Effects of Replacing Fishmeal with the Mixture of Cottonseed Protein Concentrate and Clostridium autoethanogenum Protein on the Growth, Nutrient Utilization, Serum Biochemical Indices, Intestinal and Hepatopancreas Histology of Rainbow Trout (Oncorhynchus mykiss)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experimental Diets and Design
2.3. Management of Experimental Fish and Feeding
2.4. Samples Collection
2.5. Measurement Indicators and Methods
2.5.1. Growth Performance and Physical Indices
2.5.2. The Diets and Whole-Body Composition
2.5.3. Serum Biochemical Indices
2.5.4. Nutrient Retention
2.5.5. Intestinal Digestive Enzyme Activity
2.5.6. Intestinal and Hepatopancreas Histology
2.6. Statistical Analysis
3. Results
3.1. Growth Performance and Physical Indices
3.2. Whole-Body Composition
3.3. Nutrient Utilization and Intestinal Digestive Enzyme Activity
3.4. Serum Biochemical Indices
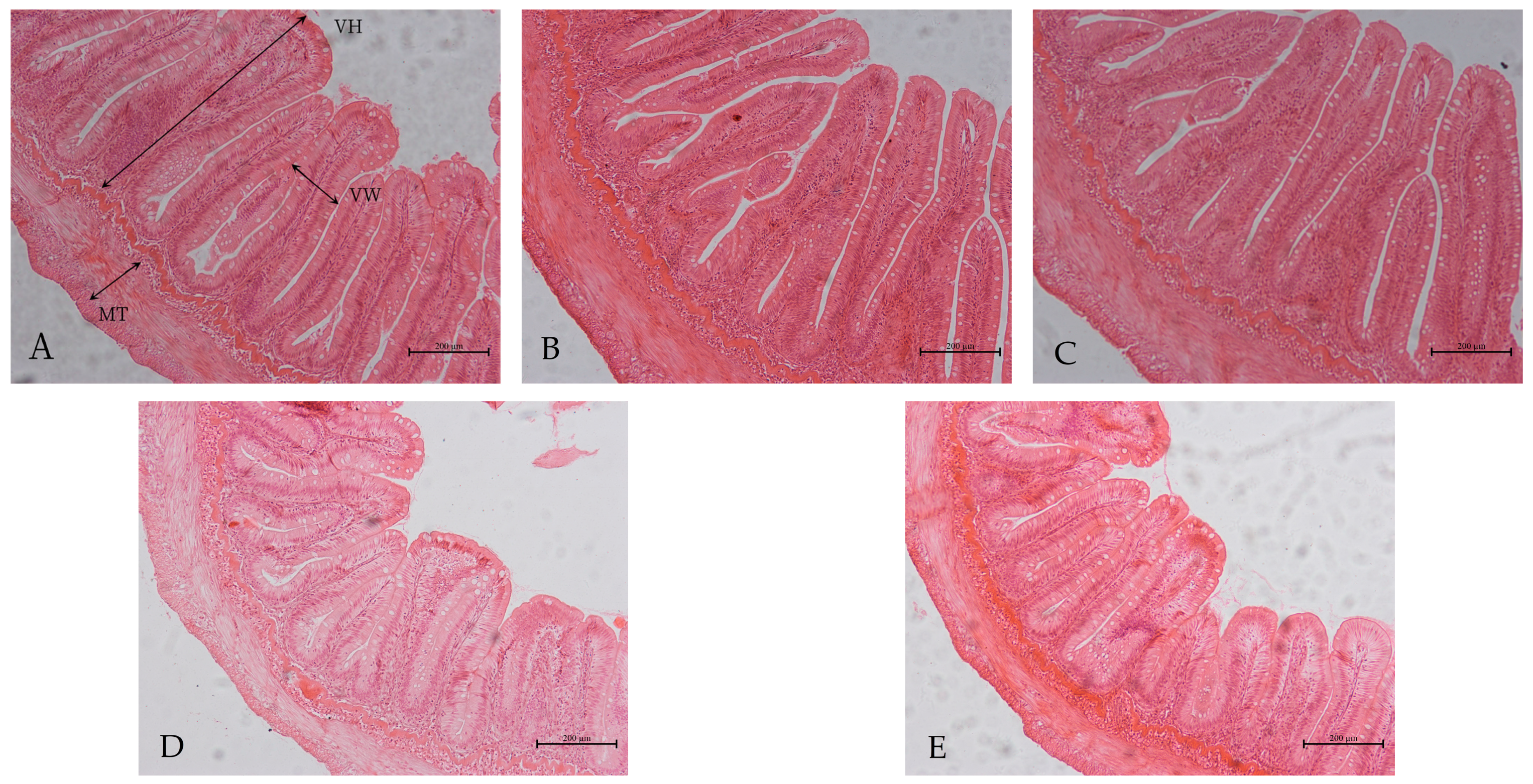
3.5. Intestinal Morphology
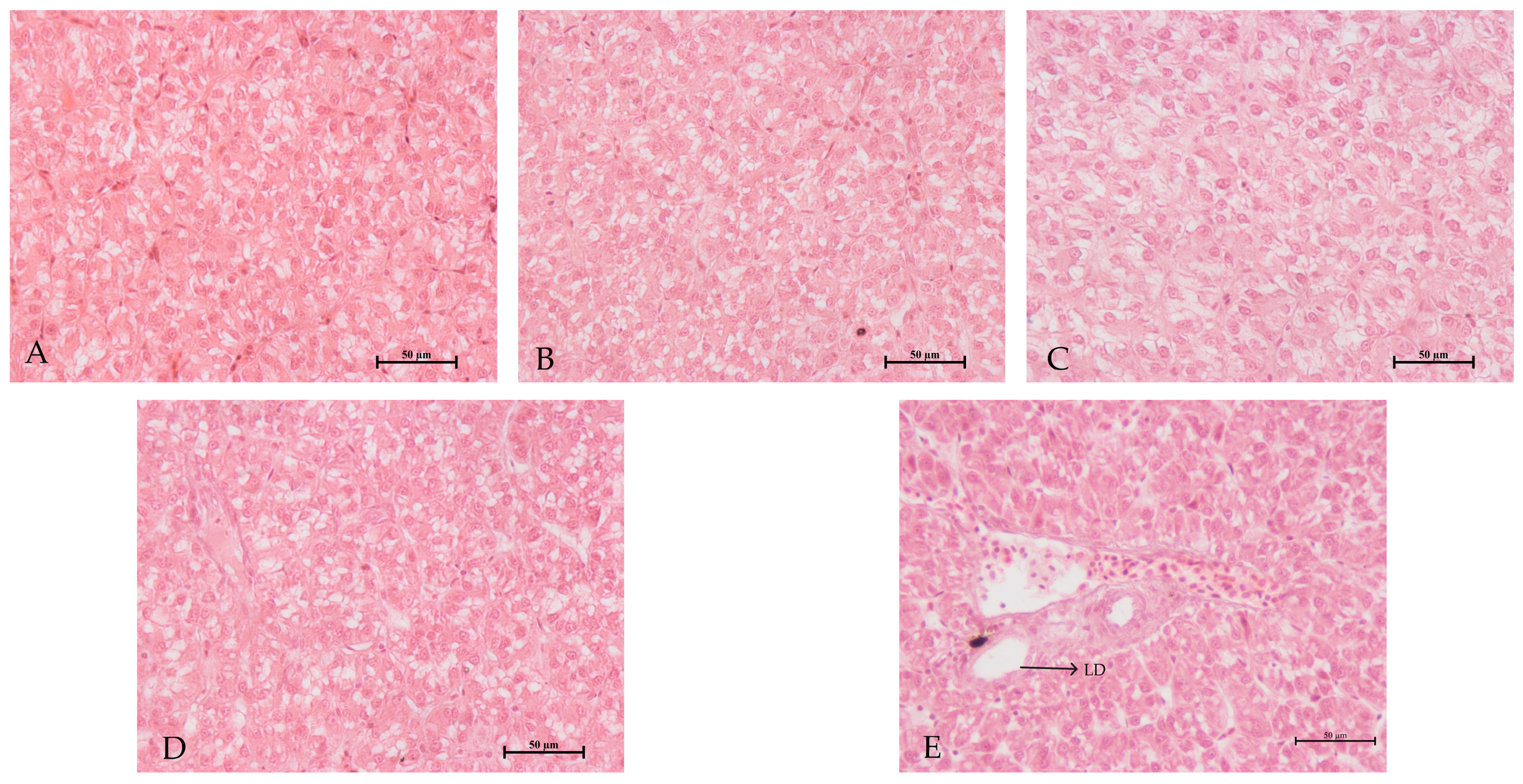
3.6. Hepatopancreas Morphology
4. Discussion
4.1. Growth Performance, Whole-Body Composition and Nutrient Utilization
4.2. Intestinal Morphology and Digestive Enzyme Activity
4.3. Serum Biochemical Indices
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ye, G.; Dong, X.; Yang, Q.; Chi, S.; Liu, H.; Zhang, H.; Tan, B.; Zhang, S. Low-gossypol cottonseed protein concentrate used as a replacement of fish meal for juvenile hybrid grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂): Effects on growth performance, immune responses and intestinal microbiota. Aquaculture 2020, 524, 735309. [Google Scholar] [CrossRef]
- Bendiksen, E.; Johnsen, C.A.; Olsen, H.J.; Jobling, M. Sustainable aquafeeds: Progress towards reduced reliance upon marine ingredients in diets for farmed Atlantic salmon (Salmo salar L.). Aquaculture 2011, 314, 132–139. [Google Scholar] [CrossRef]
- Olsen, R.L.; Hasan, M.R. A limited supply of fishmeal: Impact on future increases in global aquaculture production. Trends Food Sci. Technol. 2012, 27, 120–128. [Google Scholar] [CrossRef]
- Liu, T.; Han, T.; Wang, J.; Liu, T.; Bian, P.; Wang, Y.; Cai, X. Effects of replacing fish meal with soybean meal on growth performance, feed utilization and physiological status of juvenile redlip mullet Liza haematocheila. Aquac. Rep. 2021, 20, 100756. [Google Scholar] [CrossRef]
- Fu, S.; Qian, K.; Liu, H.; Song, F.; Ye, J. Effects of fish meal replacement with low-gossypol cottonseed meal on the intestinal barrier of juvenile golden pompano (Trachinotus ovatus). Aquac. Res. 2021, 53, 285–299. [Google Scholar] [CrossRef]
- Ye, G.; Dong, X.; Yang, Q.; Chi, S.; Liu, H.; Zhang, H.; Tan, B.; Zhang, S. Dietary replacement of fish meal with peanut meal in juvenile hybrid grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂): Growth performance, immune response and intestinal microbiota. Aquac. Rep. 2020, 17, 100327. [Google Scholar] [CrossRef]
- Slawski, H.; Nagel, F.; Wysujack, K.; Balke, D.; Franz, P.; Schulz, C. Total fish meal replacement with canola protein isolate in diets fed to rainbow trout (Oncorhynchus mykiss W.). Aquac. Nutr. 2013, 19, 535–542. [Google Scholar] [CrossRef]
- Shekarabi, S.P.H.; Mehrgan, M.S.; Banavreh, A.; Foroudi, F. Partial replacement of fishmeal with corn protein concentrate in diets for rainbow trout (Oncorhynchus mykiss): Effects on growth performance, physiometabolic responses, and fillet quality. Aquac. Res. 2020, 52, 249–259. [Google Scholar] [CrossRef]
