Simple Summary
Bovine uterine infections are common in the postpartum period and are associated with economic losses in dairy herding. Adequate immune function is key to preventing disease. Adenosine triphosphate (ATP) is a known activator of the inflammatory response, and can be produced in the endometrial microenvironment. In this study, we observed that ATP increased the proinflammatory responses in bovine endometrial cells, such as release of chemokine interleukin-8 (IL-8), intracellular calcium mobilization, and ERK1/2 phosphorylation. Additionally, we demonstrated the presence of a subtype of purinergic receptors (P2Y), specifically, high levels of P2Y1 and P2Y2 receptors. The inhibition of P2Y receptors reduced the proinflammatory responses induced by ATP. The results suggest that P2Y receptors play a role in endometrial inflammatory activation, which could be useful as a therapeutic strategy to regulate uterine inflammation through the modulation of P2Y receptors.
Abstract
The bovine endometrium has an important defensive role in the postpartum period that acts when an inflammatory process associated with tissue damage or infection by bacteria is produced. Endometrial cells release cytokines and chemokines that recruit inflammatory cells, which release danger-associated molecular patterns (DAMPs), such as adenosine triphosphate (ATP), and initiate and regulate the inflammatory response. However, the role of ATP in bovine endometrial cells is unclear. The aim of this study was to determine the effect of ATP on interleukin-8 (IL-8) release, intracellular calcium mobilization, ERK1/2 phosphorylation, and the role of P2Y receptors, in bovine endometrial cells. Bovine endometrial (BEND) cells were incubated with ATP and the IL-8 release was determined by the ELISA assay. ATP of 50 and 100 μM significantly increased IL-8 released in BEND cells (50 μM: 23.16 ± 3.82 pg/mL, p = 0.0018; 100 μM: 30.14 ± 7.43 pg/mL, p = 0.0004). ATP (50 μM) also induced rapid intracellular calcium mobilization in Fura-2AM-loaded BEND cells, as well as ERK1/2 phosphorylation (ratio 1.1 ± 0.04, p = 0.0049). Suramin (50 μM), a pan-antagonist of P2Y receptors, partially reduced the intracellular calcium mobilization, ERK1/2 phosphorylation (ratio 0.83 ± 0.08, p = 0.045), and IL-8 release (9.67 ± 0.02 pg/mL, p = 0.014) induced by ATP. Finally, BEND cells expressed higher mRNA levels of P2Y1 and P2Y2 purinergic subtype receptors, and lower levels of P2Y11 and P2Y12 receptors, as determined by RT-qPCR. In conclusion, these results showed that ATP activates pro-inflammatory responses in BEND cells, which are partially mediated via P2Y receptors, and BEND cells express the mRNA of subtypes of P2Y receptors, which could have a key role in bovine endometrial inflammation.
1. Introduction
Bovine uterine infections are a common health problem in the postpartum period and lead to economic losses due to treatment costs, lost milk production, and infertility [1,2]. The endometrium, as well as being the site of embryo implantation and responsible for the regulation of the estrous cycle through the release of prostaglandins, has a defensive role against infection through classical innate immune mechanisms [3,4,5,6,7]. After parturition, all mammals can develop an inflammatory process associated with tissue damage or infection by bacteria [6]. Diverse cytokines and chemokines are produced by endometrial cells in response to tissue damage or infection, and an influx of inflammatory cells such as polymorphonuclear (PMN) are recruited into the endometrium, all of which favor an inflammatory environment [2,8,9]. Interleukin-8 (IL-8), a strongly attractant chemokine for neutrophils, is released by bovine endometrial cells and produced at high levels in the endometrium in the postpartum period, and in cows with subclinical or clinical endometritis [10,11,12]. PMNs also contribute to inflammation with the release of pro-inflammatory mediators such as adenosine triphosphate (ATP) [13], which acts as a danger-associated molecular pattern (DAMP), and initiates and regulates the inflammatory response along with other damage signals [14,15].
ATP binds to purinergic receptors of type P2. There are 2 subtypes of P2 receptors: P2X and P2Y receptors. P2Y receptors are G protein-coupled receptors, and eight receptor subtypes have been identified in humans and rodents: P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, and P2Y14 [14,15,16]. In bovines, P2Y receptors have been identified in neutrophils, retinal endothelial cells, chromaffin cells, chondrocytes, and synoviocites [17,18,19]. However, its presence in bovine endometrial cells is unknown. Some studies have reported the presence of P2Y and P2X subtype receptors in the human endometrium [20,21,22,23,24] and the rat uterine epithelium [25].
After binding to its receptors, ATP activates different signaling pathways. ATP induced intracellular calcium mobilization in human endometrial cancer cells [24]. In human endometrial stromal cells, ATP activates the pathway PLC/PKC/ERK [22]. ERK1/2 MAPK is a signaling pathway involved in the activation of transcription factors, such as the nuclear factor-kappaB (NF-κB), and the IL-8 gene has NF-κB binding sites in its promoter region that regulate its expression [26,27]. However, little evidence exists regarding the role of ATP in endometrial innate immunity. Another nucleotide, UDP-glucose, stimulates IL-8 production in human endometrial epithelial cells, and neutrophil chemotaxis in an IL-8-dependent manner [21].
We hypothesized that ATP activates inflammatory responses such as intracellular calcium release, ERK1/2 phosphorylation, and IL-8 production in bovine endometrial cells, and these responses could be mediated via P2Y subtype receptors.
