Protein Content in the Diet Influences Growth and Diarrhea in Weaning Piglets
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics
2.2. Experimental Design
- -
- Period I: From the start of the experiment until the change of feed;
- -
- Period II: From the change of feed until the change of housing;
- -
- Period III: From the change of housing to the end of the post-weaning period (end of the experiment).
2.3. Growth Parameters
2.4. Diarrhea Scores and Corrective Interventions
2.5. Feces Chemical Characterization
2.6. DNA Extraction, Library Construction, and Sequencing
2.7. Statistical Analysis
2.8. Bioinformatic Analyses
3. Results
3.1. Growth Performance
3.2. Health Status
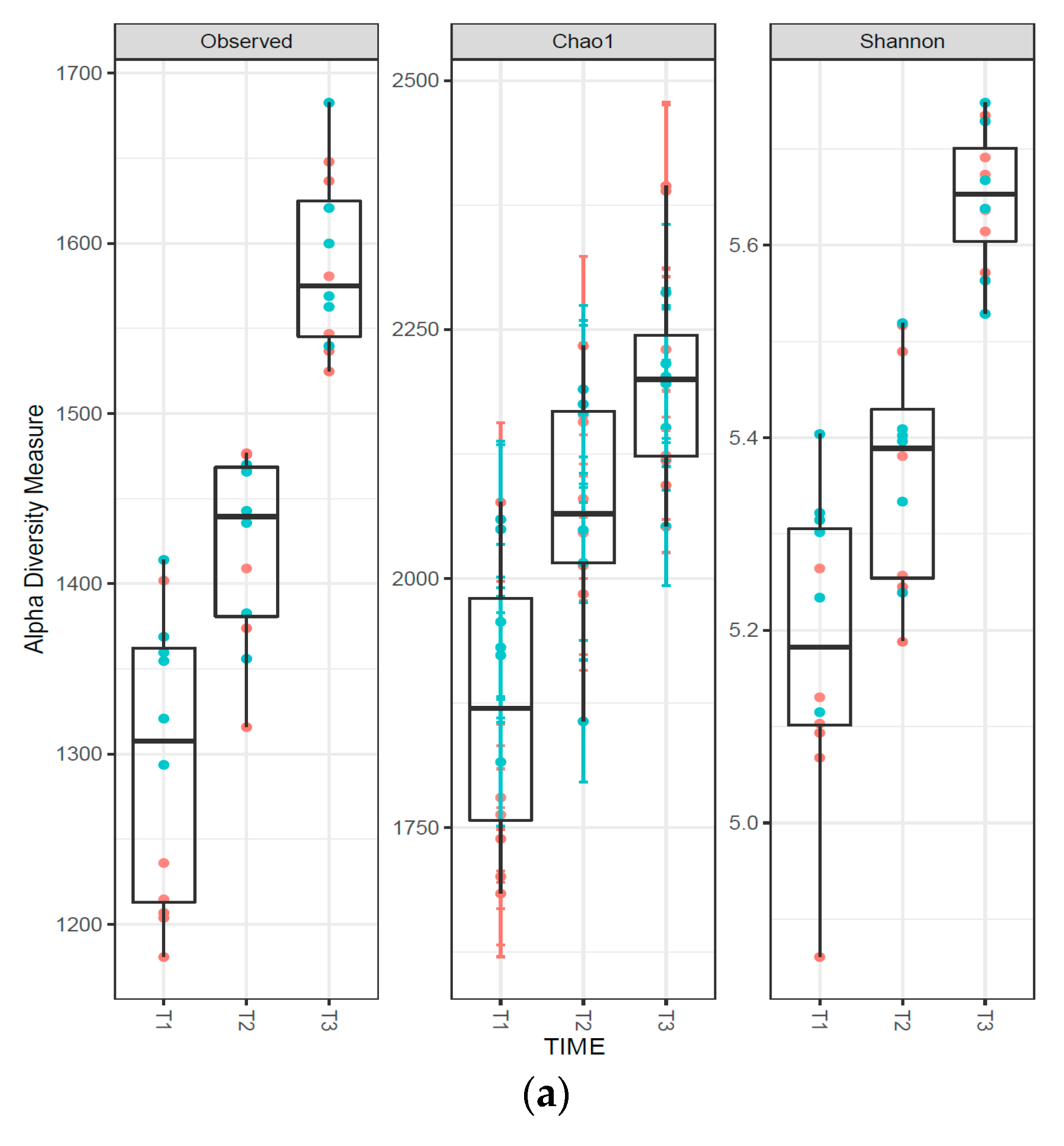
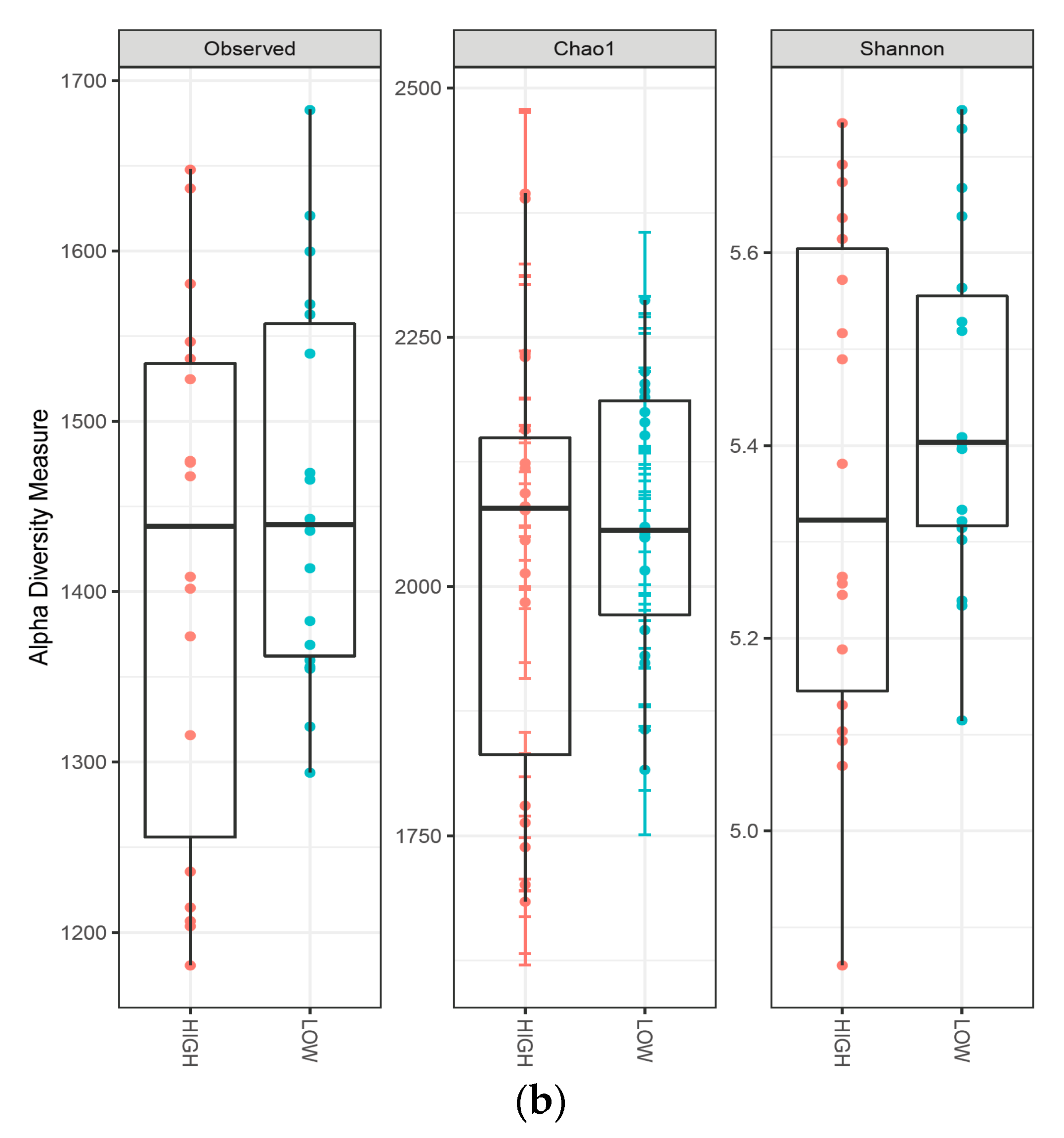
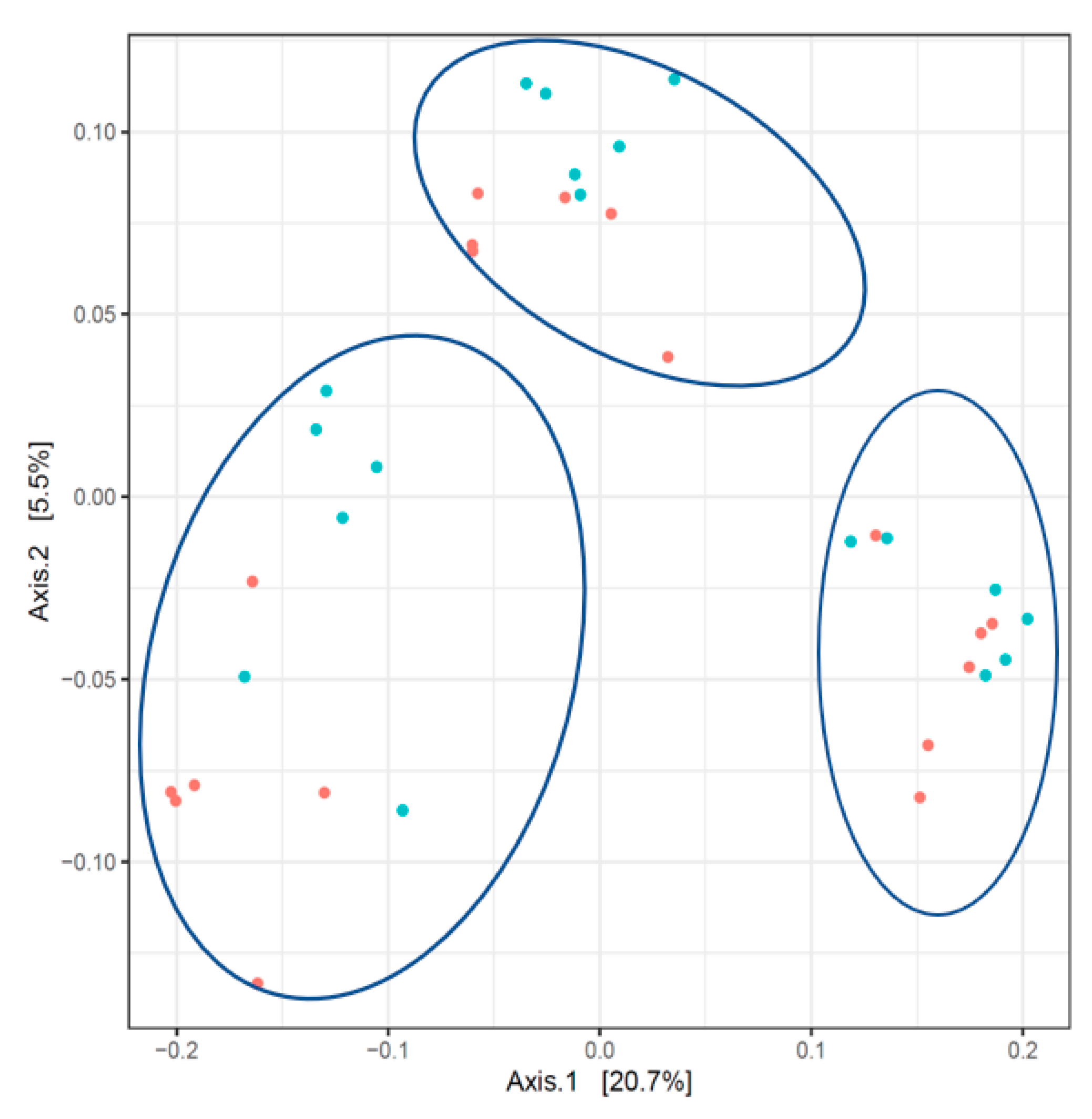
3.3. Composition of the Fecal Microbiota
3.4. Feces Composition
4. Discussion
4.1. Dietary Protein Level, Growth Performance, and Susceptibility to PWD
4.2. Piglet Fecal Microbiota in the Post-Weaning Period
4.3. Dietary Protein Level, Composition of the Fecal Microbiota, and PWD Susceptibility
4.4. Dietary Protein Level and Feces Composition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, J.; Yin, J.; Xu, K.; Han, H.; Liu, Z.M.; Wang, C.Y.; Li, T.J.; Yin, Y.L. Protein level and infantile diarrhea in a postweaning piglet model. Med. Inflamm. 2020, 2020, 1937387. [Google Scholar] [CrossRef]
- Kim, J.C.; Hansen, C.F.; Mullan, B.P.; Pluske, J.R. Nutrition and pathology of weaner pigs: Nutritional strategies to support barrier function in the gastrointestinal tract. Anim. Feed Sci. Technol. 2012, 172, 3–16. [Google Scholar] [CrossRef] [Green Version]
