Effects of Probiotics Supplementation on the Intestinal Metabolites, Muscle Fiber Properties, and Meat Quality of Sunit Lamb
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Sample Collection at Slaughter
2.3. Meat Quality Analysis
2.3.1. Color
2.3.2. pH Value
2.3.3. Cooking Loss
2.3.4. Shear Force
2.4. Histological Analysis
2.5. Muscle Enzyme Activity
2.6. Real-Time PCR
2.7. Short-Chain Fatty Acids in the Intestine
2.8. Statistical Analysis
3. Results
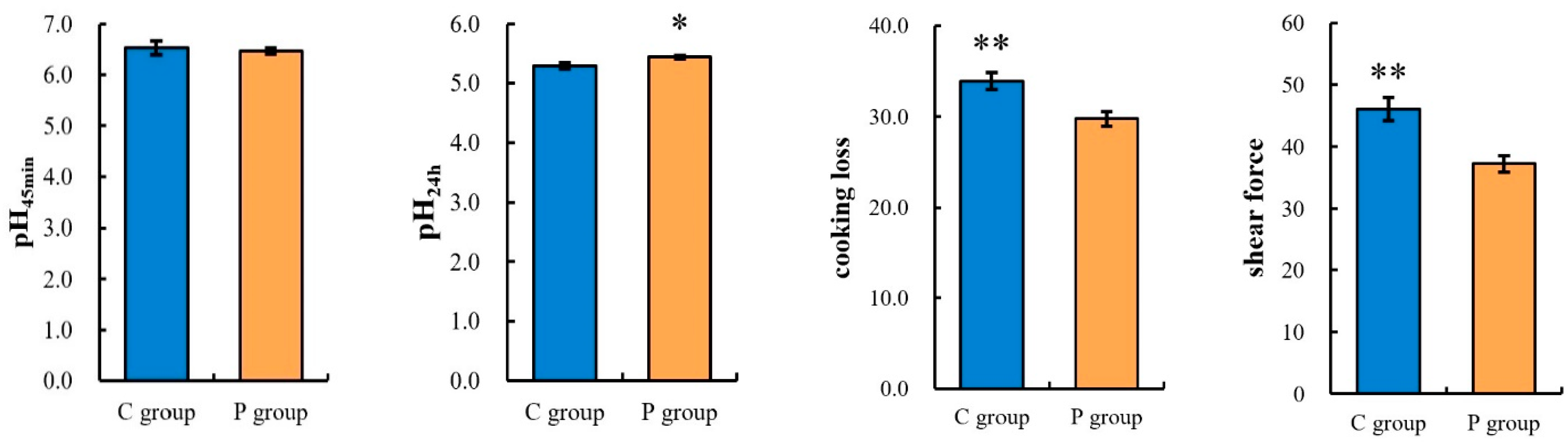
3.1. Effects of Probiotics on the Meat Quality of Sunit Lambs
3.2. Effects of Probiotics on the Muscle Fiber Properties of Sunit Lambs
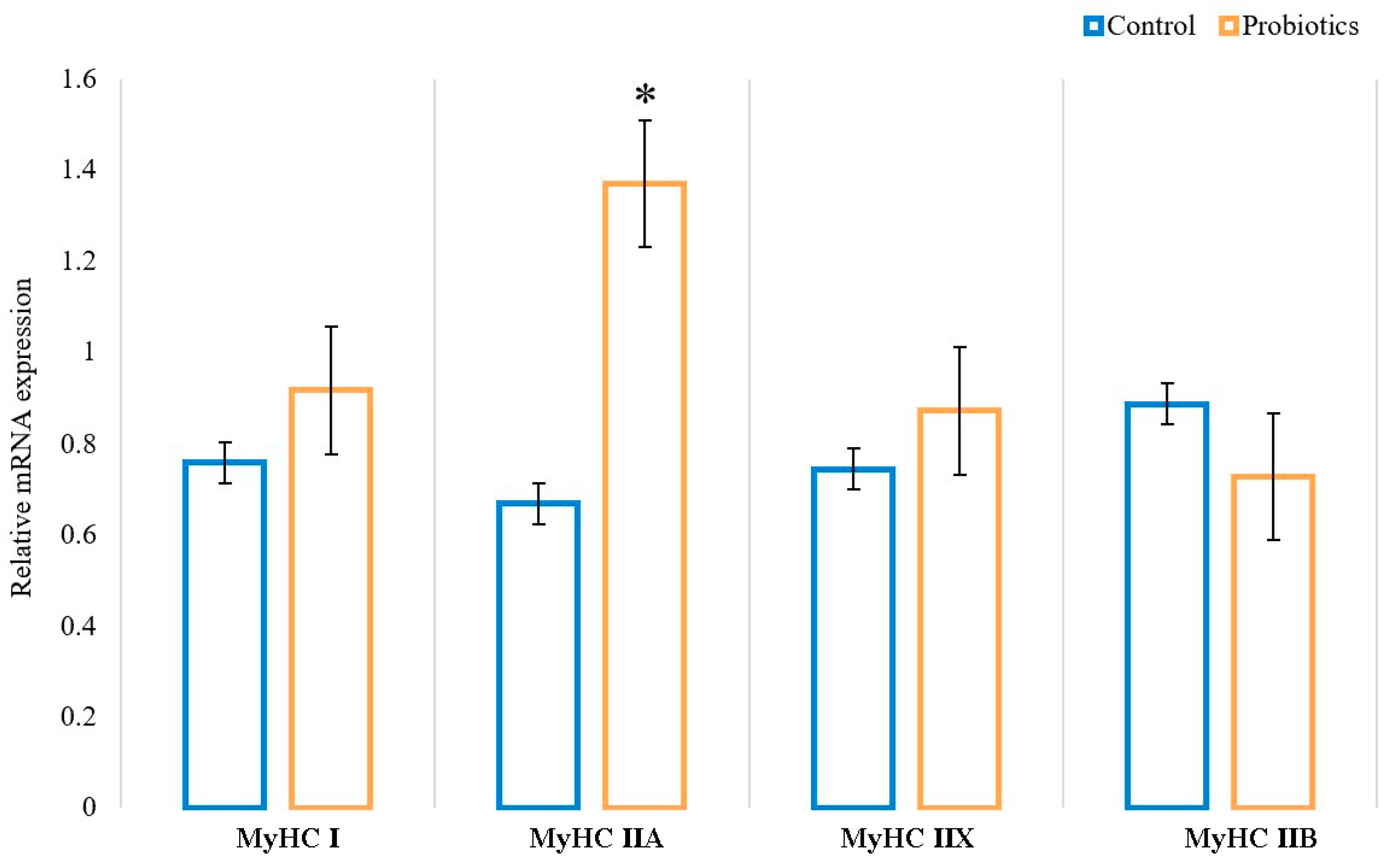
3.3. Effects of Probiotics on the Gene mRNA Expression of MyHC Isoforms
3.4. Effects of Probiotics on Muscle Oxidative Metabolism
3.5. Effects of Probiotics on Intestinal Metabolites (Short-Chain Fatty Acids)
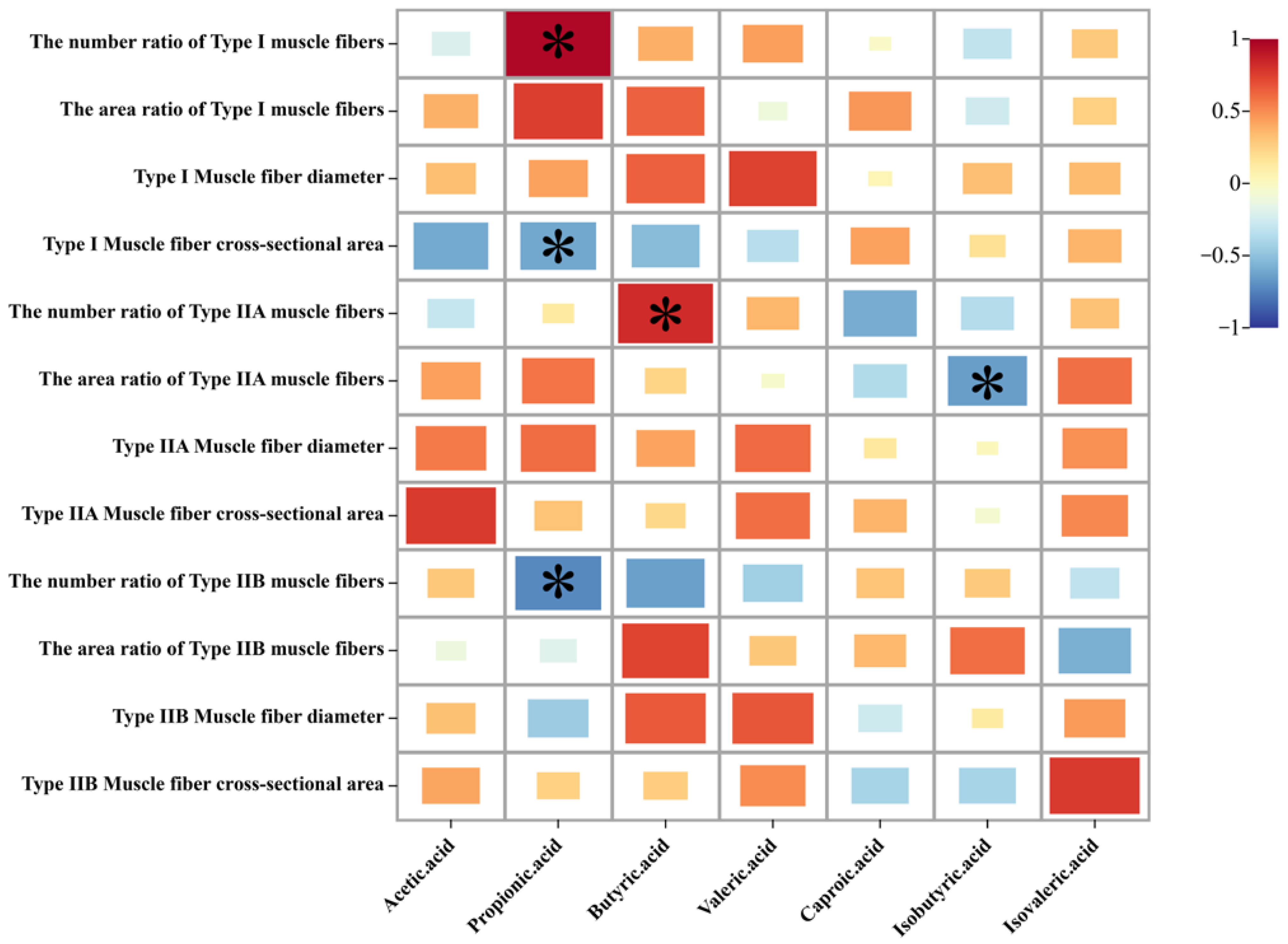
3.6. Relationship between Intestinal Metabolites (SCFAs) and Muscle Fiber Types
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, C.; Yan, X.; Bai, Y.; Sun, L.; Zhao, L.; Jin, Y.; Su, L. Lactobacillus improves meat quality in Sunit sheep by affecting mitochondrial biogenesis through the AMPK pathway. Front. Nutr. 2022, 9, 1030485. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Luo, Y.; Wang, Y.; Wang, D.; Hou, Y.; Yao, D.; Tian, J.; Jin, Y. Rumen bacteria and meat fatty acid composition of Sunit sheep reared under different feeding regimens in China. J. Sci. Food Agric. 2021, 101, 1100–1110. [Google Scholar] [CrossRef] [PubMed]
- Jiang, A.; Yin, D.; Zhang, L.; Li, B.; Li, R.; Zhang, X.; Zhang, Z.; Liu, H.; Kim, K.; Wu, W. Parsing the microRNA genetics basis regulating skeletal muscle fiber types and meat quality traits in pigs. Anim. Genet. 2021, 52, 292–303. [Google Scholar] [CrossRef]
- Matarneh, S.K.; Silva, S.L.; Gerrard, D.E. New Insights in Muscle Biology that Alter Meat Quality. Annu. Rev. Anim. Biosci. 2021, 16, 355–377. [Google Scholar] [CrossRef]
