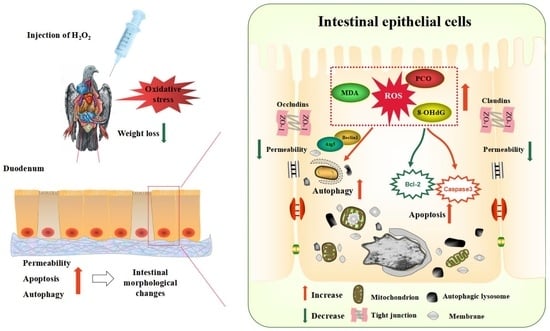
Effects of Hydrogen Peroxide-Induced Oxidative Stress on Intestinal Morphology, Redox Status, and Related Molecules in Squabs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of H2O2 Solution
2.2. Experimental Design, Animal, and Management
2.3. Sample Collection
2.4. Growth Performance
2.5. Hematoxylin-Eosin (HE) Staining
2.6. Transmission Electron Microscopy (TEM)
2.7. Detection of Oxidative Stress Markers in Serum and Duodenum
2.8. RNA Isolation and Quantitative Real-Time PCR Analysis (qPCR)
3. Results
3.1. Growth Performance
3.2. Intestinal Histomorphology under Optical Microscope
3.3. Ultrastructure of Intestinal Epithelial Cells under Transmission Electron Microscope
3.4. Content of Oxidative Stress Markers in the Serum and Duodenum
3.5. Analyses of Expression of Genes Involved in the Regulation of Intestinal Barrier, Autophagy, and Apoptosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, Q.; Li, H.; Zhou, W.; Zou, X.; Dong, X. Age-Related changes in serum lipid levels, hepatic morphology, antioxidant status, lipid metabolism related gene expression and enzyme activities of domestic pigeon squabs (Columba livia). Animals 2020, 10, 1121. [Google Scholar] [CrossRef] [PubMed]
- Sales, J.; Janssens, G.P.J. Nutrition of the domestic pigeon (Columba livia domestica). World’s Poult. Sci. J. 2003, 59, 221–232. [Google Scholar] [CrossRef] [Green Version]
- Ge, P.; Ma, H.; Li, Y.; Ni, A.; Isa, A.M.; Wang, P.; Bian, S.; Shi, L.; Zong, Y.; Wang, Y.; et al. Identification of microRNA-Associated-ceRNA networks regulating crop milk production in pigeon (Columba livia). Genes 2021, 12, 39. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.Y.; Wang, Y.M.; Dai, L.; Azzam, M.M.M.; Wang, C.; Zou, X.T. Posthatch development of intestinal morphology and digestive enzyme activities in domestic pigeons (Columba livia). Poult. Sci. 2012, 91, 1886–1892. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.Q.; Yang, J.X.; Chen, M.X.; Yan, H.C.; Wang, X.Q. Growth curves and age-related changes in carcass characteristics, organs, serum parameters, and intestinal transporter gene expression in domestic pigeon (Columba livia). Poult. Sci. 2016, 95, 867–877. [Google Scholar] [CrossRef]
- Mishra, B.; Jha, R. Oxidative stress in the poultry gut: Potential challenges and interventions. Front. Vet. Sci. 2019, 6, 60–64. [Google Scholar] [CrossRef] [Green Version]
- Surai, P.F.; Kochish, I.I.; Fisinin, V.I.; Kidd, M.T. Antioxidant defence systems and oxidative stress in poultry biology: An update. Antioxidants 2019, 8, 235. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Xing, T.; Li, J.; Zhang, L.; Jiang, Y.; Gao, F. Hydrogen peroxide-induced oxidative stress impairs redox status and damages aerobic metabolism of breast muscle in broilers. Poult. Sci. 2021, 100, 918–925. [Google Scholar] [CrossRef]
- Surai, P.F.; Kochish, I.I.; Romanov, M.N.; Griffin, D.K. Nutritional modulation of the antioxidant capacities in poultry: The case of vitamin E. Poult. Sci 2019, 98, 4030–4041. [Google Scholar] [CrossRef]