- de Lange, C.F.M.; Pluske, J.; Gong, J.; Nyachoti, C.M. Strategic use of feed ingredients and feed additives to stimulate gut health and development in young pigs. Livest. Sci. 2010, 134, 124–134. [Google Scholar] [CrossRef]
- Zhang, H.; Wielen, N.V.; Hee, B.V.; Wang, J.; Hendriks, W.; Gilbert, M. Impact of fermentable protein, by feeding high protein diets, on microbial composition, microbial catabolic activity, gut health and beyond in pigs. Microorganisms 2020, 8, 1735. [Google Scholar] [CrossRef]
- Gaskins, H.R. Intestinal bacteria and their influence on swine growth. In Swine Nutrition; Lewis, A.J., Southern, L.L., Eds.; PCRC Press LLC: Boca Raton, FL, USA, 2000; pp. 585–608. [Google Scholar]
- Macfarlane, G.T.; Macfarlane, S. Bacteria, colonic fermentation, and gastrointestinal health. J. AOAC Int. 2012, 95, 50–60. [Google Scholar] [CrossRef]
- Opapeju, F.O.; Rademacher, M.; Blank, G.; Nyachoti, C.M. Effect of low-protein amino acid-supplemented diets on the growth performance, gut morphology, organ weights and digesta characteristics of weaned pigs. Animal 2008, 2, 1457–1464. [Google Scholar] [CrossRef] [Green Version]
- Rhouma, M.; Fairbrother, J.M.; Beaudry, F.; Letellier, A. Post weaning diarrhea in pigs: Risk factors and non-colistin-based control strategies. Acta Vet. Scand. 2017, 59, 31. [Google Scholar] [CrossRef] [Green Version]
- Fouhse, J.M.; Zijlstra, R.T.; Willing, B.P. The role of gut microbiota in the health and disease of pigs. Anim. Front. 2016, 6, 30–36. [Google Scholar] [CrossRef] [Green Version]
- Gresse, R.; Chaucheyras-Durand, F.; Fleury, M.A.; Van de Wiele, T.; Forano, E.; Blanquet-Diot, S. Gut microbiota dysbiosis in postweaning piglets: Understanding the keys to health. Trends Microbiol. 2017, 25, 851–873. [Google Scholar] [CrossRef]