- Apaoblaza, A.; Gerrard, S.D.; Matarneh, S.K.; Wicks, J.C.; Kirkpatrick, L.; England, E.M.; Scheffler, T.L.; Duckett, S.K.; Hao, S.; de Silva, S.; et al. Muscle from grass- and grain-fed cattle differs energetically. Meat Sci. 2020, 161, 107996. [Google Scholar] [CrossRef]
- Ryu, Y.C.; Kim, B.C. The relationship between muscle fiber characteristics, postmortem metabolic rate, and meat quality of pig longissimus dorsi muscle. Meat Sci. 2005, 71, 351–357. [Google Scholar] [CrossRef]
- Brooke, M.H.; Kaiser, K.K. Muscle fiber types: How many and what kind? Arch. Neurol. 1970, 23, 369–379. [Google Scholar] [CrossRef]
- Schiaffino, S.; Reggiani, C. Molecular diversity of myofibrillar proteins: Gene regulation and functional significance. Physiol. Rev. 1996, 76, 371–423. [Google Scholar] [CrossRef]
- Pette, D.; Staron, R.S. Transitions of muscle fiber phenotypic profiles. Histochem. Cell Biol. 2001, 115, 359–372. [Google Scholar] [CrossRef]
- Joo, S.T.; Kim, G.D.; Hwang, Y.H.; Ryu, Y.C. Control of fresh meat quality through manipulation of muscle fiber characteristics. Meat Sci. 2013, 95, 828–836. [Google Scholar] [CrossRef]
- Lee, S.H.; Choe, J.H.; Choi, Y.M.; Jung, K.C.; Rhee, M.S.; Hong, K.C.; Lee, S.K.; Ryu, Y.C.; Kim, B.C. The influence of pork quality traits and muscle fiber characteristics on the eating quality of pork from various breeds. Meat Sci. 2012, 90, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.; Delzenne, N.M. Modulation of the gut microbiota-adipose tissue-muscle interactions by prebiotics. J. Endocrinol. 2021, 249, R1–R23. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, S.; Kim, H.; Garcia-Perez, I.; Reza, M.M.; Martin, K.A.; Kundu, P.; Cox, L.M.; Selkrig, J.; Posma, J.M.; Zhang, H.; et al. The gut microbiota influences skeletal muscle mass and function in mice. Sci. Transl. Med. 2019, 11, 5662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manickam, R.; Duszka, K.; Wahli, W. PPARs and Microbiota in Skeletal Muscle Health and Wasting. Int. J. Mol. Sci. 2020, 21, 8056. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.H.A.; Khan, R.; Cheng, G.; Long, F.; Bing, S.; Easa, A.A.; Schreurs, N.M.; Pant, S.D.; Zhang, W.; Li, A.; et al. RNA-Seq reveals the potential molecular mechanisms of bovine KLF6 gene in the regulation of adipogenesis. Int. J. Biol. Macromol. 2022, 195, 198–206. [Google Scholar] [CrossRef]