2. Materials and Methods
2.1. Cell Culture
Bovine endometrial (BEND) cells (ATCC® CRL2398™) were obtained from ATCC (Manassas, VA, USA). BEND cells, a cell line derived from the uterine endometrium of a cow on d14 of the estrous cycle [28], were cultured according to the protocol recommended by the manufacturer and as described by Valenzuela et al. [29]. Briefly, a 1:1 mixture of Ham’s F12 and Eagle’s Minimal Essential medium with Earle’s BSS (D-valine modification) containing 1.5 mM L-glutamine adjusted to contain 1.5 g/L sodium bicarbonate (Sigma-Aldrich Co, St Louis, MO, USA) was used. The culture medium was supplemented with 0.034 g/L D-valine (Sigma-Aldrich Co, St Louis, MO, USA), 10% heat-inactivated fetal bovine serum (BioWest, Kansas City, MO, USA), 10% heat-inactivated horse serum (Gibco-Brl, Grand Island, NY, USA), 100 U/mL penicillin, and 100 μg/mL streptomycin (Hyclone, Logan, UT, USA). Before each experiment, the culture medium was changed into a serum-reduced culture medium (2% serum) in the absence of antibiotics, and the cells were maintained for two hours.
2.2. IL-8 ELISA Assay
BEND cells (n = 3 independent experiments) were cultured with vehicle (PBS) or different concentrations of ATP (0.1, 1, 10, 50, or 100 μM) (Cayman Chemical, Ann Arbor, MO, USA) for 24 h at 37 °C. LPS (500 ng/mL) (Escherichia coli O111:B4, Sigma-Aldrich, Saint Louis, MO, USA) was used as a positive control for the IL-8 assay. For specific experiments, BEND cells (n = 3 independent experiments) were cultured with 50 μM suramin for 15 min and then 50 μM ATP was added, followed by incubation for 24 h at 37 °C. IL-8 in the supernatants was assessed in duplicate using the Bovine IL-8 (CXCL8) ELISA BASIC Kit (MABTECH AB, Nacka Strand, Sweden), according to the instructions provided by the manufacturer.
2.3. Intracellular Calcium Mobilization
BEND cells (1 × 107 cells/mL) (n = 3 independent experiments) were trypsinized (0.25% trypsin/EDTA), washed in Hank’s balanced salt solution (HBSS) (136 mM NaCl, 5 mM KCl, 0.9 mM CaCl2, 0.4 mM KH2PO4, 0.3 mM NaH2PO4, 6 mM glucose, pH 7.4), and loaded with Fura-2-AM, according to the method described by Valenzuela et al. [29]. Briefly, BEND cells were incubated with 2.5 μM Fura-2-AM (Invitrogen, Carlsbad, CA, USA) for 30 min at 37 °C in the dark, and then two washes of HBSS were performed. Finally, the cells were suspended in HBSS containing 1 μM probenecid (Invitrogen, Carlsbad, CA, USA). Each experiment was performed in a cuvette maintained at 37 °C with constant stirring in an LS55 spectrofluorometer (PerkinElmer Life Science, Waltham, MA, USA). After 60 s of basal measurement, every 0.2 s at 509 nm emission and 340/380 nm dual-wavelength excitation, vehicle (HBSS) or ATP (0.5, 1 or 50 μM) was added, and the signal registered for 240 s. In another set of experiments, the cells were incubated with vehicle (DMSO 0.1%) or suramin (10, 50, or 100 μM) for 15 min at 37 °C, and then the fluorescence intensity was detected for 60 s of basal measurement. ATP (50 μM ) was then added, and the signal registered for 140 s.
2.4. Immunoblot
BEND cells cultured in a 60 mm plate (n = 3 independent experiments) were treated with vehicle (0.1 % DMSO) or 50 μM suramin for 15 min, and then 50 μM ATP (or vehicle PBS) was added, followed by incubation for 5 min at 37 °C. The cells were lysed according to Manosalva et al. [30,31]. Briefly, cells were incubated with a lysis buffer (50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 10 mg/mL aprotinin, 10 mg/mL leupeptin, 5 mM phenylmethylsulfonyl fluoride (PMSF), and 1 mM DTT) on ice, and centrifuged at 18,000× g for 20 min at 4 °C. Protein concentration was determined using the Bradford method (Sigma-Aldrich, Saint Louis, MO, USA). Fifty micrograms of protein were used on 10% SDS–PAGE gels. The proteins were transferred to PVDF membranes, and the immunoblot was performed according to the protocol recommended by the manufacturer of the antibody phospho-ERK1/2 (Cell Signaling, Beverly, MA, USA). The antibody against phospho-ERK1/2 was incubated overnight at 4 °C. Then, the membranes were washed and incubated with an HRP-conjugated secondary antibody (LI-COR, Lincoln, NE, USA). The bands of phospho-ERK1/2 were visualized using an Odyssey Fc infrared/chemiluminescent detection system (LI-COR Biosciences). The primary antibody was stripped, and the membranes were incubated with total anti-ERK1/2 antibody (Cell Signaling, Beverly, MA, USA) for 2 h at room temperature. A HRP-conjugated secondary antibody was used and the signal was detected as described above for phospho-ERK1/2 antibody. The band intensity was measured using image Studio Lite v5.2 software (LI-COR Biosciences). The results are shown in a representative image of three experiments, and the band intensity of phspho-ERK1/2 and ERK1/2 is shown in a bar graph as the ratio phspho-ERK1/2/ERK1/2.