- Guevarra, R.B.; Lee, J.H.; Lee, S.H.; Seok, M.J.; Kim, D.W.; Kang, B.N.; Johnson, T.J.; Isaacson, R.E.; Kim, H.B. Piglet gut microbial shifts early in life: Causes and effects. J. Anim. Sci. Biotechnol. 2019, 10, 1. [Google Scholar] [CrossRef] [Green Version]
- Heo, J.M.; Kim, J.C.; Hansen, C.F.; Mullan, B.P.; Hampson, D.J.; Pluske, J.R. Feeding a diet with decreased protein content reduces indices of protein fermentation and the incidence of postweaning diarrhea in weaned pigs challenged with an enterotoxigenic strain of Escherichia coli. J. Anim. Sci. 2009, 87, 2833–2843. [Google Scholar] [CrossRef] [Green Version]
- Rist, V.T.; Weiss, E.; Eklund, M.; Mosenthin, R. Impact of dietary protein on microbiota composition and activity in the gastrointestinal tract of piglets in relation to gut health: A review. Animal 2013, 7, 1067–1078. [Google Scholar] [CrossRef] [Green Version]
- Luise, D.; Chalvon-Demersay, T.; Lambert, W.; Bosi, P.; Trevisi, P. Meta-analysis to evaluate the impact of the reduction of dietary crude protein on the gut health of post-weaning pigs. Ital. J. Anim. Sci. 2021, 20, 1386–1397. [Google Scholar] [CrossRef]
- Van Milgen, J.; Dourmad, J.Y. Concept and application of ideal protein for pigs. J. Anim. Sci. Biotechnol. 2015, 6, 15. [Google Scholar] [CrossRef] [Green Version]
- APHA. Standard Methods for the Examination of Water and Wastewater, 18th ed.; American Public Health Association: Washington DC, USA, 1992. [Google Scholar]
- AOAC. Official Method 978.10-1979 (1996), Fiber (Crude) in Animal Feed and Pet Food. Available online: http://www.aoacofficialmethod.org/index.php?main_page=product_info&products_id=366 (accessed on 19 February 2023).
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral-detergent fiber and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, 1. [Google Scholar] [CrossRef]