- Raza, S.H.A.; Khan, R.; Schreurs, N.M.; Guo, H.; Gui, L.S.; Mei, C.; Zan, L. Expression of the bovine KLF6 gene polymorphisms and their association with carcass and body measures in Qinchuan cattle (Bos Taurus). Genomics 2020, 112, 423–431. [Google Scholar] [CrossRef]
- Liu, C.; Cheung, W.H.; Li, J.; Chow, S.K.; Yu, J.; Wong, S.H.; Ip, M.; Sung, J.J.Y.; Wong, R.M.Y. Understanding the gut microbiota and sarcopenia: A systematic review. J. Cachexia Sarcopenia Muscle 2021, 12, 1393–1407. [Google Scholar] [CrossRef]
- Hee, B.; Wells, J.M. Microbial Regulation of Host Physiology by Short-chain Fatty Acids. Trends Microbiol. 2021, 29, 700–712. [Google Scholar]
- Zhao, Y.; Bi, J.; Yi, J.; Peng, J.; Ma, Q. Dose-dependent effects of apple pectin on alleviating high fat-induced obesity modulated by gut microbiota and SCFAs. Food Sci. Human Wellness 2022, 11, 143–154. [Google Scholar] [CrossRef]
- Wellmann, K.B.; Baggerman, J.O.; Burson, W.C.; Smith, Z.K.; Kim, J.; Hergenreder, J.E.; Rounds, W.; Bernhard, B.C.; Johnson, B.J. Effects of zinc propionate supplementation on growth performance, skeletal muscle fiber, and receptor characteristics in beef steers. J. Anim. Sci. 2020, 98, 210. [Google Scholar] [CrossRef]
- Baggerman, J.O.; Smith, Z.K.; Thompson, A.J.; Kim, J.; Hergenreder, J.E.; Rounds, W.; Johnson, B.J. Chromium propionate supplementation alters animal growth performance, carcass characteristics, and skeletal muscle properties in feedlot steers. Transl. Anim. Sci. 2020, 4, 146. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Ojima-Kato, T.; Saburi, W.; Mori, H.; Matsui, H.; Tanabe, S.; Suzuki, T. Supplemental epilactose prevents metabolic disorders through uncoupling protein-1 induction in the skeletal muscle of mice fed high-fat diets. Brit. J. Nutr. 2015, 114, 1774–1783. [Google Scholar] [CrossRef] [Green Version]