2.5. Real Time-qPCR
BEND cells (n = 3) grown in 100 mm-plates were used for total RNA isolation, using the EZNA™ Total RNA Isolation Kit (Omega Bio-Tek, Norcross, GA, USA). To ensure removal of genomic DNA, all samples of total RNA were treated with DNase. For cDNA synthesis, 250 ng of total RNA were subjected to reverse transcription with Affinity Script RT (Agilent, Santa Clara, CA, USA), and then real-time PCR was performed in duplicate using the Brilliant II SYBRGreen qPCR K it (Agilent) with primers specific for bovine P2Y receptors subtype and the housekeeping ribonucleoprotein S9 (RSP9) (Table 1) [17,32]. The following conditions were used: 95 °C for 3 min, 40 cycles of 10 s at 95 °C, and 60 s at 60 °C. Then, three additional steps (dissociation curve) were performed: 95 °C for 15 s, 60 °C for 60 s, and 95 °C for 15 s. The post-PCR melting curves confirmed the specificity of the single-target amplification. The efficiency of the reaction was determined by the StepOne software and ranged between 95 and 110% for the different primers. The abundance of each gene was calculated relative to RSP9 mRNA using the 2−ΔΔCt method [33]. The formula used is: relative abundance = 2 (−ΔCt), where ΔCt is calculated as the difference between the Ct of each P2YR (P2Y1R, P2Y2R, P2Y4R, P2Y6R, P2Y11R, P2Y12R, or P2Y13R) and RSP9 [32,34]. The results are shown in a bar graph, as the relative expression of mRNA of each P2Y receptor.

Table 1.
Primers used in the RT-qPCR assay.
2.6. Viability Assay