- Circu, M.L.; Aw, T.Y. Intestinal redox biology and oxidative stress. Semin. Cell Dev. Biol. 2012, 23, 729–737. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T. Analysis of relative gene expression data using real-time quantitative PCR and the 2-DDCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zhang, Q.; Wang, X.; Yuan, M.; Zhang, Y.; Xu, Z.; Li, G.; Liu, T. Reactive oxygen species mediated oxidative stress links diabetes and atrial fibrillation. Mol. Med. Rep. 2018, 17, 4933–4940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, J.F.; Evans, J.W.; Limoli, C.L.; Calabro-Jones, P. Radiation and hydrogen peroxide induced free radical damage to DNA. Br. J. Cancer Suppl. 1987, 55, 105–111. [Google Scholar]
- Wang, F.; Liu, J.; Hu, X.; Zhong, Y.; Wen, F.; Tang, X.; Yang, S.; Zhong, S.; Zhou, Z.; Yuan, X.; et al. The influence on oxidative stress markers, inflammatory factors and intestinal injury-related molecules in Wahui pigeon induced by lipopolysaccharide. PLoS ONE 2021, 16, e251462. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhang, Y.; Lan, J.; Liu, H.; Li, W.; Wu, Y.; Leng, Y.; Tang, L.; Hou, J.; Sun, Q.; et al. Ischemic postconditioning alleviates intestinal Ischemia-Reperfusion injury by enhancing autophagy and suppressing oxidative stress through the Akt/GSK-3β/Nrf2 pathway in mice. Oxid. Med. Cell. Longev. 2020, 2020, 6954764. [Google Scholar] [CrossRef] [Green Version]
- Hauck, A.K.; Huang, Y.; Hertzel, A.V.; Bernlohr, D.A. Adipose oxidative stress and protein carbonylation. J. Biol. Chem. 2019, 294, 1083–1088. [Google Scholar] [CrossRef] [Green Version]
- Cao, S.; Wu, H.; Wang, C.C.; Zhang, Q.; Jiao, L.; Lin, F.; Hu, C.H. Diquat-induced oxidative stress increases intestinal permeability, impairs mitochondrial function, and triggers mitophagy in piglets. J. Anim. Sci. 2018, 96, 1795–1805. [Google Scholar] [CrossRef]
- Chen, X.; Gu, R.; Zhang, L.; Li, J.; Jiang, Y.; Zhou, G.; Gao, F. Induction of nuclear factor-κB signal-mediated apoptosis and autophagy by reactive oxygen species is associated with hydrogen peroxide-impaired growth performance of broilers. Animal 2018, 12, 2561–2570. [Google Scholar] [CrossRef]
- Liu, C.; Chaudhry, M.T.; Zhao, D.; Lin, T.; Tian, Y.; Fu, J. Heat shock protein 70 protects the quail cecum against oxidant stress, inflammatory injury, and microbiota imbalance induced by cold stress. Poult. Sci. 2019, 98, 5432–5445. [Google Scholar] [CrossRef]
- Okumura, R.; Takeda, K. Maintenance of intestinal homeostasis by mucosal barriers. Inflamm. Regen. 2018, 38, 5–13. [Google Scholar] [CrossRef]
- Pi, D.; Liu, Y.; Shi, H.; Li, S.; Odle, J.; Lin, X.; Zhu, H.; Chen, F.; Hou, Y.; Leng, W. Dietary supplementation of aspartate enhances intestinal integrity and energy status in weanling piglets after lipopolysaccharide challenge. J. Nutr. Biochem. 