- Littell, R.C.; Henry, P.R.; Ammerman, C.B. Statistical analysis of repeated measures data using SAS procedures. J. Anim. Sci. 1998, 76, 1216–1231. [Google Scholar] [CrossRef] [Green Version]
- Albanese, D.; Fontana, P.; De Filippo, C.; Cavalieri, D.; Donati, C. MICCA: A complete and accurate software for taxonomic profiling of metagenomic data. Sci. Rep. 2015, 5, 9743. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [Green Version]
- Goodrich, J.K.; Di Rienzi, S.C.; Poole, A.C.; Koren, O.; Walters, W.A.; Caporaso, J.G.; Knight, R.; Ley, R.E. Conducting a microbiome study. Cell 2014, 158, 250–262. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhou, J.; Wang, G.; Cai, S.; Zeng, X.; Qiao, S. Advances in low-protein diets for swine. J. Anim. Sci. Biotechnol. 2018, 9, 60. [Google Scholar] [CrossRef] [Green Version]
- Wen, X.; Wang, L.; Zheng, C.; Yang, X.; Ma, X.; Wu, Y.; Chen, Z.; Jiang, Z. Fecal scores and microbial metabolites in weaned piglets fed different protein sources and levels. Anim. Nutr. 2018, 4, 31–36. [Google Scholar] [CrossRef]
- Rattigan, R.; Sweeney, T.; Maher, S.; Ryan, M.T.; Thornton, K.; O’Doherty, J.V. Effects of reducing dietary crude protein concentration and supplementation with either laminarin or zinc oxide on the growth performance and intestinal health of newly weaned pigs. Anim. Feed Sci. Technol. 2020, 270, 114693. [Google Scholar] [CrossRef]
- Limbach, J.R. Reducing Crude Protein Levels in Diets Fed to Young Pigs to Optimize Growth Performance and Intestinal Health. Ph.D. Thesis, University of Illinois, Champaign, IL, USA, 2020. Available online: http://hdl.handle.net/2142/108563 (accessed on 24 March 2022).