- Giudice, J.; Taylor, J.M. Muscle as a paracrine and endocrine organ. Curr. Opin. Pharmacol. 2017, 34, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Brooke, M.H.; Kaise, K.K. Three “myosin adenosine triphosphatase” systems: The nature of their pH lability and sulfhydryl dependence. J. Histochem. Cytochem. 1970, 18, 670–672. [Google Scholar] [CrossRef] [PubMed]
- Gagaoua, M.; Picard, B. Muscle fiber properties in cattle and their relationships with meat qualities: An overview. J. Agr. Food Chem. 2020, 68, 6021–6039. [Google Scholar]
- Ryu, Y.; Choi, Y.; Lee, S.; Shin, H.; Choe, J.; Kim, J.; Hong, K.; Kim, B. Comparing the histochemical characteristics and meat quality traits of different pig breeds. Meat Sci. 2008, 80, 363–369. [Google Scholar] [CrossRef]
- Pennings, B.; Groen, B.; De Lange, A.; Gijsen, A.P.; Zorenc, A.H.; Senden, J.M.G.; Van Loon, L.J.C. Amino acid absorption and subsequent muscle protein accretion following graded intakes of whey protein in elderly men. Am. J. Physiol. Endocrinol. Metabol. 2012, 302, 992. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Hong, J.; Xu, X.; Feng, Q.; Zhang, D.; Gu, Y.; Shi, J.; Zhao, S.; Liu, W.; Wang, X.; et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat. Med. 2017, 23, 859–868. [Google Scholar] [CrossRef]
- Liu, X.; Kim, S.H.; Kim, I.H. Effects of the combination of multistrain probiotics and Castanea crenata shell extract on growth performance, nutrient digestibility, fecal microbial shedding, meat quality, noxious gas emissions, and blood parameters in finishing pigs. Livest. Sci. 2020, 240, 104185. [Google Scholar] [CrossRef]
- Varian, B.J.; Goureshetti, S.; Poutahidis, T.; Lakritz, J.R.; Levkovich, T.; Kwok, C.; Teliousis, K.; Ibrahim, Y.M.; Mirabal, S.; Erdman, S.E. Beneficial bacteria inhibit cachexia. Oncotarget 2016, 7, 11803–11816. [Google Scholar] [CrossRef] [Green Version]
- Mariño, E.; Richards, J.L.; McLeod, K.H.; Stanley, D.; Yap, Y.A.; Knight, J.; McKenzie, C.; Kranich, J.; Oliveira, A.C.; Rossello, F.J.; et al. Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes. Nat. Immunol. 2017, 18, 552. [Google Scholar] [CrossRef] [PubMed]