2014, 25, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Yu, B.; He, J.; Yu, J.; Mao, X.; Luo, Y.; Luo, J.; Huang, Z.; Tian, G.; Zeng, Q.; et al. Arginine metabolism and its protective effects on intestinal health and functions in weaned piglets under oxidative stress induced by diquat. Br. J. Nutr. 2017, 117, 1495–1502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sellin, M.E.; Muller, A.A.; Felmy, B.; Dolowschiak, T.; Diard, M.; Tardivel, A.; Maslowski, K.M.; Hardt, W.D. Epithelium-intrinsic NAIP/NLRC4 inflammasome drives infected enterocyte expulsion to restrict Salmonella replication in the intestinal mucosa. Cell Host Microbe 2014, 16, 237–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gou, Z.Y.; Li, L.; Fan, Q.L.; Lin, X.J.; Jiang, Z.Y.; Zheng, C.T.; Ding, F.Y.; Jiang, S.Q. Effects of oxidative stress induced by high dosage of dietary iron ingested on intestinal damage and caecal microbiota in Chinese Yellow broilers. J. Anim. Physiol. An. N. 2018, 102, 924–932. [Google Scholar] [CrossRef]
- Zihni, C.; Mills, C.; Matter, K.; Balda, M.S. Tight junctions: From simple barriers to multifunctional molecular gates. Nat. Rev. Mol. Cell Biol. 2016, 17, 564–580. [Google Scholar] [CrossRef]
- Tsukita, S.; Furuse, M.; Itoh, M. Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell Biol. 2001, 2, 285–293. [Google Scholar] [CrossRef]
- Andrade, M.E.R.; Araújo, R.S.; de Barros, P.A.V.; Soares, A.D.N.; Abrantes, F.A.; Generoso, S.D.V.; Fernandes, S.O.A.; Cardoso, V.N. The role of immunomodulators on intestinal barrier homeostasis in experimental models. Clin. Nutr. 2015, 34, 1080–1087. [Google Scholar] [CrossRef]
- Xu, Q.; Jian, H.; Zhao, W.; Li, J.; Zou, X.; Dong, X. Early weaning stress induces intestinal microbiota disturbance, mucosal barrier dysfunction and inflammation response activation in pigeon squabs. Front. Microbiol. 2022, 13, 877866. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wu, K.; Xue, D.; Zhang, C.; Rajput, S.A.; Qi, D. Mechanism of deoxynivalenol mediated gastrointestinal toxicity: Insights from mitochondrial dysfunction. Food Chem. Toxicol. 2021, 153, 112214. [Google Scholar] [CrossRef]
- Brokatzky, D.; Dorflinger, B.; Haimovici, A.; Weber, A.; Kirschnek, S.; Vier, J.; Metz, A.; Henschel, J.; Steinfeldt, T.; Gentle, I.E.; et al. A non-death function of the mitochondrial apoptosis apparatus in immunity. EMBO J. 2019, 38, e100907. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Bonneau, B.; Prudent, J.; Popgeorgiev, N.; Gillet, G. Non-apoptotic roles of Bcl-2 family: The calcium connection. Biochim. Et Biophys. Acta BBA Mol. Cell Res. 2013, 1833, 1755–1765. [Google Scholar] [CrossRef] [Green Version]
- Crowley, L.C.; Waterhouse, N.J. Detecting cleaved caspase-3 in apoptotic cells by flow cytometry. Cold Spring Harb. Protoc. 2016, 2016, 958–962. [Google Scholar] [CrossRef] [PubMed]
- Shaoyong, W.; Zhang, W.; Wang, C.; Kou, Z.; Yong, W.; Jiao, J.; Yan, W.; Pang, W. BDE-209 caused gut toxicity through modulating the intestinal barrier, oxidative stress, autophagy, inflammation, and apoptosis in mice. Sci. Total Environ. 2021, 776, 146018–146033. [Google Scholar] [CrossRef]
- Quinn, P.M.J.; Moreira, P.I.; Ambrósio, A.F.; Alves, C.H. PINK1/PARKIN signalling in neurodegeneration and neuroinflammation. Acta Neuropathol. Commun. 2020, 8, 189–209. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Baehrecke, E.H.; Ballabio, A.; Boya, P.; Bravo-San Peo, J.M.; Cecconi, F.; Choi, A.M.; Chu, C.T.; Codogno, P.; Colombo, M.I.; et al. Molecular definitions of autophagy and related processes. EMBO J. 2017, 36, 1811–1836. [Google Scholar] [CrossRef]