- Lynegaard, J.C.; Kjeldsen, N.J.; Bache, J.K.; Weber, N.R.; Hansen, C.F.; Nielsen, J.P.; Amdi, C. Low protein diets without medicinal zinc oxide for weaned pigs reduced diarrhea treatments and average daily gain. Animal 2021, 15, 100075. [Google Scholar] [CrossRef]
- Yu, D.; Zhu, W.; Hang, S. Effects of low-protein diet on the intestinal morphology, digestive enzyme activity, blood urea nitrogen, and gut microbiota and metabolites in weaned pigs. Arch. Anim. Nutr. 2019, 73, 287–305. [Google Scholar] [CrossRef]
- Yang, Q.; Huang, X.; Wang, P.; Yan, Z.; Sun, W.; Zhao, S.; Gun, S. Longitudinal development of the gut microbiota in healthy and diarrheic piglets induced by age-related dietary changes. Microbiologyopen 2019, 8, e923. [Google Scholar] [CrossRef] [Green Version]
- Yu, D.; Zhu, W.; Hang, S. Effects of long-term dietary protein restriction on intestinal morphology, digestive enzymes, gut hormones, and colonic microbiota in pigs. Animals 2019, 9, 180. [Google Scholar] [CrossRef] [Green Version]
- Seradj, A.R.; Balcells, J.; Sarri, L.; Fraile, L.J.; de la Fuente, O.G. The impact of producing type and dietary crude protein on animal performances and microbiota together with greenhouse gases emissions in growing pigs. Animals 2020, 10, 1742. [Google Scholar] [CrossRef]
- Dou, S.; Gadonna-Widehem, P.; Rome, V.; Hamoudi, D.; Rhazi, L.; Lakhal, L.; Larcher, T.; Bahi-Jaber, N.; Pinon-Quintana, A.; Guyonvarch, A.; et al. Characterisation of early-life fecal microbiota in susceptible and healthy pigs to post-weaning diarrhoea. PLoS ONE 2017, 10, e0169851. [Google Scholar] [CrossRef] [Green Version]
- Guevarra, R.B.; Hong, S.H.; Cho, J.H.; Kim, B.R.; Shin, J.; Lee, J.H.; Kang, B.N.; Kim, Y.H.; Wattanaphansak, S.; Isaacson, R.E.; et al. The dynamics of the piglet gut microbiome during the weaning transition in association with health and nutrition. J. Anim. Sci. Biotechnol. 2018, 30, 9–54. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Xu, Y.; Chen, X.; Fang, C.; Zhao, L.; Chen, F. The maturing development of gut microbiota in commercial piglets during the weaning transition. Front. Microbiol. 2017, 8, 1688. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Liu, Y.; Liu, J.; Wang, H.; Guo, Y.; Du, M.; Cai, C.; Zhao, Y.; Lu, C.; Guo, X.; et al. Composition of the fecal microbiota of piglets at various growth stages. Front. Vet. Sci. 2021, 8, 661671. [Google Scholar] [CrossRef]
- de Vries, H.; Smidt, H. Microbiota development in piglets. In The Suckling and Weaned Piglet; Chantal Farmer Editor; Wageningen Academic Publishers: Wageningen, The Netherlands, 2020; pp. 179–205. [Google Scholar] [CrossRef]
- Pollock, J.; Hutchings, M.R.; Hutchings, K.E.K.; Gally, D.L.; Houdijk, J.G.M. Changes in the ileal, but not fecal, microbiome in response to increased dietary protein level and enterotoxigenic Escherichia coli exposure in pigs. Appl. Environ. Microbiol. 2019, 85, e01252. [Google Scholar] [CrossRef] [Green Version]
- Fan, P.; Liu, P.; Song, P.; Chen, X.; Ma, X. Moderate dietary protein restriction alters the composition of gut microbiota and improves ileal barrier function in adult pig model. Sci. Rep. 2017, 7, 43412. [Google Scholar] [CrossRef] [Green Version]
- González-Prendes, R.; Pena, R.N.; Solé, E.; Seradj, A.R.; Estany, J.; Ramayo-Caldas, Y. Modulatory effect of protein and carotene dietary levels on pig gut microbiota. Sci. Rep. 2019, 9, 14582. [Google Scholar] [CrossRef] [Green Version]
- Varel, V.H.; Yen, J.T. Microbial perspective on fiber utilization by swine. J. Anim. Sci. 1997, 75, 2715–2722. [Google Scholar] [CrossRef] [Green Version]
- Stewart, C.S.; Flint, H.J. Bacteroides (Citrobacter) succinogenes, a cellulolytic anaerobic bacterium from the gastrointestinal tract. Appl. Microbiol. Biotechnol. 1989, 30, 433–439. [Google Scholar] [CrossRef]