BEND cells (n = 3 independent experiments) were cultured with vehicle (0.1% DMSO) or suramin (1, 10, 100, or 300 μM) for 30 min or 24 h at 37 °C, and cell viability was assessed, in duplicate, using the propidium iodide method as described by Valenzuela et al. [29]. Briefly, 5 μM propidium iodide (Molecular Probes, Invitrogen, Carlsbad, CA, USA) were added and incubation was implemented for 15 min at 37 °C. A positive control for cell death was performed in cells incubated with 0.2% triton X-100 at 37 °C for 40 min. The propidium iodide signal was detected with a fluorescence multiplate reader (Varioskan® Flash, Thermo Fisher Scientific, Waltham, MA, USA) at 530 nm excitation/620 nm emission. The results are shown as % of cell viability.
2.7. Statistical Analysis
The experiments (three independent experiments) were analyzed with a one-way analysis of variance and Dunnett’s multiple comparison test (compared with the control or basal), performed with a significance level of 5%. An unpaired t-test was performed in selected groups of IL-8 assay and immunoblot; comparisons between groups are shown in each graph with square brackets and significance level. All analyses were performed with the GraphPad Prism 8.0 program. The results are shown as bar graphs of the mean ± SEM.
3. Results and Discussion
3.1. ATP Increased IL-8 Levels
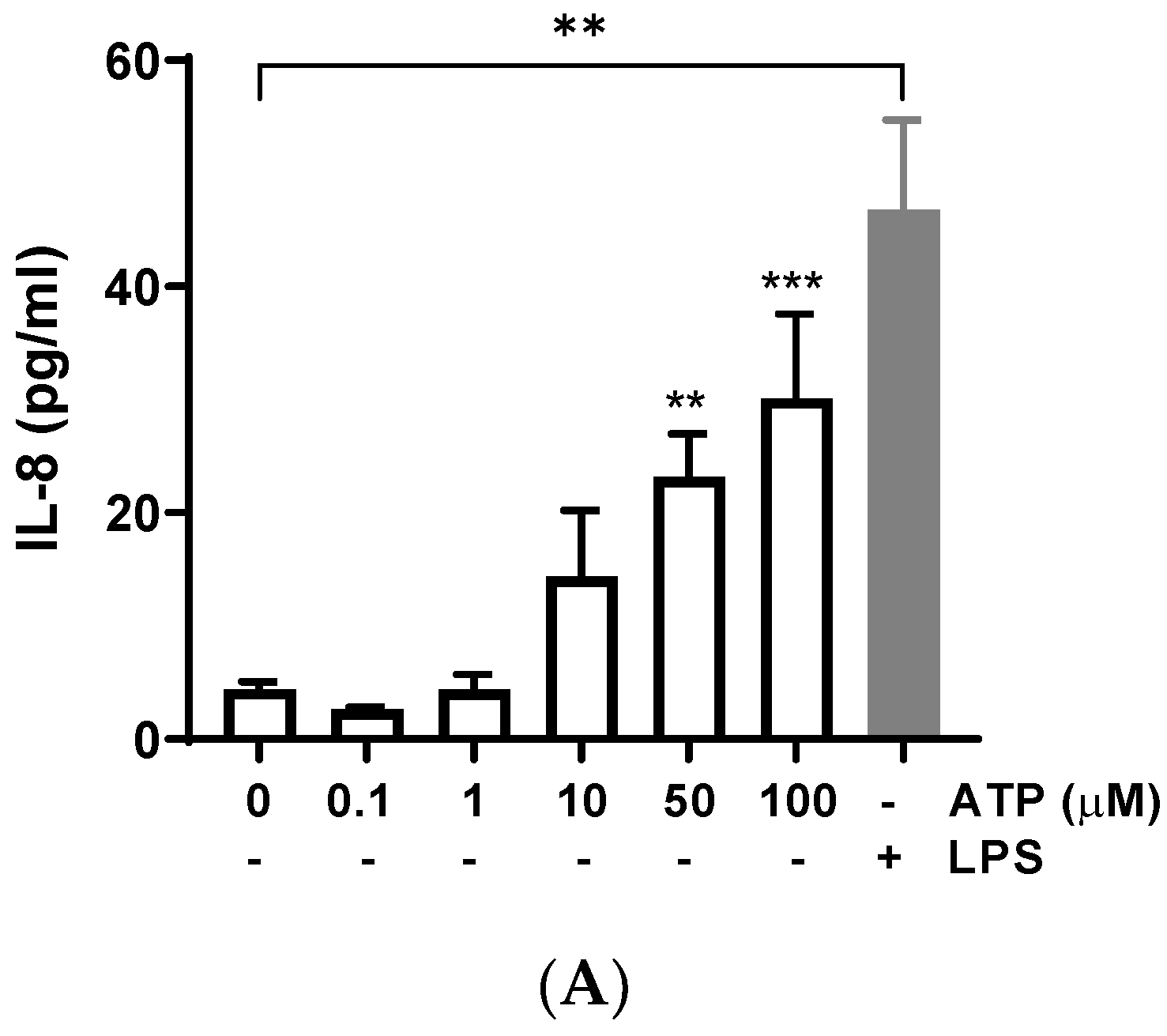
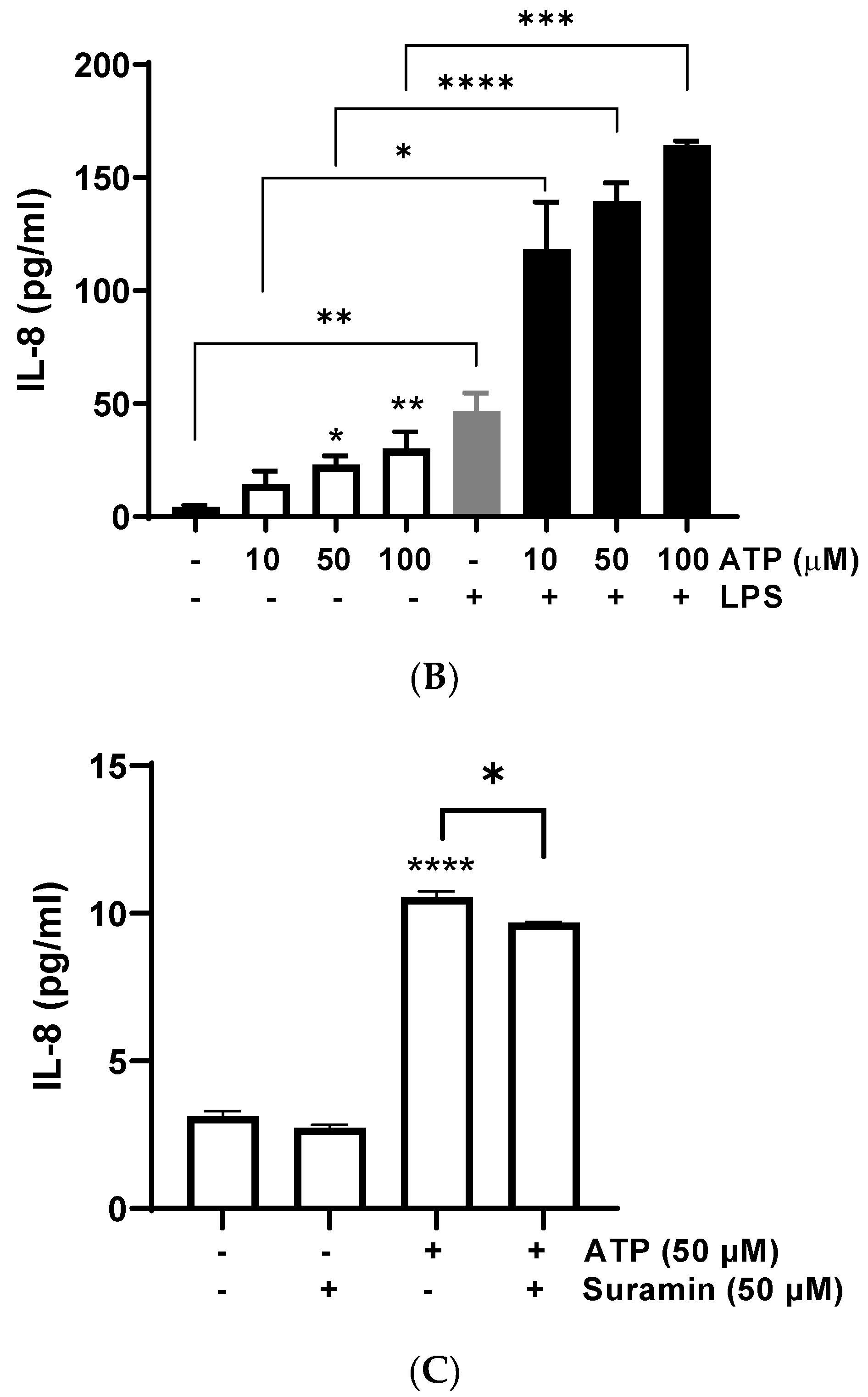
Bovine endometrial cells were treated with different concentrations of ATP for 24 h, and IL-8 release was determined in the supernatant. ATP (50 and 100 μM) significantly increased the production of IL-8 compared with the control (vehicle) (Figure 1A) (50 μM: 23.16 ± 3.82 pg/mL, p = 0.0018; 100 μM: 30.14 ± 7.43 pg/mL, p = 0.0004); similarly, the positive control LPS induced IL-8 release (p < 0.01), as has been described in several studies [35,36,37]. The treatment of BEND cells with ATP plus LPS showed an additive effect on IL-8 release (Figure 1B), for each concentration of ATP assessed.