- Wang, X.; Wu, R.; Liu, Y.; Zhao, Y.; Bi, Z.; Yao, Y.; Liu, Q.; Shi, H.; Wang, F.; Wang, Y. M6A mRNA methylation controls autophagy and adipogenesis by targetingAtg5 and Atg7. Autophagy 2020, 16, 1221–1235. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Lu, S.; Su, Y.; Ding, D.; Tao, L.; Wang, M.; Wang, Y.; Liu, X. C/EBP-α induces autophagy by binding to Beclin1 through its own acetylation modification in activated hepatic stellate cells. Exp. Cell Res. 2021, 405, 112721–112733. [Google Scholar] [CrossRef] [PubMed]







| Item | Content | Nutrient Levels 2 | |
|---|---|---|---|
| Ingredients (%) | ME/(MJ/kg) | 11.90 | |
| Corn | 40.00 | CP(%) | 22.97 |
| Wheat bran | 4.18 | EE(%) | 4.64 |
| Wheat middling | 10.00 | CF(%) | 5.73 |
| Soybean meal | 32.50 | Ca(%) | 1.09 |
| Rice bran meal | 5.00 | TP(%) | 0.59 |
| Soybean oil | 4.00 | Lys(%) | 1.26 |
| Premix 1 | 0.55 | Met(%) | 0.36 |
| CaHPO4 | 0.59 | ||
| Limestone | 1.73 | ||
| Lys | 0.12 | ||
| Met | 0.12 | ||
| NaHCO3 | 0.08 | ||
| Choline chloride | 0.08 | ||
| Antifungal agent | 0.10 | ||
| NaCl | 0.20 | ||
| Zeolite | 0.75 | ||
| Total | 100.00 |
| Item | Content | Nutrient Levels 1 | |
|---|---|---|---|
| Corn | 37.50 | ME/(MJ/kg) | 13.00 |
| Sorghum | 31.25 | CP | 12.06 |
| Wheat | 15.625 | EE | 3.21 |
| Peas | 15.625 | CF | 3.08 |
| Ca | 0.03 | ||
| TP | 0.3 | ||
| Lys | 0.47 | ||
| Total | 100 | Met | 0.15 |
| Genes | Forward Primer Sequence (5′→3′) | Reverse Primer Sequence (5′→3′) | Product Size (bp) | Accession Number |
|---|---|---|---|---|
| β-actin | F: CCCATCTACGAAGGCTACGC | R: CTTGATGTCACGCACAATTTC | 149 | XM_005504502.2 |
| Occludin | F: CAGGACGTGGCAGAGGA | R: GTGGAAGAGCTTGTTGCGT | 105 | XM_005509325.2 |
| ZO-1 | F: GAACCAAAGCCAGTGTATG | R: GGTCCCCTTCCTCTAATC | 161 | XM_021299314.1 |
| Claudin2 | F: GTGCAGATGGGAACAAGGT | R: GAGCCAAGGAAGCTACGG | 119 | XM_021283269.1 |
| Claudin3 | F: ACCTCATCCCCGTCTCCT | R: CAGCCCACGTAGAGCGA | 109 | XM_005515008.2 |
| Beclin1 | F: AGCTGGAGGACGTTGAGAAA | R: AGCTCCAGTTGCTGTCTCTT | 139 | XM_021280982.1 |
| Atg5 | F: GTCCAAGGTTTGTGGCTGTT | R: CAGAATGGGAACAGCACTGG | 188 | XM_005509471.2 |
| Caspase-3 | F: CCTACCTGCCAGCAAGTCTA | R: CTTGCAGCATCTGCATCTGT | 159 | XM_005509733.3 |
| Bcl-2 | F: TACCTCCGAGACCCTGAGAA | R: CAGCAACAGGGAGAGAGGAA | 161 | XM_005500178.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, Y.; Ma, T.; Fu, Z.; Chen, A.; Yu, J.; Huang, Y.; Fu, J. Effects of Hydrogen Peroxide-Induced Oxidative Stress on Intestinal Morphology, Redox Status, and Related Molecules in Squabs. Animals 2023, 13, 749. https://doi.org/10.3390/ani13040749
Zhong Y, Ma T, Fu Z, Chen A, Yu J, Huang Y, Fu J. Effects of Hydrogen Peroxide-Induced Oxidative Stress on Intestinal Morphology, Redox Status, and Related Molecules in Squabs. Animals. 2023; 13(4):749. https://doi.org/10.3390/ani13040749
Chicago/Turabian StyleZhong, Yajing, Tingting Ma, Zhiqi Fu, Ailing Chen, Jiahao Yu, Yanhua Huang, and Jing Fu. 2023. "Effects of Hydrogen Peroxide-Induced Oxidative Stress on Intestinal Morphology, Redox Status, and Related Molecules in Squabs" Animals 13, no. 4: 749. https://doi.org/10.3390/ani13040749