- Xie, X.; Yang, C.; Guan, L.L.; Wang, J.; Xue, M.; Liu, J.X. Persistence of cellulolytic bacteria Fibrobacter and Treponema after short-term corn stover-based dietary intervention reveals the potential to improve rumen fibrolytic function. Front. Microbiol. 2018, 9, 1363. [Google Scholar] [CrossRef]
- Zhao, Y.; Tian, G.; Chen, D.; Zheng, P.; Yu, J.; He, J.; Mao, X.; Yu, B. Effects of varying levels of dietary protein and net energy on growth performance, nitrogen balance and faecal characteristics of growing-finishing pigs. R. Bras. Zootec. 2019, 48, e20180021. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; He, T.; Bumbie, G.Z.; Hu, H.; Chen, Q.; Lu, C.; Tang, Z. Effects of dietary crude protein levels on fecal crude protein, amino acids flow amount, fecal and ileal microbial amino acids composition and amino acid digestibility in growing pigs. Animals 2020, 10, 2092. [Google Scholar] [CrossRef]
- Choct, M.; Dersjant-Li, Y.; McLeish, J.; Peisker, M. Soy oligosaccharides and soluble non-starch polysaccharides: A review of digestion, nutritive and anti-nutritive effects in pigs and poultry. Anim. Biosci. 2010, 23, 1386–1398. [Google Scholar] [CrossRef]
- Smiricky, M.R.; Grieshop, C.M.; Albin, D.M.; Wubben, J.E.; Gabert, V.M.; Fahey, G.C., Jr. The influence of soy oligosaccharides on apparent and true ileal amino acid digestibilities and fecal consistency in growing pigs. J. Anim. Sci. 2002, 80, 2433–2441. [Google Scholar] [CrossRef]
- Zhang, L.; Li, D.; Qiao, S.; Wang, J.; Bai, L.; Wang, Z.; Han, I.K. The effect of soybean galacto-oligosaccharides on nutrient and energy digestibility and digesta transit time in weanling piglets. Anim. Biosci. 2001, 14, 1598–1604. [Google Scholar] [CrossRef]
- Nakatani, M.; Inoue, R.; Tomonaga, S.; Fukuta, K.; Tsukahara, T. Production, absorption, and blood flow dynamics of short-chain fatty acids produced by fermentation in piglet hindgut during the suckling-weaning period. Nutrients 2018, 10, 1220. [Google Scholar] [CrossRef] [Green Version]
| Growth Period | Event (Initial and Final of the Period) | Time from the Start of the Experiment (Days) | Piglet Age (Days) | Measurement of Piglet Body Weight | Fecal Sampling |
|---|---|---|---|---|---|
| Weaning | 0 | 25 | + | – | |
| Period I | |||||
| Change of feed | 25 | 50 | + | T1 | |
| Period II | |||||
| Change of housing | 46 | 71 | + | T2 | |
| Period III | |||||
| End of post-weaning | 70 | 95 | + | T3 |
| Growth Phase | Protein Level (% CP) | |
|---|---|---|
| High | Low | |
| From the start of weaning to 15 kg (Period I and II) | 18.5 | 16.5 |
| From 15 kg to the end of the experiment (Period III) | 16.5 | 14.5 |
| Protein Level | |||||
|---|---|---|---|---|---|
| Feed Composition, as Is 1 | From 8 to 15 kg Body Weight | From 15 to 30 kg Body Weight | |||
| High | Low | High | Low | ||
| Ingredients | |||||
| Corn | % | 18.8 | 23.7 | 26.6 | 35.0 |
| Barley | % | 20.0 | 20.0 | 15.0 | 15.0 |
| Expanded wheat | % | 10.0 | 10.0 | 6.00 | 6.00 |
| Soybean meal (48% CP 2) | % | 8.87 | 2.73 | 8.43 | 3.07 |
| Wheat middlings, durum | % | 0.00 | 0.00 | 8.13 | 4.60 |
| Bakery byproducts | % | 8.00 | 8.00 | 4.00 | 4.00 |
| Whey powder, sweet | % | 6.00 | 6.00 | 0.00 | 0.00 |
| Soybean protein concentrate (65% CP) | % | 5.00 | 5.00 | 2.00 | 2.00 |
| Mineral vitamin premix | % | 5.00 | 5.00 | 2.50 | 2.50 |
| Wheat bran, soft | % | 4.23 | 5.00 | 4.00 | 4.00 |
| Wheat | % | 4.00 | 4.00 | 8.00 | 8.00 |
| Wheat middlings, soft | % | 0.00 | 0.00 | 8.00 | 8.00 |
| Fish meal (68% CP) | % | 3.00 | 3.00 | 2.00 | 2.00 |
| Dextrose | % | 2.50 | 2.50 | 1.00 | 1.00 |
| Monodicalcium phosphate | % | 1.57 | 1.64 | 0.00 | 0.21 |
| Chicory pulp, dehydrated | % | 1.20 | 1.20 | 1.00 | 1.00 |
| Coconut oil | % | 0.96 | 0.83 | 1.16 | 1.04 |
| Soybean oil | % | 0.00 | 0.00 | 0.50 | 0.50 |
| Acidifiers | % | 0.80 | 0.80 | 0.80 | 0.80 |
| DL-Methionine | % | 0.05 | 0.12 | 0.18 | 0.25 |
| L-Valine | % | 0.05 | 0.17 | 0.00 | 0.08 |
| L-lysine HCl | % | 0.01 | 0.22 | 0.47 | 0.68 |
