Spatial Variability of External Egg Quality in Vertical Naturally Ventilated Caged Aviaries
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
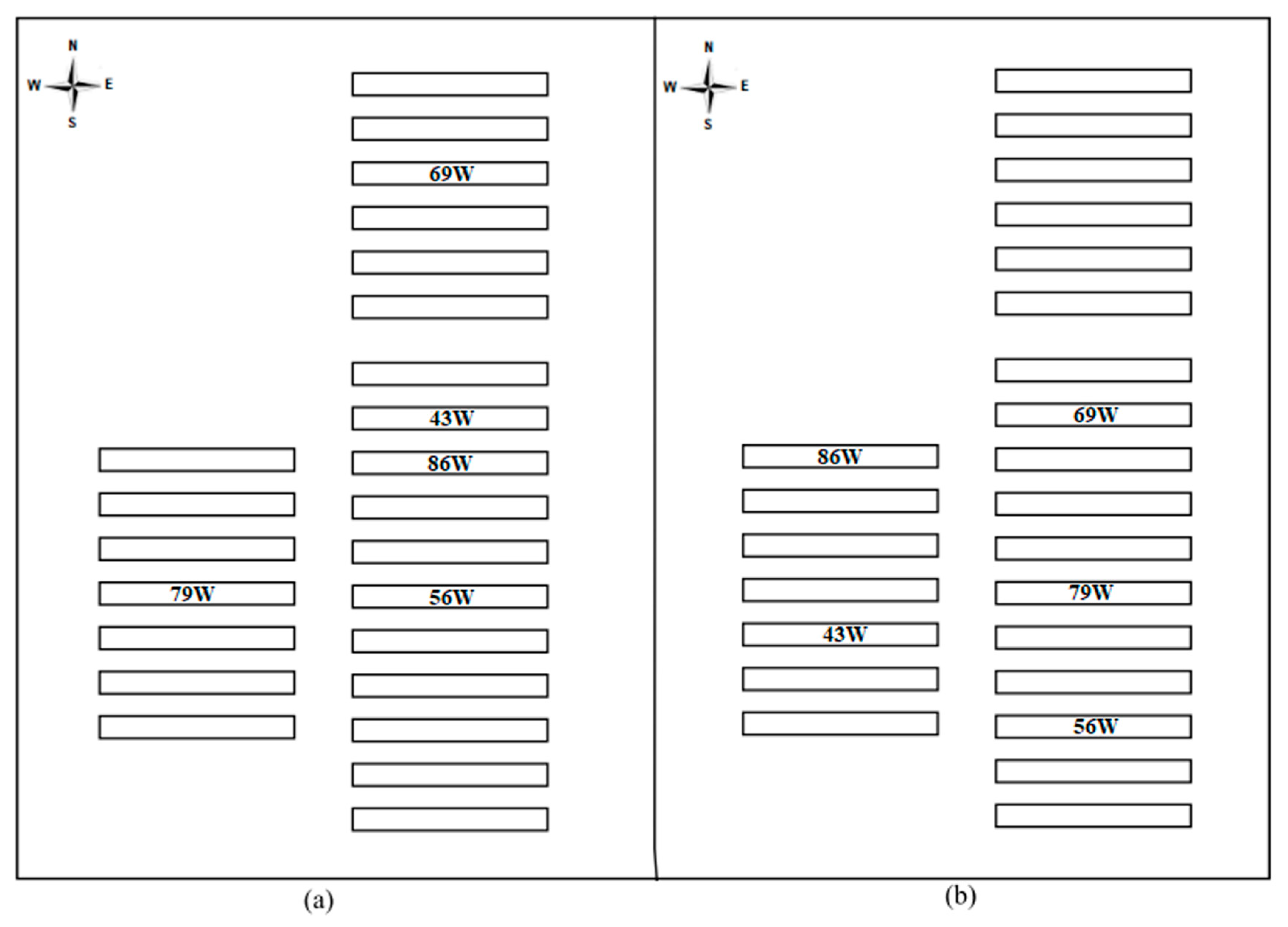
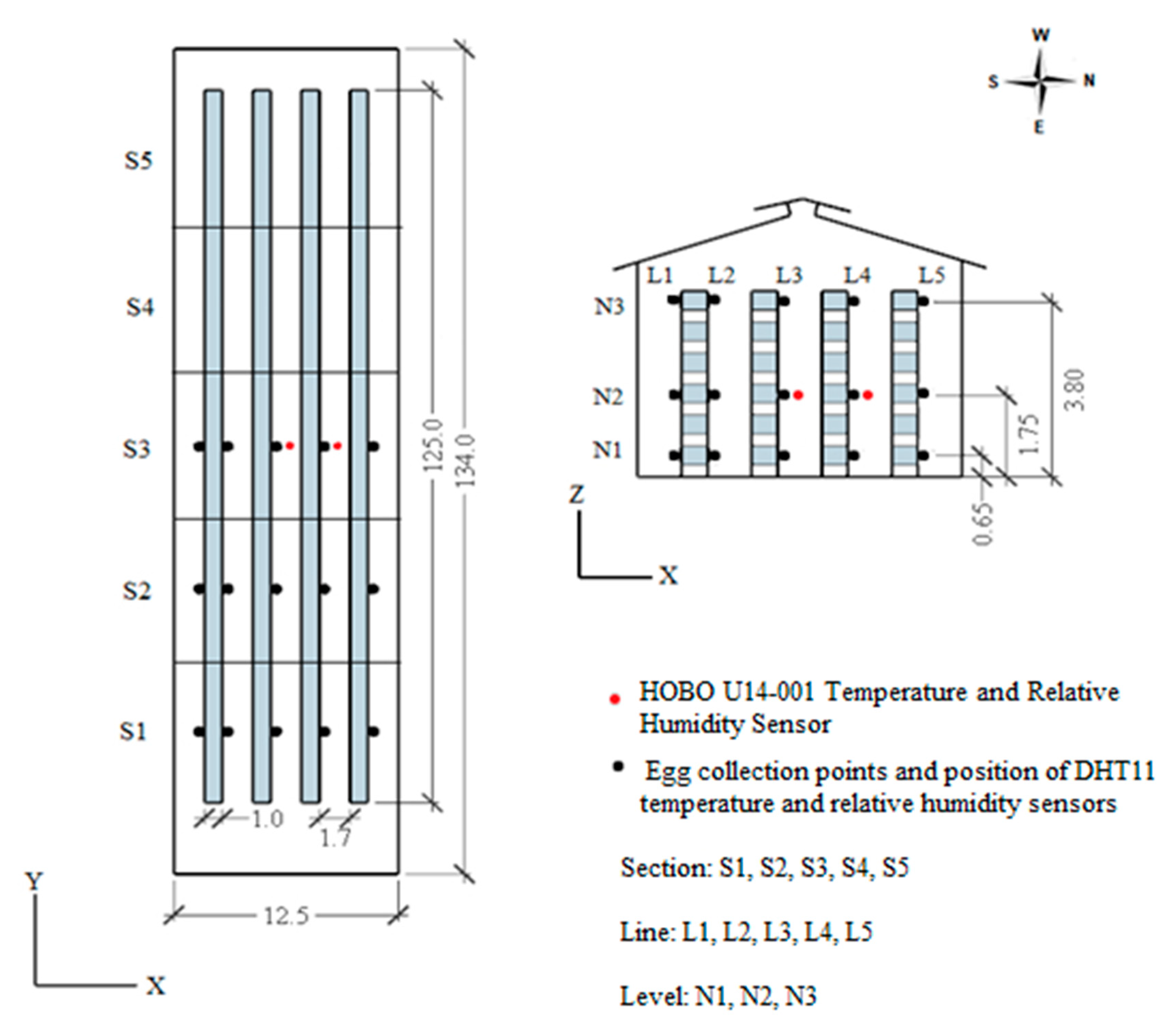
2.1. Characteristics of Facilities
2.2. Thermal Conditions and Light Intensity
2.3. External Egg Quality
2.4. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, J.Y.; Hester, P.Y.; Makagon, M.M.; Vezzoli, G.; Gates, R.S.; Xiong, Y.J.; Cheng, H.W. Cooled perch effects on performance and well-being traits in caged White Leghorn hens. Poult. Sci. 2016, 95, 2737–2746. [Google Scholar] [CrossRef]
- Duman, M.; Şekeroğlu, A.; Yildirim, A.H.; Eleroğlu, H.; Camci, Ö. Relation between egg shape index and egg quality characteristics. Europ. Poult. Sci. 2016, 80, 1612–9199. [Google Scholar] [CrossRef]
- Ketta, M.; Tůmová, E. Relationship between eggshell thickness and other eggshell measurements in eggs from litter and cages. Ital. J. Anim. Sci. 2018, 17, 234–239. [Google Scholar] [CrossRef]
- Pires, P.G.S.; Bavaresco, C.; Prato, B.S.; Wirth, M.L.; Moraes, P.O. The relationship between egg quality and hen housing systems-A systematic review. Livest. Sci. 2021, 250, 104597. [Google Scholar] [CrossRef]
- Hamilton, R.M.G. Methods and factors that affect the measurement of egg shell quality. Poult. Sci. 1982, 61, 2022–2039. [Google Scholar] [CrossRef]
- Onbaşilar, E.E.; Ünal, N.; Erdem, E. Some egg quality traits of two laying hybrids kept in different cage systems. Ankara Univ. Vet. Fak. Derg. 2018, 65, 51–55. [Google Scholar]
- Samiullah, S.; Omar, A.S.; Roberts, J.; Chousalkar, K. Effect of production system and flock age on eggshell and egg internal quality measurements. Poult. Sci. 2017, 96, 246–258. [Google Scholar] [CrossRef]
- Ferreira, R.A. Maior Produção Com Melhor Ambiente Para Aves, Suínos e Bovinos, 3rd ed.; Aprenda Fácil: Viçosa, Brazil, 2015; 528p. [Google Scholar]
- Andrade, R.R.; Tinôco, I.F.F.; Souza, C.F.; Oliveira, K.P.; Barbari, M.; Cruz, V.M.F.; Baptista, F.J.F.; Vilela, M.O.; Conti, L.; Rossi, G. Effect of thermal environment on body temperature of early-stage laying hens. Agron. Res. 2018, 16, 320–327. [Google Scholar] [CrossRef]
