anfibi e Rettili Dell’Arcipelago Toscano; Banfi, A., Giannini, F., Montauti, G., Eds.; Parco Nazionale Arcipelago Toscano: Livorno, Italy, 2006. [Google Scholar]
- Lanza, B. Sul Significato Biogeografico Delle Isole Fossili, Con Particolare Riferimento All’arcipelago Pliocenico Della Toscana. Atti Soc. Ital. Sci. Nat. 1984, 125, 145–158. [Google Scholar] [CrossRef]
- Bonardi, A.; Ficetola, G.F.; Razzetti, E.; Canedoli, C.; Falaschi, M.; Lo Parrino, E.; Rota, N.; Padoa-Schioppa, E.; Sindaco, R. ReptIslands: Mediterranean Islands and the Distribution of Their Reptile Fauna. Glob. Ecol. Biogeogr. 2022, 31, 840–847. [Google Scholar] [CrossRef]
- Rohlf, F.J. The Tps Series of Software. Hystrix 2015, 26, 1–4. [Google Scholar] [CrossRef]
- Klingenberg, C.P.; Barluenga, M.; Meyer, A. Shape Analysis of Symmetric Structures: Quantifying Variation among Individuals and Asymmetry. Evolution 2002, 56, 1909–1920. [Google Scholar] [CrossRef]
- Anderson, M.J. A New Method for Non-Parametric Multivariate Analysis of Variance. Aust. Ecol. 2006, 26, 32–46. [Google Scholar]
- Adams, D.C.; Collyer, M.L. A General Framework for the Analysis of Phenotypic Trajectories in Evolutionary Studies. Evolution 2009, 63, 1143–1154. [Google Scholar]
- Collyer, M.L.; Adams, D.C. RRPP: An r Package for Fitting Linear Models to High-Dimensional Data Using Residual Randomization. Methods Ecol. Evol. 2018, 9, 1772–1779. [Google Scholar] [CrossRef]
- Adams, D.C.; Collyer, M.L.; Kaliontzopoulou, A.; Baken, E.K. Geomorph: Software for Geometric Morphometric Analyses. R Package Version 4.0.4. Available online: https://www.semanticscholar.org/paper/geomorph%3A-Software-for-geometric-morphometric-Adams-Collyer/2fb3968b4a251d1b806ae4dd656fe34b8289c11c (accessed on 6 January 2023).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Cardini, A.; Seetah, K.; Barker, G. How Many Specimens Do I Need? Sampling Error in Geometric Morphometrics: Testing the Sensitivity of Means and Variances in Simple Randomized Selection Experiments. Zoomorphology 2015, 134, 149–163. [Google Scholar] [CrossRef]
- Millien, V. Morphological Evolution Is Accelerated among Island Mammals. PLoS Biol. 2006, 4, 1863–1868. [Google Scholar] [CrossRef]
- Alzate, A.; Etienne, R.S.; Bonte, D. Experimental Island Biogeography Demonstrates the Importance of Island Size and Dispersal for the Adaptation to Novel Habitats. Glob. Ecol. Biogeogr. 2019, 28, 238–247. [Google Scholar] [CrossRef]
- Kaliontzopoulou, A.; Carretero, M.Á.; Llorente, G.A. Head Shape Allometry and Proximate Causes of Head Sexual Dimorphism in Podarcis Lizards: Joining Linear and Geometric Morphometrics. Biol. J. Linn. Soc. 2008, 93, 111–124. [Google Scholar] [CrossRef]
- Herrel, A.; De Grauw, E.; Lemos-Espinal, J.A. Head Shape and Bite Performance in Xenosaurid Lizards. J. Exp. Zool. 2001, 290, 101–107. [Google Scholar] [CrossRef]
- Arnold, E.N. Cranial Kinesis in Lizards. Evol. Biol. 1988, 30, 323–357. [Google Scholar] [CrossRef]
- Yang, W.; Feiner, N.; Pinho, C.; While, G.M.; Kaliontzopoulou, A.; Harris, D.J.; Salvi, D.; Uller, T. Extensive Introgression and Mosaic Genomes of Mediterranean Endemic Lizards. Nat. Commun. 2021, 12, 2762. [Google Scholar] [CrossRef]

| Island | Size (Km2) | Type | Species | Males | Females |
|---|---|---|---|---|---|
| Mount Argentario | 60.23 | large | P. muralis P. siculus | 7 5 | 7 1 |
| Capraia Island | 19.24 | large | P. siculus | 3 | 5 |
| Elba Island | 223 | large | P. muralis P. siculus | 29 14 | 15 5 |
| Giannutri Island | 2.39 | small | P. siculus | 7 | 5 |
| Giglio Island | 21.47 | large | P. siculus | 3 | 7 |
| Gorgona Island | 2.27 | small | P. muralis | 10 | 10 |
| Palmaiola Islet | 0.09 | small | P. muralis | 13 | 9 |
| Pianosa Island | 10.41 | small | P. muralis P. siculus | 13 3 | 12 4 |
| Mount Massoncello | 34.59 | large | P. muralis | 10 | 5 |
| Model Predictor | df | F | p |
|---|---|---|---|
| Species | 1,164 | 9.097 | 0.001 |
| Sex | 1,164 | 0.646 | 0.741 |
| Island size | 1,164 | 5.184 | 0.001 |
| lnCS | 1,164 | 44.172 | 0.001 |
| Species × Sex | 1,164 | 0.498 | 0.919 |
| Species × Island size | 1,164 | 1.763 | 0.065 |
| Sex × Island size | 1,164 | 1.428 | 0.149 |
| Species × lnCS | 1,164 | 1.348 | 0.193 |
| Sex × lnCS | 1,164 | 1.056 | 0.355 |
| Island size × lnCS | 1,164 | 3.073 | 0.002 |
| Species × Sex × Island size | 1,164 | 2.574 | 0.004 |
| Species × Sex × lnCS | 1,164 | 0.549 | 0.882 |
| Species × Island size × lnCS | 1,164 | 1.090 | 0.338 |
| Sex × Island size × lnCS | 1,164 | 0.750 | 0.672 |
| Species × Sex × Island size × lnCS | 1,164 | 2.148 | 0.035 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sacchi, R.; Mangiacotti, M.; Scali, S.; Storniolo, F.; Zuffi, M.A.L. Species-Specific Spatial Patterns of Variation in Sexual Dimorphism by Two Lizards Settled in the Same Geographic Context. Animals 2023, 13, 736. https://doi.org/10.3390/ani13040736
Sacchi R, Mangiacotti M, Scali S, Storniolo F, Zuffi MAL. Species-Specific Spatial Patterns of Variation in Sexual Dimorphism by Two Lizards Settled in the Same Geographic Context. Animals. 2023; 13(4):736. https://doi.org/10.3390/ani13040736
Chicago/Turabian StyleSacchi, Roberto, Marco Mangiacotti, Stefano Scali, Federico Storniolo, and Marco A. L. Zuffi. 2023. "Species-Specific Spatial Patterns of Variation in Sexual Dimorphism by Two Lizards Settled in the Same Geographic Context" Animals 13, no. 4: 736. https://doi.org/10.3390/ani13040736
APA StyleSacchi, R., Mangiacotti, M., Scali, S., Storniolo, F., & Zuffi, M. A. L. (2023). Species-Specific Spatial Patterns of Variation in Sexual Dimorphism by Two Lizards Settled in the Same Geographic Context. Animals, 13(4), 736. https://doi.org/10.3390/ani13040736