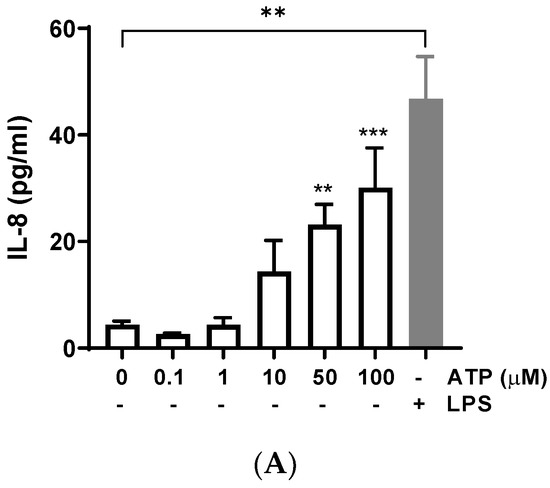
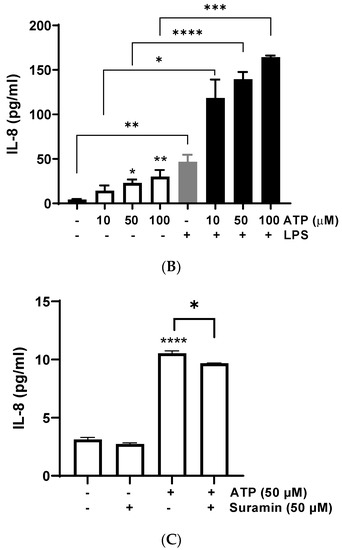
Figure 1.
ATP increases interleukin-8 (IL-8) production in BEND cells. (A) BEND cells were treated with vehicle (PBS) or different concentrations of ATP (0.1, 1, 10, 50, and 100 μM) for 24 h, and IL-8 production was assessed in the culture medium by ELISA assay. LPS (500 ng/mL) was used as a positive control. (B) BEND cells were incubated with vehicle (PBS) or ATP (10, 50, and 100 μM) without or with LPS (500 ng/mL) for 24 h, and IL-8 production was assessed in the culture medium by ELISA assay. (C) BEND cells were incubated with vehicle (DMSO 0.1%) or suramin (50 μM) for 15 min, and then ATP (50 μM) was added for 24 h, and IL-8 production was determined by ELISA assay. n = 3 independent experiments. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
The contribution of different cells producing ATP into the uterine environment could have important consequences, including reducing bovine reproductive performance. ATP is released from inflammatory cells in response to bacterial peptides and inflammatory mediators [13]. Likewise, ATP is released from endometrial epithelial cells, uterine blood vessels, and nerves innervating the uterus [38]. ATP regulates classical endometrial functions such as implantation of the fertilized oocyte, endometrial fluid functionality, cell proliferation and differentiation in post-partum, apoptosis, and sperm functionality [38]. However, few studies have evidenced the role of ATP in the endometrial inflammatory response. We observed a significant increase of IL-8 production in BEND cells stimulated with ATP, and in BEND cells treated with ATP plus LPS. High levels of IL-8 in the uterus promote an exacerbated inflammatory response, because more immune cells could be attracted. Inflammatory cells, such as neutrophils and macrophages, produce IL-8 under activation by stimuli associated with infection by bacteria or tissue damage [27,30,39,40,41]; therefore, the control of the uterine immune response is crucial for the resolution of inflammation.
To assess a possible role of purinergic receptors in the increase of IL-8 induced by ATP, BEND cells were incubated with the P2Y pan-antagonist suramin for 15 min. Then, ATP was added and incubation carried out for 24 h. The IL-8 production was partially yet significantly reduced by suramin (Figure 1C) (p < 0.05), suggesting a partial role of P2Y receptors in this response. The partial suramin-induced reduction of IL-8 can be explained because ATP does not only activate P2Y receptors. ATP also activates P2X purinergic receptors and can be hydrolyzed into adenosine and activate different signaling pathways, which may contribute to gene expression [14]. The participation of P2Y receptors in endometrial function has been previously suggested in other species. In human endometrial cells, the inhibition of the P2 receptor attenuated the ATP-induced activation of MAPK [22]. Additionally, suramin blocked the stimulatory effect of ATP on prostaglandin production in a guinea pig’s uterus [42]. Thus, our results with suramin support the participation of P2Y receptors in IL-8 production in bovine endometrial cells.