- Andrade, R.R.; Tinôco, I.D.F.; Baêta, F.C.; Albino, L.F.; Cecon, P.R. Influence of different thermal environments on the performance of laying hens during the initial stage of rearing. Eng. Agric. 2019, 39, 32–40. [Google Scholar] [CrossRef]
- Karami, M.; Torki, M.; Mohammadi, H. Effects of dietary supplemental chromium methionine, zinc oxide, and ascorbic acid on performance, egg quality traits, and blood parameters of laying hens subjected to heat stress. J. Appl. Anim. Res. 2018, 46, 1174–1184. [Google Scholar] [CrossRef]
- Sahin, N.; Hayirli, A.; Orhan, C.; Tuzcu, M.; Komorowski, J.R.; Sahin, K. Effects of the supplemental chromium form on performance and metabolic profile in laying hens exposed to heat stress. Poult. Sci. 2018, 97, 1298–1305. [Google Scholar] [CrossRef]
- Torki, M.; Karami, M.; Mohammadi, H. Effects of dietary supplemental vitamin E and chromium on egg production, egg quality and blood parameters of laying hens under thermoneutral or heat stressed conditions. Iran J. Appl. Anim. Sci. 2018, 8, 295–303. [Google Scholar]
- Albino, L.F.T.; Carvalho, B.R.; Maia, R.C.; Barros, V.R.S.M. Galinhas Poedeiras Criação e Alimentação, 1st ed.; Aprenda Fácil: Viçosa, Brazil, 2014; 376p. [Google Scholar]
- Min, J.K.; Hossan, M.S.; Nazma, A.; Jae, C.N.; Han, T.B.; Hwan, K.K.; Dong, W.K.; Hyun, S.C.; Hee, C.C.; Ok, S.S. Effect of monochromatic light on sexual maturity, production performance and egg quality of laying hens. Avian. Biol. Res. 2012, 5, 69–74. [Google Scholar] [CrossRef]
- Renema, R.A.; Robinson, F.E.; Feddes, J.J.; Fasenko, G.M.; Zuidhoft, M.J. Effects of Light Intensity from Photostimulation in Four Strains of Commercial Egg Layers: 2. Egg Production Parameters. Poult. Sci. 2001, 80, 1121–1131. [Google Scholar] [CrossRef]
- Yildiz, A.; Laçin, E.; Hayirli, A.; Macit, M. Effects of cage location and tier level with respect to light intensity in semiconfined housing on egg production and quality during the late laying period. J. Appl. Poul. Res. 2006, 15, 355–361. [Google Scholar] [CrossRef]
- Yildirim, A.; Sekeroglu, A.; Koç, H.; Eleroglu, H.; Duman, M.; Tahtali, Y.; Elmastas, M.; Mutlu, M.I.S. Egg production and quality characteristics of laying hens fed diets supplemented with dry caper (Capparis spinosa) leaf powder. Indian J. Anim. Res. 2018, 52, 72–78. [Google Scholar] [CrossRef]