| L-tryptophan | % | 0.01 | 0.04 | 0.05 | 0.09 |
| L-threonine | % | 0.00 | 0.02 | 0.14 | 0.23 |
| Calculated values | |||||
| Crude protein | % | 18.5 | 16.6 | 16.5 | 14.6 |
| Crude fat | % | 5.00 | 5.00 | 4.50 | 4.50 |
| Crude fiber | % | 2.96 | 2.85 | 3.90 | 3.50 |
| Ash | % | 6.07 | 5.87 | 5.50 | 5.31 |
| Digestible Energy | kcal kg−1 | 3472 | 3450 | 3324 | 3320 |
| Metabolizable Energy | kcal kg−1 | 3289 | 3277 | 3176 | 3180 |
| Net energy | kcal kg−1 | 2480 | 2510 | 2401 | 2447 |
| Lysine | % | 1.40 | 1.40 | 1.20 | 1.20 |
| Methionine | % | 0.54 | 0.58 | 0.45 | 0.49 |
| Methionine + Cystine | % | 0.84 | 0.84 | 0.72 | 0.72 |
| Threonine | % | 0.92 | 0.84 | 0.72 | 0.72 |
| Tryptophan | % | 0.28 | 0.28 | 0.24 | 0.24 |
| Valine | % | 0.98 | 0.98 | 0.86 | 0.84 |
| Isoleucine | % | 0.78 | 0.65 | 0.68 | 0.56 |
| Calcium | % | 0.65 | 0.65 | 0.59 | 0.62 |
| Phosphorus | % | 0.78 | 0.77 | 0.50 | 0.50 |
| Fraction of Litter 1 Suffering from Diarrhea | Diarrhea Intensity | ||
|---|---|---|---|
| Mild | Medium | Serious | |
| 0 | 0 | 0 | 0 |
| 1/3 | 1 | 4 | 7 |
| 2/3 | 2 | 5 | 8 |
| 3/3 | 3 | 6 | 9 |
| Protein Level | |||
|---|---|---|---|
| Growth Parameter | High | Low | Significance of the Difference |
| Initial body weight (kg) | 7.80 | 7.77 | NS |
| From weaning to change of housing | |||
| Period I 1 | 17.2 | 15.6 | NS |
| Period II 2 | 29.8 | 28.0 | NS |
| Period I + Period II | 23.5 | 21.8 | NS |
| Average daily gain (g) | |||
| Period I | 377 | 313 | p < 0.01 |
| Period II | 599 | 593 | NS |
| Period I + Period II | 479 | 440 | p < 0.05 |
| Average daily feed intake (g) | |||
| Period I | 585 | 536 | NS |
| Period II | 1181 | 1138 | NS |
| Period I + Period II | 857 | 810 | NS |
| Feed conversion ratio (-/-) | |||
| Period I | 1.55 | 1.72 | p < 0.01 |
| Period II | 1.97 | 1.92 | NS |
| Period I + Period II | 1.79 | 1.84 | p < 0.05 |
| From change of housing to the end of post-weaning (period III) 3 | |||
| Average daily gain (g) | 457 | 437 | NS |
| Average daily feed intake (g) | 952 | 952 | NS |
| Feed conversion ratio (-/-) | 2.11 | 2.18 | NS |
| Final body weight (kg) | 40.7 | 38.5 | NS |
| Diarrhea Score Summations 1 | ||||
|---|---|---|---|---|
| Protein Level | Significance of the Difference | |||
| Growth period | High | Low | Total in the Period | |
| Period I | 91 (85.0) | 16 (15.0) | 107 (45.7) | |
| Period II | 5 (83.3) | 1 (16.7) | 6 (2.6) | |
| Total (Period I + Period II) | 96 (85.0) | 17 (15.0) | 113 (48.3) | p < 0.01 |
| Period III | 71 (58.7) | 50 (41.3) | 121 (51.7) | NS |
| Total score | 167 (71.4) | 67 (28.6) | 234 | |
| Phylum | Sampling Time and Protein Level | Protein Level | Significance Level 3 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | |||||||||||||
| High | Low | Mean | High | Low | Mean | High | Low | Mean | High | Low | Overall Mean | Sampling Time | Protein Level | Sampling Time × Protein Level | |
| Actinobacteria | 0.50 | 0.37 | 0.43A | 0.23 | 0.18 | 0.21B | 0.20 | 0.20 | 0.20B | 0.31A | 0.25A | 0.28 | ** | NS | NS |
| Bacteroidetes | 40.7 | 41.9 | 41.3B | 49.4 | 50.7 | 50.1A | 46.6 | 48.6 | 47.6A | 45.6A | 47.0A | 46.3 | ** | NS | NS |
| Fibrobacteres | 0.15 | 0.28 | 0.22B | 0.38 | 0.72 | 0.55A | 0.35 | 0.52 | 0.43A | 0.29B | 0.51A | 0.38 | ** | ** | NS |
| Firmicutes | 54.4 | 50.6 | 52.5A | 40.6 | 37.2 | 38.9B | 43.0 | 39.7 | 41.4B | 46.0A | 42.5B | 44.3 | ** | * | NS |
| Proteobacteria | 1.55 | 2.40 | 1.97B | 3.02 | 3.27 | 3.14A | 2.52 | 3.15 | 2.83A | 2.36B | 2.94A | 2.67 | ** | * | NS |
| Spirochaetes | 0.90 | 2.43 | 1.67B | 3.93 | 5.38 | 4.66A | 4.25 | 4.83 | 4.54A | 3.03B | 4.27A | 3.60 | ** | * | NS |
| Unclassified | 1.63 | 1.78 | 1.71B | 1.73 | 1.83 | 1.78AB | 1.98 | 1.97 | 1.97A | 1.78A | 1.86A | 1.82 | * | NS | NS |
| Verrucomicrobia | 0.00 | 0.08 | 0.04C | 0.25 | 0.35 | 0.30B | 0.57 | 0.57 | 0.57A | 0.27A | 0.33A | 0.32 | ** | NS | NS |
| Phylum | Family | Genus | Protein Level of Prevalent Abundance | ||