- Francis, G.; Makkar, H.P.S.; Becker, K. Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture 2001, 199, 197–227. [Google Scholar] [CrossRef]
- Xu, X.; Yang, H.; Zhang, C.; Bian, Y.; Yao, W.; Xu, Z.; Wang, Y.; Li, X.; Leng, X. Effects of replacing fishmeal with cottonseed protein concentrate on growth performance, flesh quality and gossypol deposition of largemouth bass (Micropterus salmoides). Aquaculture 2021, 548, 737551. [Google Scholar] [CrossRef]
- Anderson, A.; Alam, M.; Watanabe, W.; Carroll, P.; Wedegaertner, T.; Dowd, M. Full replacement of menhaden fish meal protein by low-gossypol cottonseed flour protein in the diet of juvenile black sea bass Centropristis striata. Aquaculture 2016, 464, 618–628. [Google Scholar] [CrossRef]
- Shen, J.; Liu, H.; Tan, B.; Dong, X.; Yang, Q.; Chi, S.; Zhang, S. Effects of replacement of fishmeal with cottonseed protein concentrate on the growth, intestinal microflora, haematological and antioxidant indices of juvenile golden pompano (Trachinotus ovatus). Aquac. Nutr. 2020, 26, 1119–1130. [Google Scholar] [CrossRef]
- Tian, S.; Wu, Y.; Yuan, J.; Zhang, Z.; Huang, D.; Zhou, H.; Zhang, W.; Mai, K. Replacement of dietary fishmeal by cottonseed protein concentrate on growth performance, feed utilization and protein metabolism of large yellow croaker Larimichthys crocea. Aquac. Rep. 2022, 26, 101313. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, W.; Dan, Z.; Zhuang, Y.; Liu, Y.; Mai, K.; Ai, Q. Replacement of dietary fish meal with Clostridium autoethanogenum meal on growth performance, intestinal amino acids transporters, protein metabolism and hepatic lipid metabolism of juvenile turbot (Scophthalmus maximus L.). Front. Physiol. 2022, 13, 981750. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Li, X.; Song, B.; He, M.; Wu, C.; Leng, X. The potential of Clostridium autoethanogenum, a new single cell protein, in substituting fish meal in the diet of largemouth bass (Micropterus salmoides): Growth, feed utilization and intestinal histology. Aquac. Fish. 2023, 8, 67–75. [Google Scholar] [CrossRef]
- Cui, X.; Ma, Q.; Duan, M.; Xu, H.; Liang, M.; Wei, Y. Effects of fishmeal replacement by Clostridium autoethanogenum protein on the growth, digestibility, serum free amino acid and gene expression related to protein metabolism of obscure pufferfish (Takifugu obscurus). Anim. Feed. Sci. Technol. 2022, 292, 115445. [Google Scholar] [CrossRef]
- Zhang, J.; Dong, Y.; Song, K.; Wang, L.; Li, X.; Lu, K.; Tan, B.; Zhang, C. Substituting Fish Meal with a Bacteria Protein (Clostridium autoethanogenum Protein) Derived from Industrial-Scale Gas Fermentation: Effects on Growth and Gut Health of Juvenile Large Yellow Croakers (Larimichthys crocea). Fishes 2022, 7, 228. [Google Scholar] [CrossRef]
- Zheng, C.; Gong, S.; Cao, J.; Dong, X.; Chi, S.; Yang, Q.; Liu, H.; Zhang, S.; Xie, S.; Tan, B. Effects of Dietary Lipid Sources on Alleviating the Negative Impacts Induced by the Fishmeal Replacement with Clostridium autoethanogenum Protein in the Diet of Pacific White Shrimp (Litopenaeus vannamei). Front. Mar. Sci. 2022, 9, 879364. [Google Scholar] [CrossRef]
- Yao, W.; Yang, P.; Zhang, X.; Xu, X.; Zhang, C.; Li, X.; Leng, X. Effects of replacing dietary fish meal with Clostridium autoethanogenum protein on growth and flesh quality of Pacific white shrimp (Litopenaeus vannamei). Aquaculture 2021, 549, 737770. [Google Scholar] [CrossRef]