3.2. ATP Induces Intracellular Calcium Mobilization and ERK1/2 Phosphorylation
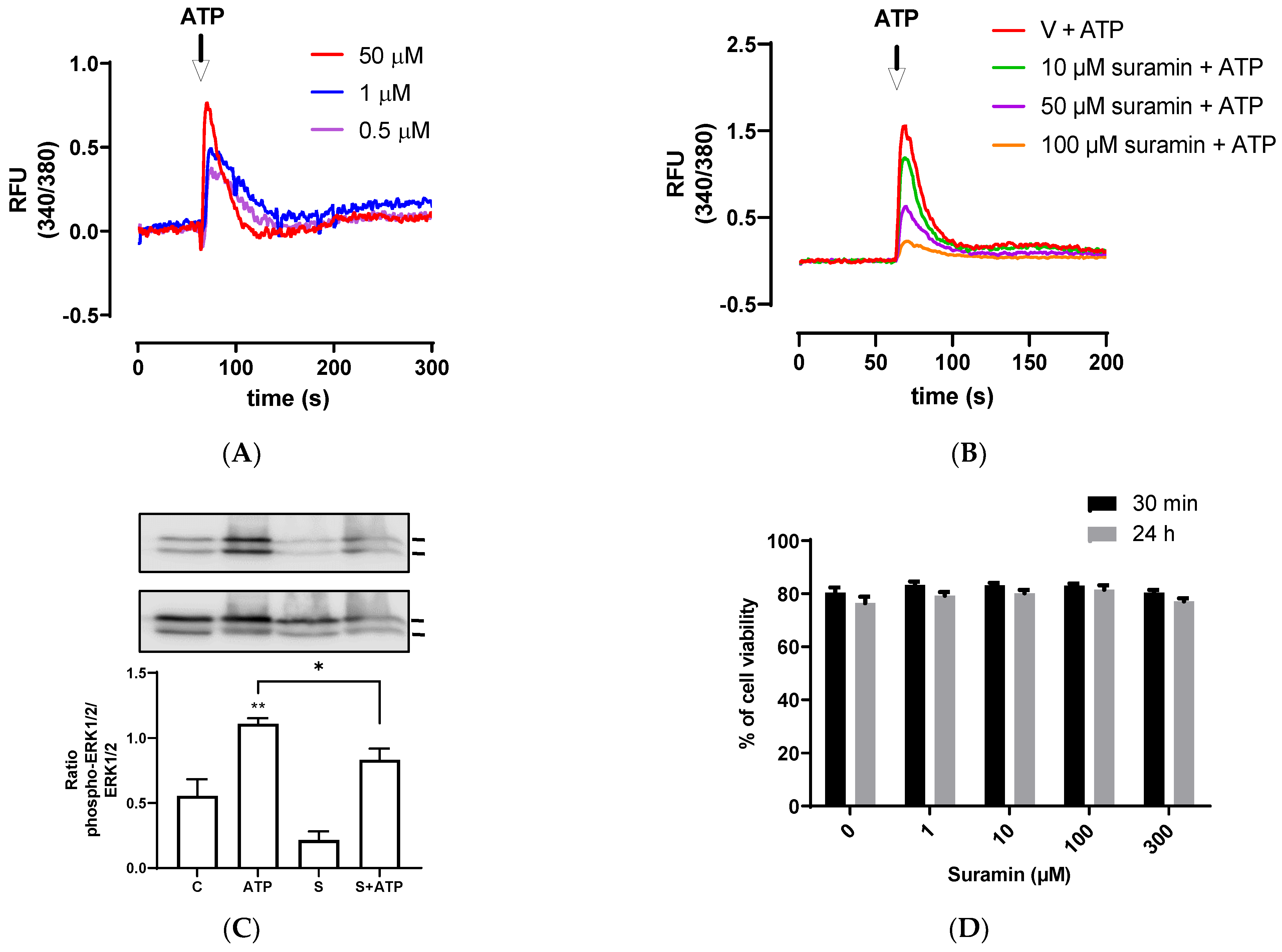
Since P2Y receptors are G-protein coupled receptors that activate second messengers, such as inositol triphosphate (IP3), and then lead to an fast increase of intracellular calcium released from the endoplasmic reticulum [43], we assessed intracellular calcium mobilization induced by different ATP concentrations. BEND cells rapidly increased intracellular calcium mobilization after ATP addition, ranging between 0.5–50 μM (Figure 2A). To assess the participation of P2Y receptors, BEND cells were incubated with suramin for 15 min. Then, ATP was added and intracellular calcium was measured. Suramin reduced the intracellular calcium mobilization induced by ATP (Figure 2B), suggesting the participation of P2Y receptors in the effect stimulated by ATP.
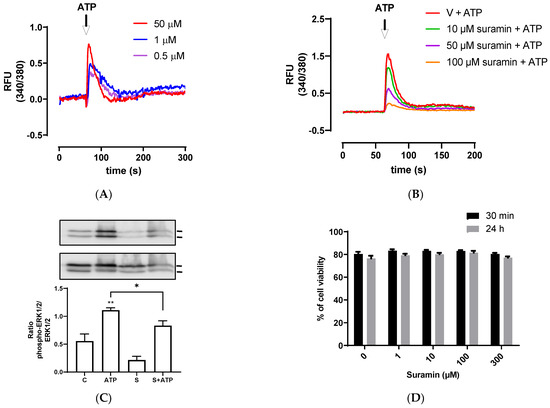
Figure 2.