- Yuri, F.M.; Souza, C.; Schneider, A.F.; Gewehr, C.E. Intermittent lighting programs for layers with different photophases in the beginning of the laying phase. Cienc. Rur. 2016, 46, 2012–2017. [Google Scholar] [CrossRef]
- Cotta, T. Galinha produção de ovos, 2nd ed.; Aprenda Fácil: Viçosa, Brazil, 2014; 250p. [Google Scholar]
- Jácome, I.M.T.D.; Rossi, L.A.; Borille, R. Influence of Artificial Lighting on the Performance and Egg Quality of Commercial Layers: A Review. Ver. Bras. Cienc. Avic. 2014, 16, 337–344. [Google Scholar] [CrossRef]
- Management Guide Lohmann LSL-Lite (s.d). 2015. Available online: http://www.hylinena.com/UserDocs/products/Lohmann_LSL-Lite.pdf (accessed on 1 January 2023).
- Freitas, L.C.S.R.; Tinôco, I.F.F.; Gates, R.S.; Souza, C.F.; Barbari, M.; Teles Junior, C.G.S. Spatial behavior of the thermo-luminous conditions of facility laying hens in naturally ventilated vertical system. Ciênc. Agrotec. 2018, 42, 550–560. [Google Scholar] [CrossRef]
- Zheng, W.; Xiong, Y.; Gates, R.S.; Wang, Y.; Koelkebeck, K.W. Air temperature, carbon dioxide, and ammonia assessment inside a commercial cage layer barn with manure-drying tunnels. Poult. Sci. 2020, 99, 3885–3896. [Google Scholar] [CrossRef]
- Li, Z.; Xiong, Y.; Wang, S.; Wang, C.; Ji, B.; Liu, Y.; Liang, C.; Tong, Q. Assessing particulate matter concentration level and its limit exceedance based on year-round field measurements of different laying hen building systems. Biosyst. Eng. 2023, 226, 266–279. [Google Scholar] [CrossRef]
- Freitas, L.C.S.R.; Tinôco, I.F.F.; Baêta, F.C.; Barbari, M.; Conti, L.; Teles Júnior, C.G.S.; Sousa, F.C. Correlation between egg quality parameters, housing thermal conditions and age of laying hens. Agron. Res. 2017, 15, 687–693. [Google Scholar]
- Almeida, J.G.; Dahlke, F.; Maiorka, A.; Faria Filho, D.E.; Oelke, C.A. Efeito da idade da matriz no tempo de eclosão, tempo de permanência do neonato no nascedouro e o peso do pintainho. Arch. Vet. Sci. 2006, 11, 45–49. [Google Scholar] [CrossRef]
- Burnham, M.R.; Peebles, E.D.; Gardner, C.W.; Brake, J.; Bruzual, J.J.; Gerard, P.D. Effects of Incubator Humidity and Hen Age on Yolk Composition in Broiler Hatching Eggs from Young Breeders. Poult. Sci. 2021, 80, 1444–1450. [Google Scholar] [CrossRef]