- Chen, Y.; Sagada, G.; Xu, B.; Chao, W.; Zou, F.; Ng, W.; Sun, Y.; Wang, L.; Zhong, Z.; Shao, Q. Partial replacement of fishmeal with Clostridium autoethanogenum single-cell protein in the diet for juvenile black sea bream (Acanthopagrus schlegelii). Aquac. Res. 2019, 51, 1000–1011. [Google Scholar] [CrossRef]
- Bureau, D.; Harris, A.; Cho, C. Apparent digestibility of rendered animal protein ingredients for rainbow trout (Oncorhynchus mykiss). Aquaculture 1999, 180, 345–358. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). The State of World Fisheries and Aquaculture 2020: Sustainability in Action; FAO: Rome, Italy, 2020; ISBN 9789251326923. [Google Scholar]
- Hang, Y.; Fu, Y.; Jin, C.; Hua, X. Effects of supplemental amino acids and bile acid in a completely replaced fish meal by enzymatically hydrolysed soybean meal diet on growth performance, liver health and fillet quality of rainbow trout (Oncorhynchus mykiss). Aquac. Res. 2022, 53, 3297–3308. [Google Scholar] [CrossRef]
- Choi, D.G.; He, M.; Fang, H.; Wang, X.L.; Li, X.Q.; Leng, X.J. Replacement of fish meal with two fermented soybean meals in diets for rainbow trout (Oncorhynchus mykiss). Aquac. Nutr. 2019, 26, 37–46. [Google Scholar] [CrossRef]
- Drew, M.; Racz, V.; Gauthier, R.; Thiessen, D. Effect of adding protease to coextruded flax: Pea or canola: Pea products on nutrient digestibility and growth performance of rainbow trout (Oncorhynchus mykiss). Anim. Feed. Sci. Technol. 2005, 119, 117–128. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, Z.-L.; Niu, J. Growth performance, intestinal histomorphology, body composition, hematological and antioxidant parameters of Oncorhynchus mykiss were not detrimentally affected by replacement of fish meal with concentrated dephenolization cottonseed protein. Aquac. Rep. 2020, 19, 100557. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Communities International, 18th ed.; AOAC: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Su, X.; Li, X.; Leng, X.; Tan, C.; Liu, B.; Chai, X.; Guo, T. The improvement of growth, digestive enzyme activity and disease resistance of white shrimp by the dietary citric acid. Aquac. Int. 2014, 22, 1823–1835. [Google Scholar] [CrossRef]
- Chen, J.; Li, X.; Xu, H.; Sun, W.; Leng, X. Substitute of soy protein concentrate for fish meal in diets of white shrimp (Litopenaeus vannamei Boone). Aquac. Int. 2017, 25, 1303–1315. [Google Scholar] [CrossRef]
- Luo, L.; Xue, M.; Wu, X.; Cai, X.; Cao, H.; Liang, Y. Partial or total replacement of fishmeal by solvent-extracted cottonseed meal in diets for juvenile rainbow trout (Oncorhynchus mykiss). Aquac. Nutr. 2006, 12, 418–424. [Google Scholar] [CrossRef]
- Wang, H.; Hu, X.; Zheng, Y.; Chen, J.; Tan, B.; Shi, L.; Zhang, S. Effects of replacing fish meal with cottonseed protein concentrate on the growth, immune responses, digestive ability and intestinal microbial flora in Litopenaeus vannamei. Fish Shellfish Immunol. 2022, 128, 91–100. [Google Scholar] [CrossRef]
- Boonyoung, S.; Haga, Y.; Satoh, S. Preliminary study on effects of methionine hydroxy analog and taurine supplementation in a soy protein concentrate-based diet on the biological performance and amino acid composition of rainbow trout (Oncorhynchus mykiss). Aquac. Res. 2012, 44, 1339–1347. [Google Scholar] [CrossRef]
- Gaylord, T.G.; Barrows, F.T.; Teague, A.M.; Johansen, K.A.; Overturf, K.E.; Shepherd, B. Supplementation of taurine and methionine to all-plant protein diets for rainbow trout (Oncorhynchus mykiss). Aquaculture 2007, 269, 514–524. [Google Scholar] [CrossRef]