- Canfora, E.E.; Meex, R.C.R.; Venema, K.; Blaak, E.E. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 2019, 15, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yi, C.-X.; Katiraei, S.; Kooijman, S.; Zhou, E.; Chung, C.K.; Gao, Y.; van den Heuvel, J.K.; Meijer, O.C.; Berbée, J.F.P.; et al. Butyrate reduces appetite and activates brown adipose tissue via the gut-brain neural circuit. Gut 2018, 67, 1269–1279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, S.; Chen, X.; Huang, Z.; Chen, D.; Yu, B.; Chen, H.; He, J.; Luo, J.; Zheng, P.; Yu, J.; et al. Dietary sodium butyrate supplementation promotes oxidative fiber formation in mice. Anim. Biotechnol. 2018, 29, 212–215. [Google Scholar] [CrossRef]
- Trinchese, G.; Cavaliere, G.; Penna, E.; De Filippo, C.; Cimmino, F.; Catapano, A.; Musco, N.; Tudisco, R.; Lombardi, P.; Infascelli, F.; et al. Milk from cow fed with high forage/concentrate ratio diet: Beneficial effect on rat skeletal muscle inflammatory state and oxidative stress through modulation of mitochondrial functions and AMPK activity. Front. Physiol. 2018, 9, 1969. [Google Scholar] [CrossRef] [Green Version]
- Conte, G.; Serra, A.; Casarosa, L.; Ciucci, F.; Cappucci, A.; Bulleri, E.; Corrales-Retana, L.; Buccioni, A.; Mele, M. Effect of linseed supplementation on total longissimus muscle lipid composition and shelf-life of beef from young maremmana bulls. Front. Vet. Sci. 2018, 5, 326. [Google Scholar] [CrossRef]
- Nie, Y.F.; Hu, J.; Yan, X.H. Cross-talk between bile acids and intestinal microbiota in host metabolism and health. J. Zhejiang Univ. Sci. B 2015, 16, 436–446. [Google Scholar] [CrossRef] [Green Version]
- Ng, S.Y.; Diffey, I.; Matsuka, J.; Ljubicic, V. Ampk Activation Normalizes Myogenic Regulatory Gene Expression in the Skeletal Muscle of Dystrophic Animals: 377. Med. Sci. Sport. Exerc. 2021, 53, 117–118. [Google Scholar] [CrossRef]
- Naseri, N.N.; Bonica, J.; Xu, H.; Park, L.C.; Arjomand, J.; Chen, Z.; Gibson, G.E. Novel metabolic abnormalities in the tricarboxylic acid cycle in peripheral cells from Huntington’s disease patients. PLoS ONE 2016, 11, e0160384. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Ren, X.; Gao, B.; Yu, X.; Zhang, X.; Zhang, J.; Liu, P. Comparison of carbohydrate metabolism key enzymes in different generations of growth-selected Portunus trituberculatus families. Aquaculture 2017, 477, 6–14. [Google Scholar] [CrossRef]