- Dikmen, B.Y.; İpek, A.; Şahan, Ü.; Sözcü, A.; Baycan, S.C. Impact of different housing systems and age of layers on egg quality characteristics. Turk. J. Vet. Anim. Sci. 2017, 41, 77–84. [Google Scholar] [CrossRef]
- Fouad, A.M.; Chen, W.; Ruan, D.; Wang, S.; XIA, W.G.; Zheng, C.T. Impact of Heat Stress on Meat, Egg Quality, Immunity and Fertility in Poultry and Nutritional Factors That Overcome These Effects: A Review. Int. J. Poult. Sci. 2016, 15, 81–95. [Google Scholar] [CrossRef]
- Castro, F.L.S.; Kim, H.Y.; Hong, Y.G.; Kim, W.K. The effect of total sulfur amino acid levels on growth performance, egg quality, and bone metabolism in laying hens subjected to high environmental temperature. Poult. Sci. 2019, 98, 4982–4993. [Google Scholar] [CrossRef]
- Akbari, M.; Torki, M.; Kaviani, K. Single and combined effects of peppermint and thyme essential oils on productive performance, egg quality traits, and blood parameters of laying hens reared under cold stress condition (6.8 ± 3 °C). Int. J. Biometeorol. 2016, 60, 447–454. [Google Scholar] [CrossRef]
- Star, L.; Arsiwalla, T.; Molist, F.; Leushuis, R.; Dalim, M.; Paul, A. Gradual Provision of Live Black Soldier Fly (Hermetia illucens) Larvae to Older Laying Hens: Effect on Production Performance, Egg Quality, Feather Condition and Behavior. Animals 2020, 10, 216. [Google Scholar] [CrossRef]
- Mashaly, M.M.; Hendricks, G.L.; Kalama, M.A.; Gehad, A.E.; Abbas, A.O.; Patterson, P.H. Effect of Heat Stress on Production Parameters and Immune Responses of Commercial Laying Hens. Poult. Sci. 2004, 83, 889–894. [Google Scholar] [CrossRef]
- Curtis, S.E. Environmental Management in Animal Agriculture; Iowa State University Press: Ames, IA, USA, 1983; 409p. [Google Scholar]
- Hu, J.Y.; Hester, P.Y.; Makagon, M.M.; Xiong, Y.; Gates, R.S.; Cheng, H.W. Effect of cooled perches on performance, plumage condition, and foot health of caged White Leghorn hens exposed to cyclic heat. Poult. Sci. 2019, 98, 2705–2718. [Google Scholar] [CrossRef]
- Hu, J.; Xiong, Y.; Gates, R.S.; Cheng, H.-W. Perches as Cooling Devices for Reducing Heat Stress in Caged Laying Hens: A Review. Animals 2021, 11, 3026. [Google Scholar] [CrossRef]