- Murillo-Gurrea, D.P.; Coloso, R.M.; Borlongan, I.G.; Serrano, A.E. Lysine and arginine requirements of juvenile Asian sea bass (Lates calcarifer). J. Appl. Ichthyol. 2001, 17, 49–53. [Google Scholar] [CrossRef]
- Wu, Z.; Yu, X.; Guo, J.; Fu, Y.; Guo, Y.; Pan, M.; Zhang, W.; Mai, K. Effects of replacing fish meal with corn gluten meal on growth performance, intestinal microbiota, mTOR pathway and immune response of abalone Haliotis discus hannai. Aquac. Rep. 2022, 23, 101007. [Google Scholar] [CrossRef]
- He, G.; Zhang, T.; Zhou, X.; Liu, X.; Sun, H.; Chen, Y.; Tan, B.; Lin, S. Effects of cottonseed protein concentrate on growth performance, hepatic function and intestinal health in juvenile largemouth bass, Micropterus salmoides. Aquac. Rep. 2022, 23, 101052. [Google Scholar] [CrossRef]
- Lu, Q.; Xi, L.; Liu, Y.; Gong, Y.; Su, J.; Han, D.; Yang, Y.; Jin, J.; Liu, H.; Zhu, X.; et al. Effects of Dietary Inclusion of Clostridium autoethanogenum Protein on the Growth Performance and Liver Health of Largemouth Bass (Micropterus salmoides). Front. Mar. Sci. 2021, 8, 764964. [Google Scholar] [CrossRef]
- Lim, S.-J.; Oh, D.-H.; Khosravi, S.; Cha, J.-H.; Park, S.-H.; Kim, K.-W.; Lee, K.-J. Taurine is an essential nutrient for juvenile parrot fish Oplegnathus fasciatus. Aquaculture 2013, 414, 274–279. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, H.; Wang, X.; Ren, X. Taurine supplementation increases the potential of fishmeal replacement by soybean meal in diets for largemouth bass Micropterus salmoides. Aquac. Nutr. 2021, 27, 691–699. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Ren, X.; Li, W.Y.; Wang, L.; Wang, Y. Influence of taurine and selenium yeast supplementation on replacing fish meal with cottonseed protein concentrate in black sea bass (Centropristis striata) diet. Chin. J. Anim. Nutr. 2020, 32, 3291–3302. [Google Scholar] [CrossRef]
- Liu, Y.; He, G.; Wang, Q.; Mai, K.; Xu, W.; Zhou, H. Hydroxyproline supplementation on the performances of high plant protein source based diets in turbot (Scophthalmus maximus L.). Aquaculture 2014, 433, 476–480. [Google Scholar] [CrossRef]
- Aksnes, A.; Mundheim, H.; Toppe, J.; Albrektsen, S. The effect of dietary hydroxyproline supplementation on salmon (Salmo salar L.) fed high plant protein diets. Aquaculture 2008, 275, 242–249. [Google Scholar] [CrossRef]
- Storebakken, T.; Baeverfjord, G.; Skrede, A.; Olli, J.J.; Berge, G.M. Bacterial protein grown on natural gas in diets for Atlantic salmon, Salmo salar, in freshwater. Aquaculture 2004, 241, 413–425. [Google Scholar] [CrossRef]
- Aas, T.S.; Hatlen, B.; Grisdale-Helland, B.; Terjesen, B.F.; Bakke-McKellep, A.M.; Helland, S.J. Effects of diets containing a bacterial protein meal on growth and feed utilisation in rainbow trout (Oncorhynchus mykiss). Aquaculture 2006, 261, 357–368. [Google Scholar] [CrossRef]
- Bian, F.; Zhou, H.; He, G.; Wang, C.; Peng, H.; Pu, X.; Jiang, H.; Wang, X.; Mai, K. Effects of replacing fishmeal with different cottonseed meals on growth, feed utilization, haematological indexes, intestinal and liver morphology of juvenile turbot (Scophthalmus maximus L.). Aquac. Nutr. 2017, 23, 1429–1439. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Zheng, C.; Chi, S.; Zhang, S.; Tan, B.; Xie, S. Evaluation of Six Novel Protein Sources on Apparent Digestibility in Pacific White Shrimp, Litopenaeus vannamei. Aquac. Nutr. 2022, 2022, 8225273. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, Q.; Xi, L.; Gong, Y.; Su, J.; Han, D.; Zhang, Z.; Liu, H.; Jin, J.; Yang, Y.; et al. Effects of Replacement of Dietary Fishmeal by Cottonseed Protein Concentrate on Growth Performance, Liver Health, and Intestinal Histology of Largemouth Bass (Micropterus salmoides). Front. Physiol. 2021, 12, 764987. [Google Scholar] [CrossRef]