- Fang, C. Study on the Differences of Meat Quality, Muscle Fiber and Enzyme Activity of Longlin Cattle, Holstein Cattle and Xilin Buffalo; Guangxi University: Nanning, China, 2018. [Google Scholar]
- Bäckhed, F.; Manchester, J.K.; Semenkovich, C.F.; Gordon, J.I. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc. Natl. Acad. Sci. USA 2007, 104, 979–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, H.; Diao, H.; Xiao, Y.; Li, W.; Yu, B.; He, J.; Yu, J.; Zheng, P.; Mao, X.; Luo, Y.; et al. Gut microbiota can transfer fiber characteristics and lipid metabolic profiles of skeletal muscle from pigs to germ-free mice. Sci. Rep. UK 2016, 6, 31786. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Genes | Primer Sequences (5′→3′) | Product Size, bp | Accession NO. |
|---|---|---|---|
| MyHC I | F:AAGAACCTGCTGCGGCTG | 250 | XM_012129251.1 |
| R:CCAAGATGTGGCACGGCT | |||
| MyHC IIA | F:GAGGAACAATCCAATACAAATCTATCT | 173 | XM_027974884.1 |
| R:CCCATAGCATCAGGACACGA | |||
| MyHC IIB | F:GACAACTCCTCTCGCTTTGG | 247 | XM_027974883.1 |
| R:GGACTGTGATCTCCCCTTGA | |||
| MyHC IIX | F:GGAGGAACAATCCAATGTCAAC | 178 | XM_004012706.4 |
| R:GTCACTTTTTAGCATTTGGATGAGTTA | |||
| 18S rRNA | F:GTAACCCGTTGAACCCCATT | 112 | |
| R:CCATCCAATCGGTAGTAGCG |
| C Group | P Group | p-Value | |
|---|---|---|---|
| Muscle fiber density (number/mm2) | 747.43 ± 14.63 | 828.60 ± 51.45 | 0.068 |
| Type I muscle fiber | |||
| The number ratio of muscle fibers (%) | 9.05 ± 0.81 b | 12.08 ± 1.18 a | 0.028 |
| The area ratio of muscle fibers (%) | 7.53 ± 0.57 b | 10.57 ± 0.99 a | 0.013 |
| Muscle fiber diameter (μm) | 34.54 ± 0.84 | 37.07 ± 1.81 | 0.164 |
| Muscle fiber cross-sectional area (μm2) | 1019.59 ± 43.21 | 1069.66 ± 84.33 | 0.568 |
| Type IIA muscle fiber | |||
| The number ratio of muscle fibers (%) | 32.23 ± 1.49 | 31.13 ± 1.65 | 0.666 |
| The area ratio of muscle fibers (%) | 37.26 ± 2.41 | 37.15 ± 1.70 | 0.977 |
| Muscle fiber diameter (μm) | 42.46 ± 0.75 | 43.48 ± 1.44 | 0.496 |