- Xiong, Y.; Gates, R.S.; Hu, J.; Hester, P.Y.; Cheng, H.W. Design and performance of an experimental cooled perch system for heat stress relief of laying hens. Trans. ASABE 2020, 63, 1109–1121. [Google Scholar] [CrossRef]
- Khatibi, S.M.R.; Heydar, Z.; Golian, A. Effect of diet nutrients density on performance and egg quality of laying hens during the post-peak production phase of the first laying cycle under subtropical climate. Ital. J. Anim. Sci. 2021, 20, 559–570. [Google Scholar] [CrossRef]
- Oguz, F.K.; Gumus, H.; Oguz, M.N.; Bugdayci, K.E.; Dagli, H.; Ozturk, Y. Effects of different levels of expanded perlite on the performance and egg quality traits of laying hens. Rev. Bras. Zootec. 2017, 46, 20–24. [Google Scholar] [CrossRef]
- Shaker, A.S.; Kirkuki, S.M.S.; Aziz, S.R.; Jalal, B.J. Influence of Genotype and Hen Age on the Egg Shape Index. Int. J. Biochem. Biophys. Mol. Biol. 2017, 2, 68–70. [Google Scholar] [CrossRef]
- Netto, D.A.; Lima, H.J.D.; Alves, J.R.; Morais, B.C.; Rosa, M.S.; Bittencourt, T.M. Production of laying hens in different rearing systems under hot weather. Acta Sci. Anim. Sci. 2018, 40, e37677. [Google Scholar] [CrossRef]
- Yan, F.F.; Murugesan, G.R.; Cheng, H.W. Effects of probiotic supplementation on performance traits, bone mineralization, cecal microbial composition, cytokines and corticosterone in laying hens. Animal 2019, 13, 33–41. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, Y.K.; Lee, S.D.; Kim, S.H.; Lee, S.R.; Lee, H.G.; Lee, K.W. Changes in Production Parameters, Egg Qualities, Fecal Volatile Fatty Acids, Nutrient Digestibility, and Plasma Parameters in Laying Hens Exposed to Ambient Temperature. Front. Vet. Sci. 2020, 7, 412. [Google Scholar] [CrossRef]
| Aviaries | Temperature (°C) | Relative Humidity (%) | ||
|---|---|---|---|---|
| Winter | Summer | Winter | Summer | |
| Aviary 43W | 21.8 ± 1.0 | 26.2 ± 0.5 | 58 ± 3.0 | 64 ± 1.7 b |
| Aviary 56W | 21.9 ± 0.9 | 27.1 ± 0.6 | 57 ± 2.7 | 67 ± 1.5 a |
| Aviary 69W | 21.2 ± 1.0 | 26.9 ± 0.5 | 61 ± 2.6 | 68 ± 1.6 a |
| Aviary 79W | 22.2 ± 0.8 | 26.9 ± 0.5 | 56 ± 2.6 | 66 ± 1.6 ab |
| Aviary 86W | 21.4 ± 0.9 | 26.8 ± 0.5 | 59 ± 2.9 | 68 ± 1.4 a |
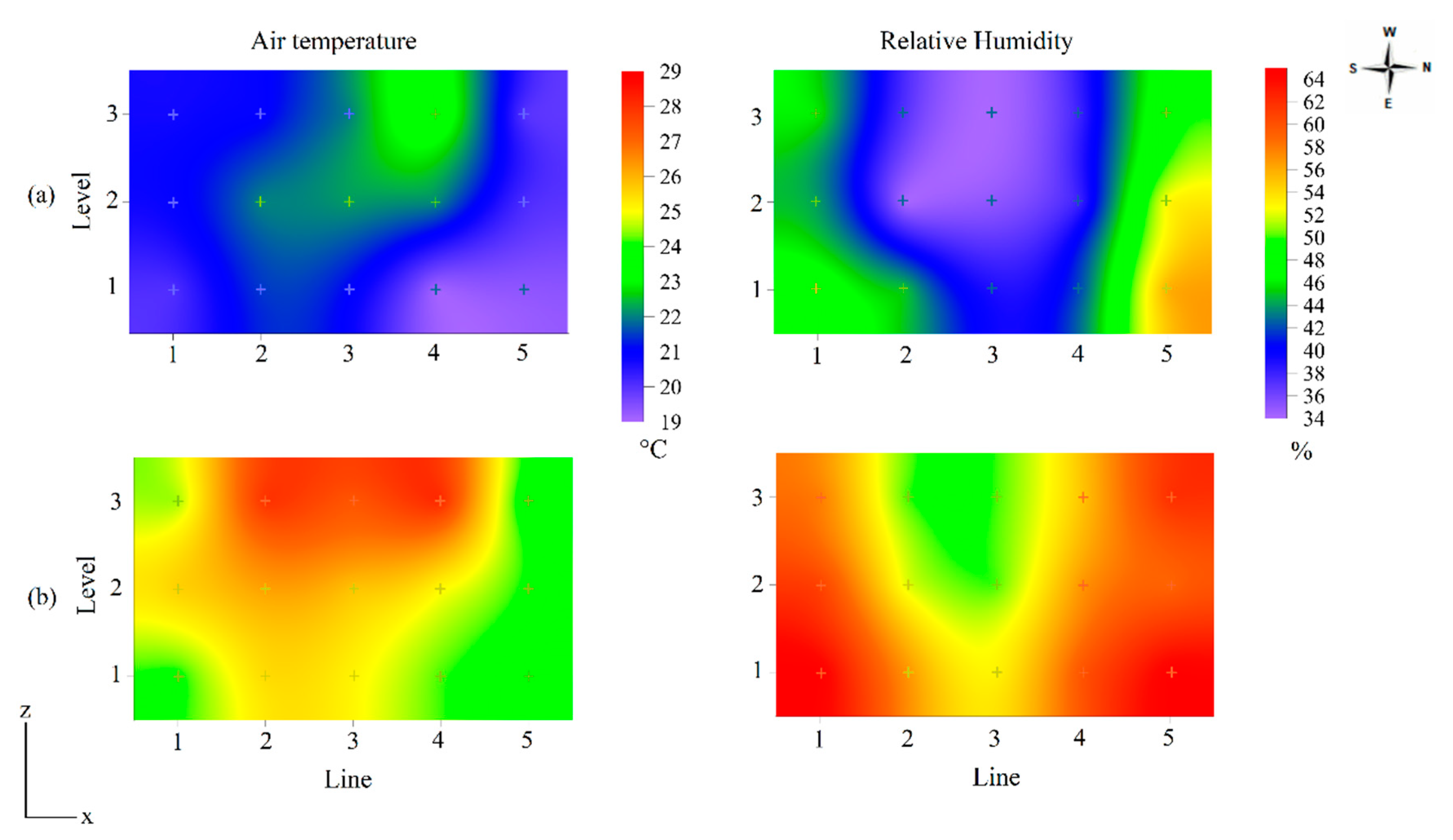
| Temperature (°C) | ||||||
|---|---|---|---|---|---|---|