ATP increases intracellular calcium mobilization and ERK1/2 phosphorylation. (A) Fura 2AM-loaded BEND cells were treated with vehicle (PBS) and basal fluorescence was registered for 60 s; then, different concentrations of ATP (0.1, 1, or 50 μM) were added and the fluorescence was determined for 240 s. RFU = relative fluorescence units. (B) Fura 2AM-loaded BEND cells were incubated with vehicle (0.1% DMSO) or suramin (10, 50, or 100 μM) for 15 min. Then, the basal fluorescence was registered for 60 s and ATP (50 μM) was added, and the fluorescence registered for 140 s. V = vehicle. (C) BEND cells were incubated with vehicle (0.1% DMSO) or suramin (50 μM) for 15 min, and then vehicle (PBS) or ATP (50 μM) was added, and incubation was carried out for 5 min. Total proteins were analyzed by immunoblot with antibodies against phospho-ERK1/2 and total ERK1/2. c = control (0.1% DMSO). S = Suramin. Bands’ intensities are shown as the ratio phospho-ERK1/2/ERK1/2 in the bar graph. (D) BEND cells were incubated with vehicle (0.1% DMSO) or suramin (1, 10, 100, or 300 μM) for 30 min or 24 h, and the cellular viability was measured by propidium iodide assay. * p < 0.05, ** p < 0.01 compared with the control. Images are representative of three independents experiments.
Calcium has diverse functions in the uterus, such as the production of developing follicle hormones, estrogen synthesis, and contraction and relaxation [44,45]. A study in bovines showed that the treatment of endometrial explant with the calcium ionophore A23187 increased the prostaglandin F2α synthesis induced by estradiol, thus demonstrating the role of calcium in hormone synthesis [46]. At a cellular level, calcium regulates different cellular processes, such as cell proliferation and apoptosis, gene expression, and inflammasome activation [47,48]. In bovine endometrial cells, it was shown that infection with E. coli increased the cytoplasmatic calcium, and the treatment with intracellular calcium chelators reduced the expression of IL-1β and IL-18, proposing the importance of calcium in pro-inflammatory gene expression [49]. In addition, prostaglandin F2α induced IL-8 expression in endometrial adenocarcinoma cells via the PKC/calcium/NFAT signaling pathway [50].
The reduction of intracellular calcium mobilization in BEND cells treated with suramin and ATP suggest the participation of P2Y subtype receptors in this response. Several previous studies done with suramin showed a reduction in ATP-induced calcium response in different cell types, such as human endocervical cell line, bovine trophoblast cell line, and canine macrophage cell line, and suggested the participation of P2 receptors in the rise of calcium [51,52,53].
Calcium is a second messenger that activates intracellular signaling pathways, such as MAPK in human endometrial cells [54,55]. Therefore, we assessed whether ATP could induce ERK1/2 phosphorylation in BEND cells. We observed that 50 μM ATP incubated for 5 min significantly increased ERK1/2 phosphorylation (p < 0.01) compared with the control, and this effect was partially but significantly reduced by the antagonist suramin (p < 0.05) (Figure 2C). The band sizes detected in all experiments correspond to the size expected for ERK1/2 (44 and 42 kDa). Antibody against total ERK1/2 (independent of the phosphorylation status) was used as loading control, therefore, differences in the amount of protein loaded in each line of the gel do not interfere in the result, because the bands of each line were normalized to total ERK. In human endometrial stromal cells, it was shown for first time that ATP activates ERK1/2, and the ERK1/2 signaling pathway was involved in mediating ATP actions in the human reproductive system [56]. Additionally, it was demonstrated that ATP activated ERK1/2 through the P2Y2 purinoceptor/PLC/PKC signaling pathway [22]. In human endometriotic stromal cells, ATP (100 μM) increased ERK1/2 phosphorylation, which was involved in endometriosis pain [20]. MAPK ERK1/2 has a key role in the expression of proinflammatory cytokines. The inhibition of the MAPK pathway in bovine endometrial epithelia and stromal cells reduced LPS-induced IL-8, IL-6, and IL-1β expression [7,57].
The effect of suramin on cellular viability of BEND cells also was evaluated. BEND cells were incubated with vehicle (0.1% DMSO) or different concentrations of suramin (1, 10, 100, or 300 μM) for 30 min or 24 h. We observed that suramin did not reduce cell viability when compared with the vehicle (Figure 2D). Therefore, the attenuation of IL-8 production, intracellular calcium mobilization, and ERK1/2 phosphorylation produced by suramin were effects mediated through the P2Y receptors.