- Alam, M.; Watanabe, W.; Carroll, P.; Gabel, J.; Corum, M.; Seaton, P.; Wedegaertner, T.; Rathore, K.; Dowd, M. Evaluation of genetically-improved (glandless) and genetically-modified low-gossypol cottonseed meal as alternative protein sources in the diet of juvenile southern flounder Paralichthys lethostigma reared in a recirculating aquaculture system. Aquaculture 2018, 489, 36–45. [Google Scholar] [CrossRef]
- Yang, H.; Bian, Y.; Huang, L.; Lan, Q.; Ma, L.; Li, X.; Leng, X. Effects of replacing fish meal with fermented soybean meal on the growth performance, intestinal microbiota, morphology and disease resistance of largemouth bass (Micropterus salmoides). Aquac. Rep. 2021, 22, 100954. [Google Scholar] [CrossRef]
- Duan, Z.; Zhang, C.; Huang, L.; Lan, Q.; Hu, J.; Li, X.; Leng, X. An Evaluation of Replacing Fish Meal with Fermented Soybean Meal in Diet of Hybrid Snakehead (Channa argus × Channa maculata): Growth, Nutrient Utilization, Serum Biochemical Indices, Intestinal Histology, and Microbial Community. Aquac. Nutr. 2022, 2022, 2964779. [Google Scholar] [CrossRef]
- Wu, Y.; Tian, S.; Yuan, J.; Zhang, Z.; Zhou, H.; Gao, W.; Zhang, W.; Mai, K. Effects of Clostridium autoethanogenum protein as substitute for dietary fishmeal on the growth, feed utilization, intestinal health and muscle quality of large yellow croaker Larimichthys crocea. Aquaculture 2022, 561, 738591. [Google Scholar] [CrossRef]
- Zheng, C.; Cao, J.; Chi, S.; Dong, X.; Yang, Q.; Liu, H.; Zhang, S.; Xie, S.; Tan, B. Dietary phosphorus supplementation in the diet of Pacific white shrimp (Litopenaeus vannamei) alleviated the adverse impacts caused by high Clostridium autoethanogenum protein. Fish Shellfish Immunol. 2022, 131, 137–149. [Google Scholar] [CrossRef]
- Liu, H.; Dong, X.; Tan, B.; Du, T.; Zhang, S.; Yang, Y.; Chi, S.; Yang, Q.; Liu, H. Effects of fish meal replacement by low-gossypol cottonseed meal on growth performance, digestive enzyme activity, intestine histology and inflammatory gene expression of silver sillago (Sillago sihama Forsskál) (1775). Aquac. Nutr. 2020, 26, 1724–1735. [Google Scholar] [CrossRef]
- Salze, G.; McLean, E.; Craig, S.R. Dietary taurine enhances growth and digestive enzyme activities in larval cobia. Aquaculture 2012, 362, 44–49. [Google Scholar] [CrossRef]
- Liu, J.-X.; Guo, H.-Y.; Zhu, K.-C.; Liu, B.-S.; Zhang, N.; Zhang, D.-C. Effects of exogenous taurine supplementation on the growth, antioxidant capacity, intestine immunity, and resistance against Streptococcus agalactiae in juvenile golden pompano (Trachinotus ovatus) fed with a low-fishmeal diet. Front. Immunol. 2022, 13, 1036821. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Liu, B.; Ge, X.; Xie, J.; Xu, P. Effect of dietary carbohydrate on the growth performance, immune response, hepatic antioxidant abilities and heat shock protein 70 expression of Wuchang bream, Megalobrama amblycephala. J. Appl. Ichthyol. 2013, 29, 1348–1356. [Google Scholar] [CrossRef]