| Winter | L1 | L2 | L3 | L4 | L5 | |
| N1 | 19.6 ± 2.8 a A | 21.7 ± 1.5 a A | 21.3 ± 1.2 a A | 19.1 ± 0.9 b A | 19.2 ± 1.9 a A | |
| N2 | 20.9 ± 2.0 a A | 22.3 ± 1.4 a A | 22.5 ± 1.1 a A | 22.3 ± 1.6 a A | 20.1 ± 1.7 a A | |
| N3 | 20.9 ± 1.79 a B | 21.2 ± 1.3 a AB | 22.2 ± 1.3 a AB | 23.8 ± 1.9 a A | 19.9 ± 0.9 a B | |
| Summer | L1 | L2 | L3 | L4 | L5 | |
| N1 | 24.1 ± 1.6 b BC | 25.6 ± 1.7 b A | 25.2 ± 1.2 b AB | 24.6 ± 1.6 b AB | 23.5 ± 1.5 a C | |
| N2 | 25.9 ± 0.9 a AB | 26.3 ± 1.4 b A | 25.8 ± 0.8 b AB | 25.1 ± 1.7 b BC | 24.1 ± 1.1 a C | |
| N3 | 24.6 ± 0.7 b C | 28.2 ± 1.1 a AB | 27.3 ± 2.1 a B | 28.7 ± 0.7 a A | 23.0 ± 0.8 a D | |
| Relative humidity (%) | ||||||
| Winter | L1 | L2 | L3 | L4 | L5 | |
| N1 | 48 ± 5.1 a B | 44 ± 4.7 a BC | 39 ± 5.1 a C | 42 ± 5.1 a BC | 56 ± 6.1 a A | |
| N2 | 43 ± 3.9 a B | 33 ± 4.5 b C | 35 ± 4.6 a C | 37 ± 4.1 a BC | 52 ± 7.1 a A | |
| N3 | 46 ± 4.9 a AB | 37 ± 4.3 ab BC | 34 ± 5.1 a C | 38 ± 4.1 a BC | 50 ± 6.7 a A | |
| Summer | L1 | L2 | L3 | L4 | L5 | |
| N1 | 65 ± 6.7 a AB | 57 ± 6.1 a CB | 54 ± 4.9 a C | 59 ± 3.8 a BC | 67 ± 3.3 a A | |
| N2 | 62 ± 6.4 ab A | 53 ± 5.7 a B | 50 ± 4.0 a B | 60 ± 7.2 a A | 61 ± 8.1 a A | |
| N3 | 59 ± 6.8 b AB | 51 ± 6.5 a C | 50 ± 1.9 a C | 55 ± 2.6 a BC | 64 ± 4.6 a A | |
| N × L | L1 | L2 | L3 | L4 | L5 | |
| Winter | N1 | 52,630 ± 4647 a A | 20 ± 1 a B | 11 ± 2 a B | 32 ± 3 a B | 3402 ± 384 a B |
| N2 | 49,607 ± 5389 a A | 43 ± 7 a B | 18 ± 4 a B | 29 ± 4 a B | 4341 ± 418 a B | |
| N3 | 9904 ± 878 b A | 448 ± 24 a B | 784 ± 55 a B | 1300 ± 59 a B | 5002 ± 462 a B | |
| L1 | L2 | L3 | L4 | L5 | ||
| Summer | N1 | 10,626 ± 2291 a A | 54 ± 4 a B | 64 ± 48 a B | 39 ± 25 a B | 9893 ± 2535 a A |
| N2 | 10,656 ± 2175 a A | 82 ± 46 a B | 74 ± 78 a B | 56 ± 37 a B | 10,385 ± 2326 a A | |
| N3 | 7056 ± 998 b A | 170 ± 73 a B | 229 ± 77 a B | 880 ± 151 a B | 7774 ± 1545 a A |
| Egg Weight (g) | |||||
|---|---|---|---|---|---|
| Season × Age (weeks) | |||||
| 43 | 56 | 69 | 79 | 86 | |
| Winter | 62.4 ± 0.5 a B | 62.9 ± 0.3 a B | 65.6 ± 0.8 a A | 66.2 ± 0.8 a A | 66.9 ± 0.6 a A |
| Summer | 59.8 ± 0.9 b B | 60.9 ± 0.4 a AB | 60.7 ± 0.9 b AB | 61.6 ± 0.7 b AB | 62.5 ± 0.7 b A |
| Specific Gravity (g·mL−1) | |||||
| Season | Winter | Summer | |||
| 1.086 ± 0.008 b | 1.090 ± 0.004 a | ||||