|---|---|---|---|---|---|
| Classes of Relative Abundance | |||||
| >1% | 0.1–1% | Low | High | ||
| Fibrobacteres | Fibrobacteraceae | Fibrobacter | + | ||
| Firmicutes | Clostridiaceae 1 | Clostridium sensu stricto | + (T1) | ||
| Firmicutes | Eubacteriaceae | Eubacterium | + | ||
| Firmicutes | Lachnospiraceae | Roseburia Blautia Clostridium XlVa | Lachnospiracea_incertae_sedis, Coprococcus, Anaerostipes, Ruminococcus2, Dorea, Fusicatenibacter | + | |
| Firmicutes | Lactobacillaceae | Lactobacillus | + (T1) | ||
| Firmicutes | Peptostreptococcaceae | Clostridium XI | + (T1) | ||
| Firmicutes | Erysipelotrichaceae | Catenibacterium, Turicibacter, Erysipelotrichaceae_incertae_sedis | + (T1) | ||
| Proteobacteria | Succinivibrionaceae | Succinivibrio | + | ||
| Spirochaetes | Spirochaetaceae | Treponema | Sphaerochaeta | + | |
| Parameter | Sampling Time | Protein Level | Significance Level 2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | ||||||||||||
| High | Low | Mean | High | Low | Mean | High | Low | Mean | High | Low | Sampling Time | Protein Level | Sampling Time × Protein Level | |
| Physico-chemical parameters: | ||||||||||||||
| Dry matter (DM) | 24.2 | 26.1 | 25.2C | 26.5 | 27.6 | 27.1B | 27.8 | 29.4 | 28.6A | 26.2B | 27.7A | ** | ** | NS |
| Organic matter (% DM) | 87.6 | 88.8 | 88.2B | 89.1 | 89.1 | 89.1A | 89.2 | 89.8 | 89.5A | 88.7B | 89.2A | ** | ** | ** |
| Total N (% DM) | 4.53 | 4.08 | 4.30A | 3.58 | 3.29 | 3.43B | 3.01 | 2.94 | 2.98C | 3.70A | 3.44B | ** | ** | NS |
| Ammonium N (% DM) | 0.61 | 0.56 | 0.59A | 0.61 | 0.51 | 0.56A | 0.49 | 0.45 | 0.47B | 0.57A | 0.51B | ** | ** | NS |
| pH | 6.31 | 6.32 | 6.31B | 6.59 | 6.55 | 6.57A | 6.62 | 6.52 | 6.57A | 6.51A | 6.46A | ** | NS | NS |
| Crude fiber (% DM) | 15.4 | 14.8 | 15.1B | 16.4 | 17.1 | 16.7A | 17.6 | 17.2 | 17.4A | 16.5A | 16.4A | ** | NS | NS |
| Hemicellulose | 19.9 | 23.0 | 21.4C | 25.0 | 27.9 | 26.1B | 30.3 | 32.1 | 31.1A | 25.0B | 27.7A | ** | ** | NS |
| Cellulose | 14.4 | 15.6 | 15.0B | 16.5 | 16.6 | 16.6A | 16.1 | 16.3 | 16.2A | 15.7B | 16.5A | ** | * | NS |
| Volatile fatty acids (mmol kg−1 DM): | ||||||||||||||
| Acetic | 183 | 197 | 190A | 237 | 214 | 225A | 181 | 189 | 185A | 200A | 200A | NS | NS | NS |
| Propionic | 62.1 | 60.3 | 61.2B | 76.7 | 75.3 | 76.0A | 66.1 | 66.1 | 66.1AB | 68.3A | 67.2A | * | NS | NS |
| Iso-butyric | 13.5 | 26.6 | 21.1A | 29.7 | 22.6 | 26.1A | 18.5 | 20.2 | 19.4A | 21.2A | 23.1A | NS | NS | NS |
| Butyric | 51.3 | 43.4 | 47.3A | 53.7 | 47.2 | 50.5A | 40.7 | 42.7 | 41.7A | 48.5A | 44.4A | NS | NS | NS |
| Isovaleric | 10.7 | 12.1 | 11.4B | 17.2 | 14.1 | 15.6A | 15.1 | 12.7 | 13.9A | 14.3A | 13.0B | ** | * | * |
| Valeric | 10.1 | 11.3 | 10.7B | 14.0 | 11.7 | 12.9A | 10.9 | 10.3 | 10.6B | 11.6A | 11.1A | * | NS | NS |
| Isocaproic | 4.38 | 6.13 | 5.26A | 4.42 | 5.43 | 4.92A | 3.92 | 4.80 | 4.36A | 4.24A | 5.46A | NS | NS | NS |
| Caproic | 3.18 | 3.18 | 3.18A | 4.10 | 2.48 | 3.29A | 2.33 | 2.32 | 2.32A | 3.21A | 2.66A | NS | NS | NS |
| Total | 341 | 361 | 351A | 441 | 395 | 418A | 344 | 358 | 351A | 400A | 367A | NS | NS | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchetti, R.; Faeti, V.; Gallo, M.; Pindo, M.; Bochicchio, D.; Buttazzoni, L.; Della Casa, G. Protein Content in the Diet Influences Growth and Diarrhea in Weaning Piglets. Animals 2023, 13, 795. https://doi.org/10.3390/ani13050795
Marchetti R, Faeti V, Gallo M, Pindo M, Bochicchio D, Buttazzoni L, Della Casa G. Protein Content in the Diet Influences Growth and Diarrhea in Weaning Piglets. Animals. 2023; 13(5):795. https://doi.org/10.3390/ani13050795
Chicago/Turabian StyleMarchetti, Rosa, Valerio Faeti, Maurizio Gallo, Massimo Pindo, Davide Bochicchio, Luca Buttazzoni, and Giacinto Della Casa. 2023. "Protein Content in the Diet Influences Growth and Diarrhea in Weaning Piglets" Animals 13, no. 5: 795. https://doi.org/10.3390/ani13050795