- Yin, B.; Liu, H.; Tan, B.; Dong, X.; Chi, S.; Yang, Q.; Zhang, S.; Chen, L. Cottonseed protein concentrate (CPC) suppresses immune function in different intestinal segments of hybrid grouper ♀ Epinephelus fuscoguttatus × ♂ Epinephelus lanceolatu via TLR-2/MyD88 signaling pathways. Fish Shellfish Immunol. 2018, 81, 318–328. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Y.; Che, Z.; Zhu, H.; Meng, G.; Hou, Y.; Ding, B.; Yin, Y.; Chen, F. Dietary L-arginine supplementation alleviates liver injury caused by Escherichia coli LPS in weaned pigs. J. Endotoxin Res. 2012, 18, 804–814. [Google Scholar] [CrossRef]
- Jahanbakhshi, A.; Imanpoor, M.R.; Taghizadeh, V.; Shabani, A. Hematological and serum biochemical indices changes induced by replacing fish meal with plant protein (sesame oil cake and corn gluten) in the Great sturgeon (Huso huso). Comp. Clin. Pathol. 2012, 22, 1087–1092. [Google Scholar] [CrossRef]
- Wei, H.C.; Yu, H.H.; Chen, X.M.; Chao, W.; Zou, F.Q.; Chen, P.; Zhen, Y.Y.; Wu, X.F.; Liang, X.F.; Xue, M. Effect of soybean meal replaced by Clostridium autoethanogenum protein on growth performance, plasma biochemical indexes and hepatopancreas and intestinal histopathology of grass carp (Ctenopharyngodon idllus). Chin. J. Anim. Nutr. 2018, 30, 4190–4201. [Google Scholar] [CrossRef]
- Hemre, G.-I.; Mommsen, T.; Krogdahl, Å. Carbohydrates in fish nutrition: Effects on growth, glucose metabolism and hepatic enzymes. Aquac. Nutr. 2002, 8, 175–194. [Google Scholar] [CrossRef]
- Ming, J.-H.; Ye, J.-Y.; Zhang, Y.-X.; Xu, P.; Xie, J. Effects of dietary reduced glutathione on growth performance, non-specific immunity, antioxidant capacity and expression levels of IGF-I and HSP70 mRNA of grass carp (Ctenopharyngodon idella). Aquaculture 2015, 438, 39–46. [Google Scholar] [CrossRef]
- Xu, X.; Li, X.; Xu, Z.; Yao, W.; Leng, X. Dietary Azomite, a natural trace mineral complex, improved the growth, immunity response, intestine health and resistance against bacterial infection in largemouth bass (Micropterus salmoides). Fish Shellfish Immunol. 2020, 108, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Wang, Y.; Li, M.; Zhang, C.; Lahaye, L.; Chowdhury, M.A.K.; Li, X.; Leng, X. Supplementation of a Multienzyme Complex, an Organic Acid-Essential Oil Complex, and Prebiotic Alone or in Combination Affects Growth, Nutrient Utilization, and Immune Function of Rainbow Trout (Oncorhynchus mykiss). Aquac. Nutr. 2022, 2022, 1068537. [Google Scholar] [CrossRef]
- Parvez, S.; Tabassum, H.; Banerjee, B.D.; Raisuddin, S. Taurine Prevents Tamoxifen-Induced Mitochondrial Oxidative Damage in Mice. Basic Clin. Pharmacol. Toxicol. 2008, 102, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Che, C.; Cai, M.; Hu, Y. Taurine improves health of juvenile rice field eel (Monopterus albus) fed with oxidized fish oil: Involvement of lipid metabolism, antioxidant capacity, inflammatory response. Aquac. Rep. 2022, 27, 101388. [Google Scholar] [CrossRef]
- Wu, D.; Xu, H.; Yang, Y.; Feng, W.; Han, T.; Wang, J. Can Taurine Supplementation in a Diet with Soybean Meal Instead of Fish Meal Improve the Growth Performance, Feed Utilization, and Antioxidant Capacity of Spotted Knifejaw (Oplegnathus punctatus)? Water 2022, 14, 3393. [Google Scholar] [CrossRef]
- Aitken, R.; Muscio, L.; Whiting, S.; Connaughton, H.; Fraser, B.; Nixon, B.; Smith, N.; De Iuliis, G. Analysis of the effects of polyphenols on human spermatozoa reveals unexpected impacts on mitochondrial membrane potential, oxidative stress and DNA integrity; implications for assisted reproductive technology. Biochem. Pharmacol. 2016, 121, 78–96. [Google Scholar] [CrossRef]
| Ingredients 1 | CON | FM-15 | FM-10 | FM-5 | FM-0 |