| Age (weeks) | 43 | 56 | 69 | 79 | 86 |
| 1.089 ± 0.001 ns | 1.090 ± 0.003 ns | 1.089 ± 0.004 ns | 1.084 ± 0.008 ns | 1.087 ± 0.010 ns | |
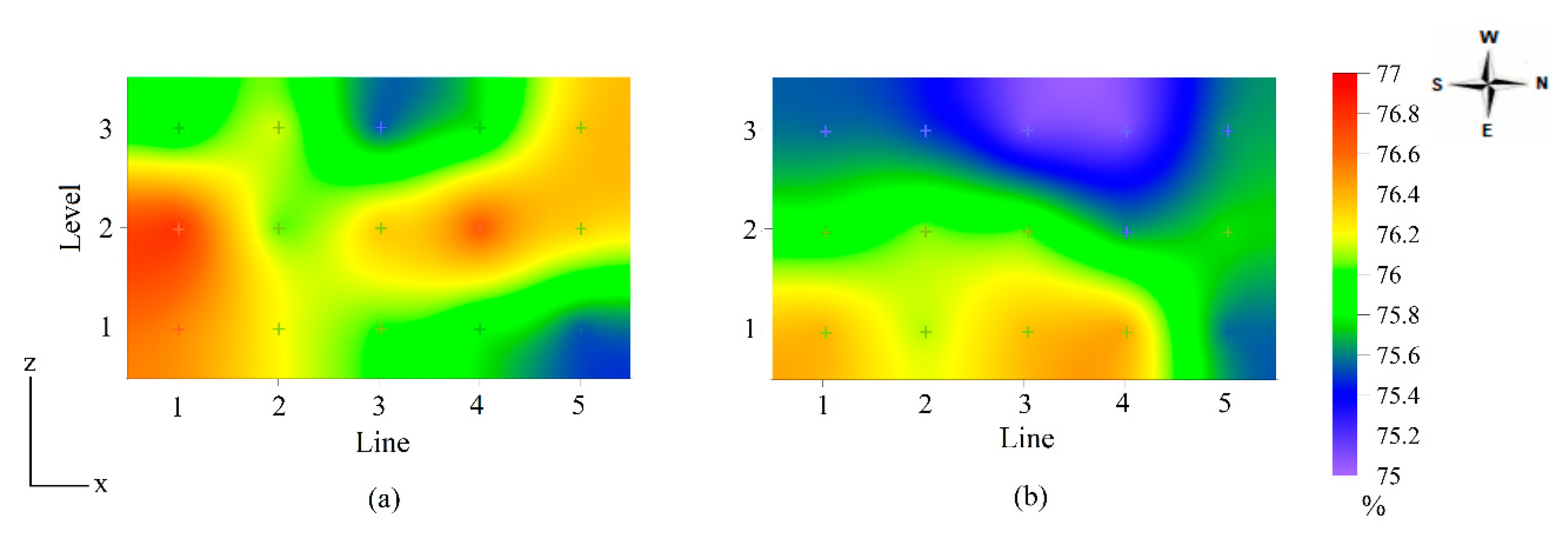
| Egg Shape Index (%) | |||||
| Season | Winter | Summer | |||
| 76.2 ± 0.5 a | 75.8 ± 0.8 b | ||||
| Age (weeks) | 43 | 56 | 69 | 79 | 86 |
| 76.0 ± 0.5 ns | 76.5 ± 0.4 ns | 75.9 ± 0.7 ns | 75.7 ± 0.5 ns | 75.6 ± 0.9 ns | |
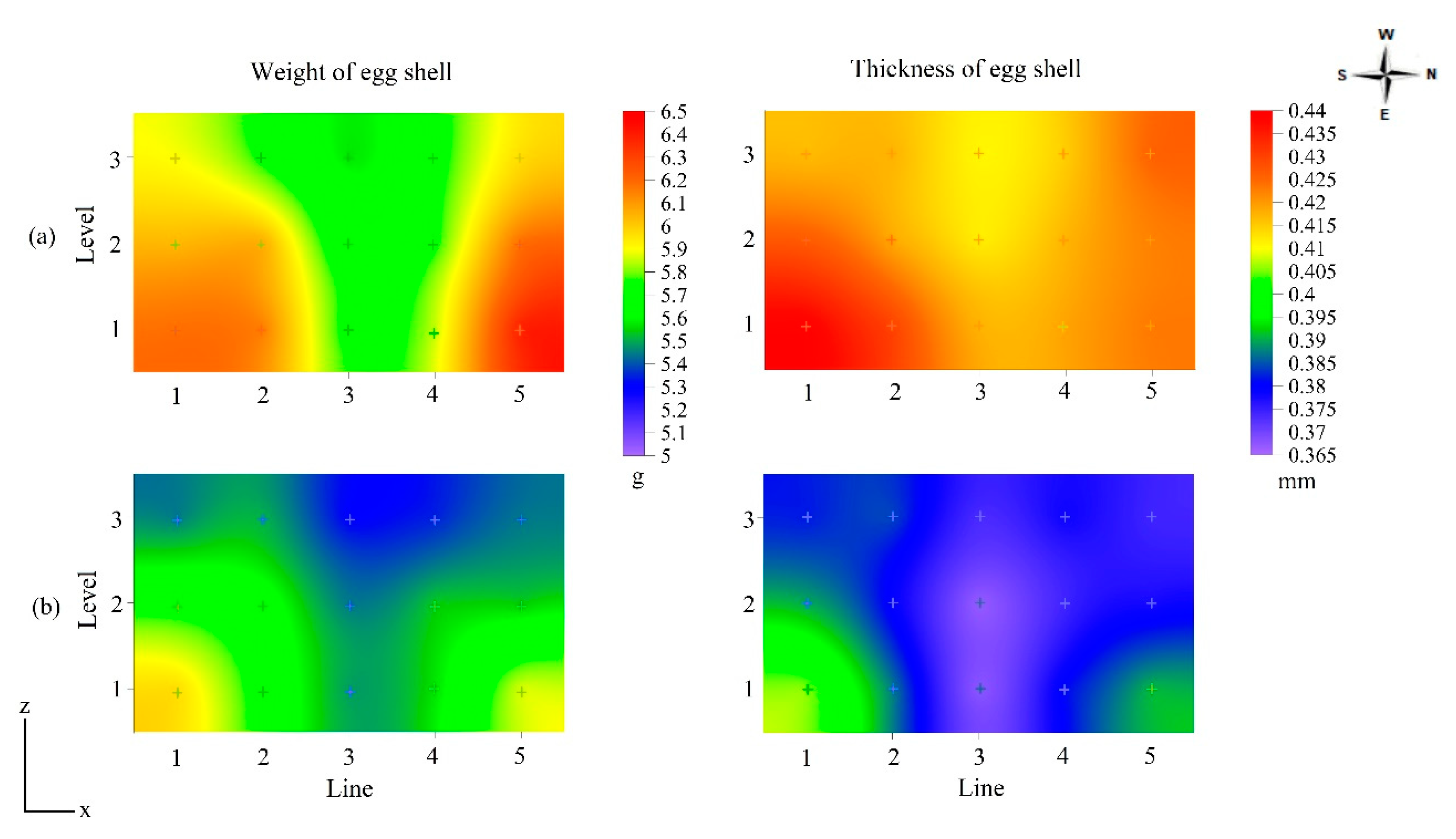
| Shell Weight (g) | |||||
| Season | Winter | Summer | |||
| 5.9 ± 0.2 a | 5.6 ± 0.1 b | ||||
| Age (weeks) | 43 | 56 | 69 | 79 | 86 |
| 5.6 ± 0.2 ns | 5.7 ± 0.1 ns | 5.8 ± 0.3 ns | 5.8 ± 0.2 ns | 5.8 ± 0.3 ns | |
| Percentage of Shell (%) | |||||
| Season | Winter | Summer | |||
| 9.2 ± 0.2 ns | 9.1 ± 0.2 ns | ||||
| Age (weeks) | 43 | 56 | 69 | 79 | 86 |
| 9.2 ± 0.2 ab | 9.3 ± 0.1 a | 9.2 ± 0.1 ab | 9.1 ± 0.1 ab | 8.9 ± 0.2 b | |
| Shell Thickness (mm) | |||||
| Season | Winter | Summer | |||
| 0.421 ± 0.06 a | 0.381 ± 0.03 b | ||||
| Age (weeks) | 43 | 56 | 69 | 79 | 86 |
| 0.391 ± 0.04 ns | 0.424 ± 0.06 ns | 0.391 ± 0.04 ns | 0.385 ± 0.03 ns | 0.413 ± 0.07 ns | |
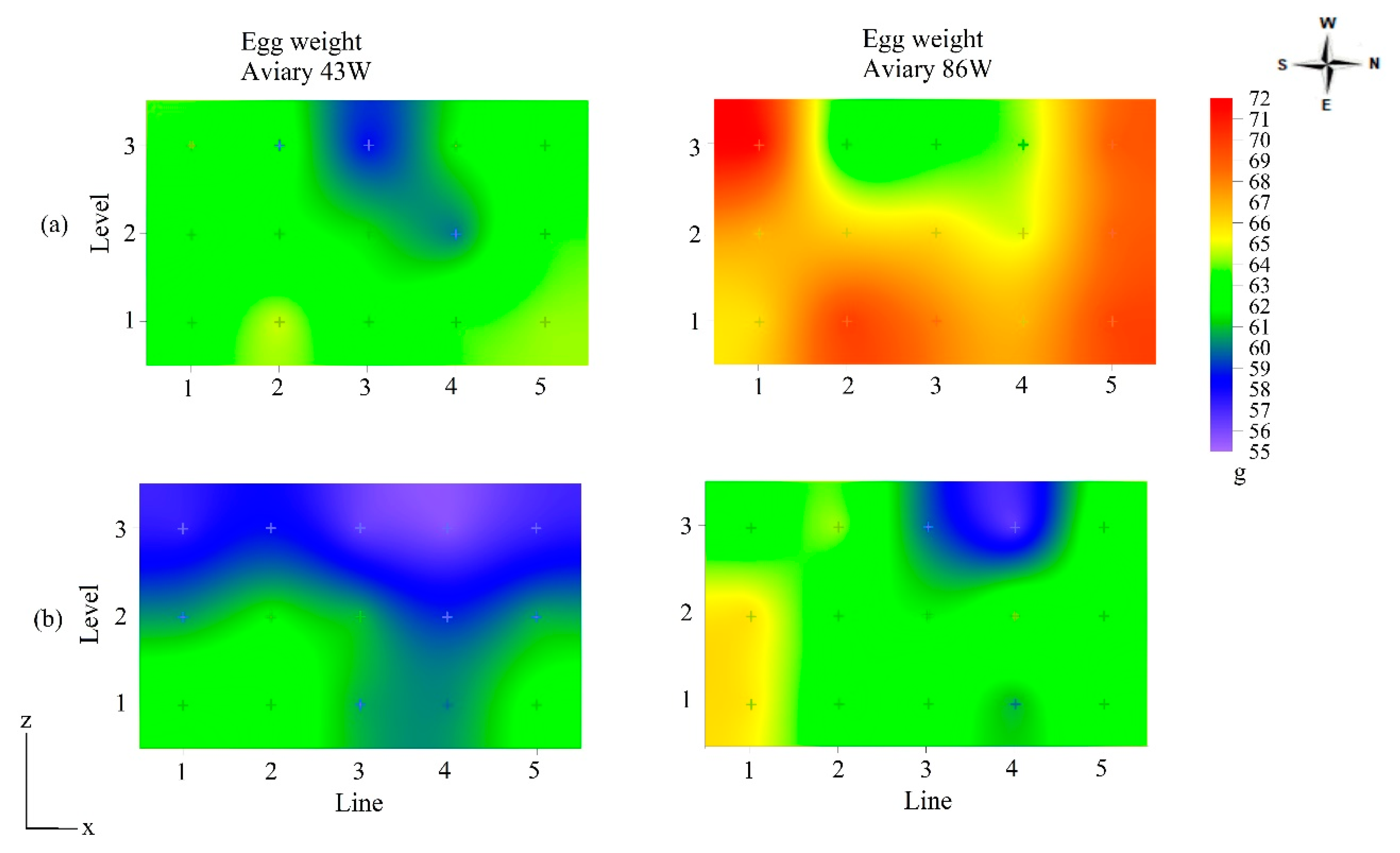
| Egg Weight (g) | ||||||
|---|---|---|---|---|---|---|
| Aviary 43W | ||||||
| Winter | Level (N) | N1 | N2 | N3 | ||
| 63.4 ± 4.8 a | 61.8 ± 4.3 b | 61.9 ± 4.4 ab | ||||
| Line (L) | L1 | L2 | L3 | L4 | L5 | |
| 62.6 ± 4.0 ab | 63.2 ± 4.2 a | 60.6 ± 4.9b | 61.8 ± 4.0 ab | 63.6 ± 5.0 a | ||
| Summer | Level (N) | N1 | N2 | N3 | ||
| 61.8 ± 4.3 a | 60.5 ± 4.6 a | 57.0 ± 4.5 b | ||||
| Line (L) | L1 | L2 | L3 | L4 | L5 | |
| 60.2 ± 5.6 ns | 61.1 ± 4.3 ns | 59.3 ± 4.3 ns | 58.3 ± 4.7 ns | 60.1 ± 5.0 ns | ||
| Aviary 86W | ||||||
| N × L | L1 | L2 | L3 | L4 | L5 | |
| Winter | N1 | 65.9 ± 5.0 a A | 70.0 ± 3.9 b A | 68.0 ± 3.9 a A | 66.9 ± 6.6 a A | 69.7 ± 4.3 a A |
| N2 | 67.1 ± 5.3 a A | 66.2 ± 6.9 ab A | 66.1 ± 4.6 a A | 64.8 ± 2.6 a A | 68.8 ± 7.6 a A | |
| N3 | 71.8 ± 7.4 a A | 61.8 ± 7.4 a B | 63.3 ± 3.8 a B | 64.2 ± 4.7 a AB | 69.0 ± 7.0 a AB | |
| Summer | N1 | 65.8 ± 3.8 a A | 62.1 ± 4.2 a A | 62.9 ± 3.0 a A | 60.8 ± 3.4 ab A | 63.4 ± 5.9 a A |
| N2 | 65.9 ± 5.5 a A | 62.3 ± 3.9 a A | 61.7 ± 4.7 a A | 63.7 ± 3.7 a A | 62.8 ± 3.3 a A | |