| Muscle fiber cross-sectional area (μm2) | 1574.39 ± 49.76 | 1476.37 ± 107.16 | 0.353 |
| Type IIB muscle fiber | |||
| The number ratio of muscle fibers (%) | 57.75 ± 1.49 | 56.79 ± 2.11 | 0.652 |
| The area ratio of muscle fibers (%) | 54.51 ± 2.36 | 52.29 ± 2.14 | 0.582 |
| Muscle fiber diameter (μm) | 32.20 ± 0.58 | 33.30 ± 1.11 | 0.350 |
| Muscle fiber cross-sectional area (μm2) | 1242.41 ± 32.80 | 1126.43 ± 58.17 | 0.083 |
| Short Chain Fatty Acid (μg/g) | C Group | P Group | p-Value |
|---|---|---|---|
| Acetic acid | 10.28 ± 0.11 | 10.29 ± 0.09 | 0.929 |
| Propionic acid | 27.11 ± 0.10 b | 27.91 ± 0.27 a | 0.031 |
| Butyric acid | 57.16 ± 0.15 b | 58.27 ± 0.41 a | 0.043 |
| Valeric acid | 27.98 ± 0.34 b | 29.33 ± 0.40 a | 0.031 |
| Caproic acid | 15.97 ± 2.05 | 15.23 ± 0.26 | 0.645 |
| Isobutyric acid | 9.68 ± 0.09 | 9.75 ± 0.05 | 0.467 |
| Isovaleric acid | 26.15 ± 0.17 | 26.36 ± 0.15 | 0.404 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Bai, Y.; Wang, C.; Zhang, T.; Su, R.; Wang, B.; Duan, Y.; Sun, L.; Jin, Y.; Su, L. Effects of Probiotics Supplementation on the Intestinal Metabolites, Muscle Fiber Properties, and Meat Quality of Sunit Lamb. Animals 2023, 13, 762. https://doi.org/10.3390/ani13040762
Liu T, Bai Y, Wang C, Zhang T, Su R, Wang B, Duan Y, Sun L, Jin Y, Su L. Effects of Probiotics Supplementation on the Intestinal Metabolites, Muscle Fiber Properties, and Meat Quality of Sunit Lamb. Animals. 2023; 13(4):762. https://doi.org/10.3390/ani13040762
Chicago/Turabian StyleLiu, Ting, Yanping Bai, Chenlei Wang, Taiwu Zhang, Rina Su, Bohui Wang, Yan Duan, Lina Sun, Ye Jin, and Lin Su. 2023. "Effects of Probiotics Supplementation on the Intestinal Metabolites, Muscle Fiber Properties, and Meat Quality of Sunit Lamb" Animals 13, no. 4: 762. https://doi.org/10.3390/ani13040762
APA StyleLiu, T., Bai, Y., Wang, C., Zhang, T., Su, R., Wang, B., Duan, Y., Sun, L., Jin, Y., & Su, L. (2023). Effects of Probiotics Supplementation on the Intestinal Metabolites, Muscle Fiber Properties, and Meat Quality of Sunit Lamb. Animals, 13(4), 762. https://doi.org/10.3390/ani13040762