|---|---|---|---|---|---|
| Fishmeal | 200.0 | 150.0 | 100.0 | 50.0 | 0.0 |
| Clostridium autoethanogenum protein (CAP) | 0.0 | 24.0 | 47.0 | 71.0 | 94.0 |
| Cottonseed protein concentrate (CPC) | 0.0 | 24.0 | 47.0 | 71.0 | 94.0 |
| Soy bean meal | 70.0 | 70.0 | 70.0 | 70.0 | 70.0 |
| Soy protein concentrate | 120.0 | 120.0 | 120.0 | 120.0 | 120.0 |
| Wheat gluten | 30.0 | 30.0 | 30.0 | 30.0 | 30.0 |
| Wheat flour | 270.0 | 268.0 | 268.0 | 266.0 | 266.0 |
| Corn gluten meal | 80.0 | 80.0 | 80.0 | 80.0 | 80.0 |
| Meat meal | 80.0 | 80.0 | 80.0 | 80.0 | 80.0 |
| Beer yeast | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 |
| Fish oil | 20.0 | 24.0 | 28.0 | 32.0 | 36.0 |
| Soybean oil | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| Soybean lecithin | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| Ca(H2PO4)2 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| Vitamin premix 2 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Mineral premix 3 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Total | 1000.0 | 1000.0 | 1000.0 | 1000.0 | 1000.0 |
| Proximate composition (g/kg) | |||||
| Moisture | 63.0 | 61.1 | 59.5 | 71.7 | 73.7 |
| Crude protein | 438.0 | 439.5 | 435.7 | 429.0 | 430.1 |
| Crude lipid | 77.9 | 74.9 | 77.1 | 82.7 | 83.7 |
| Crude ash | 111.6 | 105.0 | 98.0 | 95.5 | 90.4 |
| Items | CAP | CPC | CPC + CAP (1:1) | FM |
|---|---|---|---|---|
| Proximate composition (air-dry basis) | ||||
| Crude protein | 842.1 | 615.1 | 728.6 | 682.1 |
| Crude lipid | 1.9 | 23.6 | 12.8 | 90.0 |
| Crude ash | 32.7 | 62.0 | 47.4 | 149.5 |
| Moisture | 71.4 | 52.5 | 62.0 | 68.0 |
| Essential amino acids (dry-matter basis) | ||||
| Lysine | 87.0 | 24.7 | 55.9 | 52.1 |
| Methionine | 22.9 | 8.5 | 15.7 | 20.3 |
| Arginine | 34.0 | 78.9 | 56.5 | 40.9 |
| Histidine | 16.8 | 18.0 | 17.4 | 20.7 |
| Isoleucine | 52.8 | 18.9 | 35.9 | 27.5 |
| Leucine | 63.8 | 34.4 | 49.1 | 52.6 |
| Phenylalanine | 33.0 | 35.3 | 34.2 | 35.9 |
| Threonine | 40.2 | 19.0 | 29.6 | 28.7 |
| Tryptophan | 6.2 | 8.1 | 7.2 | 7.0 |
| Valine | 54.4 | 26.6 | 40.5 | 33.7 |
| Non-essential amino acids (dry-matter basis) | ||||
| Aspartic acid | 95.4 | 56.6 | 76.0 | 61.0 |
| Serine | 32.1 | 26.5 | 29.3 | 26.1 |
| Glutamic acid | 97.8 | 123.7 | 110.8 | 87.5 |
| Glycine | 38.7 | 25.0 | 31.9 | 41.3 |
| Alanine | 46.3 | 23.6 | 35.0 | 44.2 |
| Cysteine | 7.1 | 9.5 | 8.3 | 4.1 |
| Proline | 24.0 | 21.7 | 22.9 | 28.4 |
| Tyrosine | 31.4 | 20.2 | 25.8 | 22.9 |
| Total amino acids | 783.9 | 579.2 | 681.6 | 634.9 |
| Items | CON | FM-15 | FM-10 | FM-5 | FM-0 |
|---|---|---|---|---|---|
| IBW (g) | 35.05 ± 0.05 | 34.97 ± 0.06 | 35.00 ± 0.05 | 34.95 ± 0.05 | 35.02 ± 0.03 |
| FBW (g) | 125.73 ± 0.46 a | 125.47 ± 2.16 a | 122.47 ± 1.77 ab | 119.93 ± 0.88 b | 117.8 ± 1.41 b |
| WG (%) | 258.72 ± 1.42 a | 258.82 ± 5.75 a | 249.90 ± 5.03 ab | 242.89 ± 2.18 bc | 236.57 ± 4.04 c |
| FCR | 1.19 ± 0.01 c | 1.20 ± 0.03 c | 1.24 ± 0.02 bc | 1.28 ± 0.01 ab | 1.31 ± 0.02 a |
| FI (g/fish/day) | 1.93 | 1.93 | 1.93 | 1.93 | 1.93 |
| SGR (% BW/day) | 2.28 ± 0.01 a | 2.28 ± 0.03 a | 2.24 ± 0.03 ab | 2.2 ± 0.01 bc | 2.17 ± 0.02 c |
| Survival (%) | 100 | 100 | 100 | 100 | 100 |
| K (g/cm3) | 1.63 ± 0.01 | 1.55 ± 0.1 | 1.61 ± 0.08 | 1.64 ± 0.12 | 1.55 ± 0.01 |
| VSI (%) | 11.00 ± 0.50 | 10.13 ± 0.75 | 10.89 ± 0.99 | 10.42 ± 0.59 | 9.87 ± 0.7 |
| HSI (%) | 1.26 ± 0.09 | 1.23 ± 0.13 | 1.37 ± 0.13 | 1.28 ± 0.06 | 1.31 ± 0.02 |
| Items | CON | FM-15 | FM-10 | FM-5 | FM-0 |
|---|---|---|---|---|---|