| N3 | 62.3 ± 5.9 a AB | 64.3 ± 4.0 a A | 59.7 ± 3.2 a A | 56.4 ± 4.3 b B | 63.3 ± 5.5 a A | |
| Shell Weight (g) | ||||||
|---|---|---|---|---|---|---|
| Winter | Level (N) | N1 | N2 | N3 | ||
| 6.1 ± 0.4 a | 5.9 ± 0.3 ab | 5.8 ± 0.2 b | ||||
| Line (L) | L1 | L2 | L3 | L4 | L5 | |
| 6.0 ± 0.3 a | 6.0 ± 0.3 ab | 5.6 ± 0.2 c | 5.8 ± 0.2 bc | 6.2 ± 0.3 a | ||
| Summer | Level (N) | N1 | N2 | N3 | ||
| 5.7 ± 0.3 a | 5.6 ± 0.5 ab | 5.4 ± 0.3 b | ||||
| Line (L) | L1 | L2 | L3 | L4 | L5 | |
| 5.7 ± 0.3 a | 5.6 ± 0.2 ab | 5.4 ± 0.2 b | 5.5 ± 0.3 ab | 5.6 ± 0.3 ab | ||
| Shell Thickness (mm) | ||||||
| Winter | Level (N) | N1 | N2 | N3 | ||
| 0.426 ± 0.05 ns | 0.419 ± 0.05 ns | 0.417 ± 0.05 ns | ||||
| Line (L) | L1 | L2 | L3 | L4 | L5 | |
| 0.428 ± 0.06 ns | 0.422 ± 0.06 ns | 0.413 ± 0.05 ns | 0.417 ± 0.05 ns | 0.424 ± 0.04 ns | ||
| Summer | Level (N) | N1 | N2 | N3 | ||
| 0.387 ± 0.03 ns | 0.378 ± 0.03 ns | 0.378 ± 0.03 ns | ||||
| Line (L) | L1 | L2 | L3 | L4 | L5 | |
| 0.393 ± 0.03 a | 0.384 ± 0.03 ab | 0.369 ± 0.02 b | 0.378 ± 0.03 ab | 0.381 ± 0.03 ab | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitas, L.C.d.S.R.; Tinôco, I.d.F.F.; Gates, R.S.; dos Santos, T.C.; Andrade, R.R.; Barbari, M.; Bambi, G. Spatial Variability of External Egg Quality in Vertical Naturally Ventilated Caged Aviaries. Animals 2023, 13, 750. https://doi.org/10.3390/ani13040750
Freitas LCdSR, Tinôco IdFF, Gates RS, dos Santos TC, Andrade RR, Barbari M, Bambi G. Spatial Variability of External Egg Quality in Vertical Naturally Ventilated Caged Aviaries. Animals. 2023; 13(4):750. https://doi.org/10.3390/ani13040750
Chicago/Turabian StyleFreitas, Letícia Cibele da Silva Ramos, Ilda de Fátima Ferreira Tinôco, Richard Stephen Gates, Tatiany Carvalho dos Santos, Rafaella Resende Andrade, Matteo Barbari, and Gianluca Bambi. 2023. "Spatial Variability of External Egg Quality in Vertical Naturally Ventilated Caged Aviaries" Animals 13, no. 4: 750. https://doi.org/10.3390/ani13040750
APA StyleFreitas, L. C. d. S. R., Tinôco, I. d. F. F., Gates, R. S., dos Santos, T. C., Andrade, R. R., Barbari, M., & Bambi, G. (2023). Spatial Variability of External Egg Quality in Vertical Naturally Ventilated Caged Aviaries. Animals, 13(4), 750. https://doi.org/10.3390/ani13040750