| Moisture | 708.5 ± 3.7 | 713.4 ± 15.7 | 714.8 ± 20.1 | 706.4 ± 15.7 | 700.2 ± 19.2 |
| Crude ash | 22.4 ± 1.6 | 22.7 ± 0.2 | 20.7 ± 0.2 | 21.0 ± 0.4 | 22.0 ± 1.7 |
| Crude lipid | 64.9 ± 2.7 b | 63.2 ± 2.5 b | 61.2 ± 5.0 b | 64.0 ± 3.9 b | 71.1 ± 1.0 a |
| Crude protein | 180.4 ± 1.6 | 181.5 ± 2.1 | 179.5 ± 6.4 | 178.4 ± 3.2 | 181.0 ± 1.8 |
| Items | CON | FM-15 | FM-10 | FM-5 | FM-0 |
|---|---|---|---|---|---|
| Protease (U/mg prot) | 16.10 ± 0.75 a | 14.26 ± 4.90 ab | 10.32 ± 0.67 bc | 8.16 ± 0.71 cd | 5.12 ± 0.21 d |
| Amylase (U/mg prot) | 0.70 ± 0.02 a | 0.60 ± 0.01 ab | 0.62 ± 0.03 ab | 0.44 ± 0.08 bc | 0.34 ± 0.02 c |
| PER | 1.91 ± 0.01 a | 1.90 ± 0.04 ab | 1.85 ± 0.04 abc | 1.83 ± 0.01 bc | 1.78 ± 0.02 c |
| PR (%) | 41.02 ± 0.18 a | 41.08 ± 0.81 a | 39.76 ± 0.67 ab | 39.12 ± 0.24 b | 38.99 ± 0.45 b |
| LR (%) | 80.42 ± 4.02 a | 81.00 ± 4.49 a | 77.35 ± 2.46 a | 68.43 ± 2.31 b | 77.59 ± 1.59 a |
| Items | CON | FM-15 | FM-10 | FM-5 | FM-0 |
|---|---|---|---|---|---|
| AST (U/mL) | 1.13 ± 0.07 | 1.09 ± 0.16 | 1.12 ± 0.19 | 1.17 ± 0.11 | 1.39 ± 0.26 |
| ALT (U/mL) | 6.78 ± 0.54 | 6.16 ± 0.73 | 6.40 ± 0.54 | 7.11 ± 0.09 | 7.40 ± 0.66 |
| TG (mmol/L) | 2.55 ± 0.07 | 2.56 ± 0.07 | 2.42 ± 0.12 | 2.68 ± 0.06 | 2.44 ± 0.05 |
| GLU (mmol/L) | 4.67 ± 0.34 b | 4.84 ± 0.28 ab | 4.58 ± 0.39 b | 5.09 ± 0.17 ab | 5.47 ± 0.23 a |
| TCHO (mmol/L) | 7.11 ± 0.48 b | 6.70 ± 0.25 b | 7.01 ± 0.11 b | 7.31 ± 0.33 b | 8.32 ± 0.22 a |
| TP (g/L) | 27.41 ± 2.11 | 28.48 ± 1.26 | 27.41 ± 2.41 | 26.56 ± 2.00 | 29.35 ± 0.84 |
| MDA (nmol/mL) | 14.03 ± 0.29 b | 14.68 ± 0.88 ab | 14.46 ± 1.15 ab | 16.45 ± 0.91 a | 16.29 ± 1.19 a |
| CAT (U/mL) | 4.24 ± 0.92 | 3.73 ± 0.50 | 3.72 ± 0.37 | 3.17 ± 0.61 | 2.76 ± 0.64 |
| SOD (U/mL) | 49.93 ± 1.38 a | 50.78 ± 5.18 a | 50.57 ± 2.55 a | 40.49 ± 1.04 b | 42.16 ± 1.46 b |
| T-AOC (mmol/L) | 0.79 ± 0.04 a | 0.78 ± 0.05 a | 0.80 ± 0.05 a | 0.67 ± 0.03 b | 0.68 ± 0.01 b |
| Items | CON | FM-15 | FM-10 | FM-5 | FM-0 |
|---|---|---|---|---|---|
| Villus height (μm) | 734.9 ± 27.0 a | 737.2 ± 39.2 a | 764.4 ± 37.8 a | 670.4 ± 32.8 b | 603.1 ± 58.4 c |
| Villus width (μm) | 161.9 ± 17.6 a | 165.6 ± 4.9 a | 168.4 ± 11.7 a | 167.0 ± 23.5 a | 127.6 ± 9.0 b |
| Muscle thickness (μm) | 140.6 ± 5.8 a | 141.1 ± 6.4 a | 139.2 ± 20.1 a | 120.0 ± 9.3 b | 119.4 ± 6.0 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.; Li, X.; Cao, K.; Leng, X. Effects of Replacing Fishmeal with the Mixture of Cottonseed Protein Concentrate and Clostridium autoethanogenum Protein on the Growth, Nutrient Utilization, Serum Biochemical Indices, Intestinal and Hepatopancreas Histology of Rainbow Trout (Oncorhynchus mykiss). Animals 2023, 13, 817. https://doi.org/10.3390/ani13050817
Huang H, Li X, Cao K, Leng X. Effects of Replacing Fishmeal with the Mixture of Cottonseed Protein Concentrate and Clostridium autoethanogenum Protein on the Growth, Nutrient Utilization, Serum Biochemical Indices, Intestinal and Hepatopancreas Histology of Rainbow Trout (Oncorhynchus mykiss). Animals. 2023; 13(5):817. https://doi.org/10.3390/ani13050817
Chicago/Turabian StyleHuang, Hongfei, Xiaoqin Li, Kailin Cao, and Xiangjun Leng. 2023. "Effects of Replacing Fishmeal with the Mixture of Cottonseed Protein Concentrate and Clostridium autoethanogenum Protein on the Growth, Nutrient Utilization, Serum Biochemical Indices, Intestinal and Hepatopancreas Histology of Rainbow Trout (Oncorhynchus mykiss)" Animals 13, no. 5: 817. https://doi.org/10.3390/ani13050817