3.3. Endometrial Cells Express P2Y mRNA Receptors
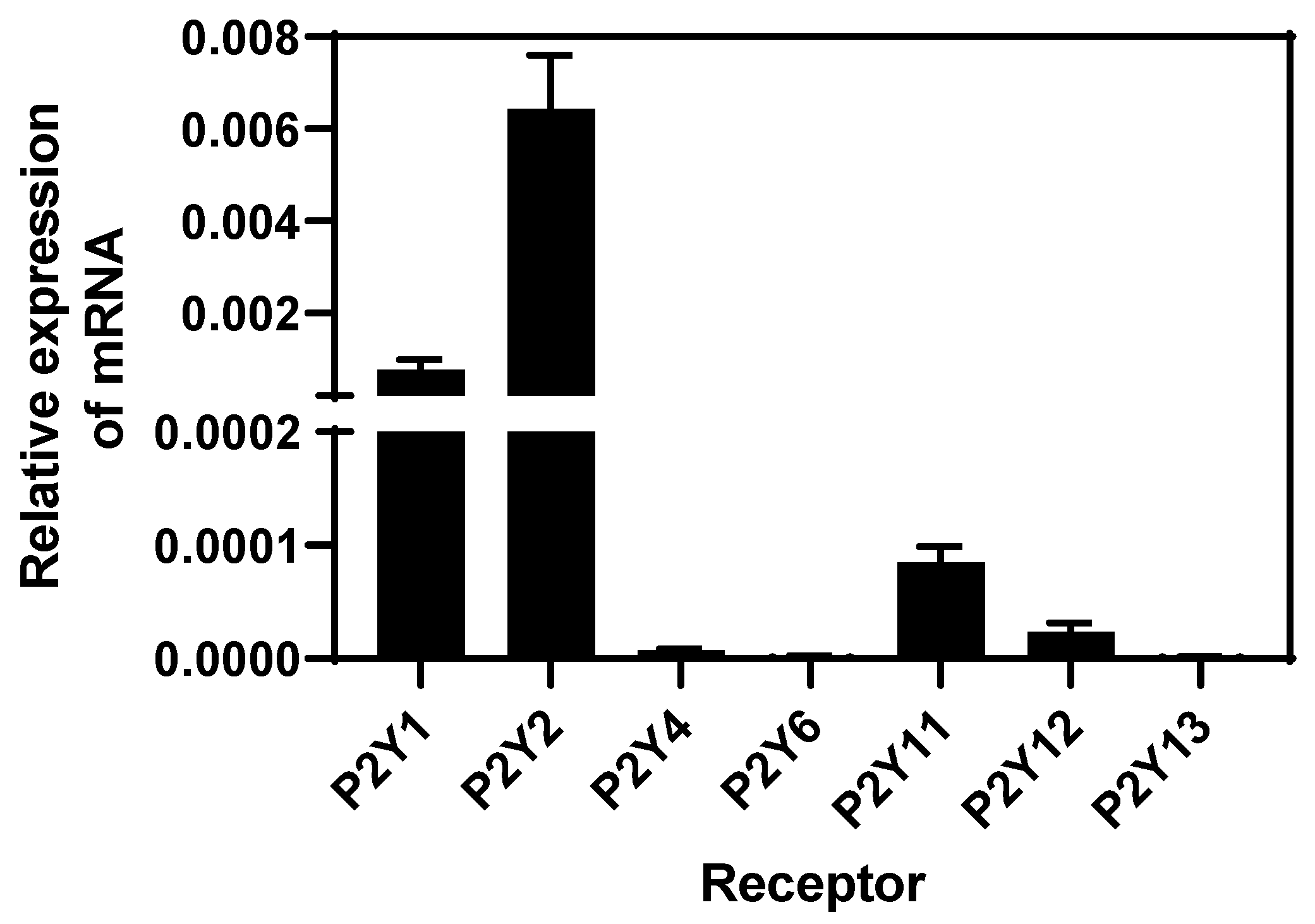
Since extracellular ATP mediates its functions through binding purinergic type P2 receptors, we assessed the presence of mRNA of subtype P2Y receptors in BEND cells through RT-qPCR and analysis with the 2−ΔΔCt method. BEND cells expressed higher levels of P2Y1 and P2Y2 purinergic subtype receptors, lower levels of P2Y11 and P2Y12 receptors, and P2Y4, P2Y6, and P2Y13 mRNA receptors were undetectable (Figure 3). P2Y receptors are predominantly activated by ATP or ADP, specifically P2Y-1, -2, -4, and -11 by ATP [14]. A P2Y1 receptor can be activated by ATP, which is considered a partial agonist, but ADP present highest affinity by the receptor [58]. Although the presence of P2Y receptors has been studied in human endometrial cells, in bovines, the expression has only been studied in other cell types (as mentioned in the Introduction). Human endometrial stromal cells express the P2Y2 receptor [56]. Additionally, P2Y2, P2Y14, and P2X7 are expressed in the human endometrium [21,22,23] with P2Y2 in human endometrial cancer cell lines [24], and P2Y1, P2Y2, P2Y4, and P2X7 in the rat uterine epithelium [25]. Thus, our research shows, for the first time, the presence of P2Y receptor subtypes in bovine endometrial cells.
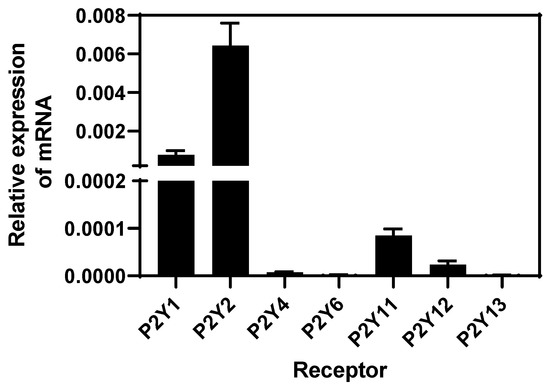
Figure 3.
Levels of expression of mRNA P2Y subtype receptors in BEND cells. Total RNA was isolated from BEND cells and RT-qPCR for P2Y subtype receptors was performed. Level of expression of each subtype receptor was determined by the 2−ΔΔCt method. n = 3 independent experiments.
P2Y subtype receptors couple to different G-protein. P2Y1 and P2Y2 are receptors linked to Gαq protein, and their activation stimulates the pathway of PLC/DAG/IP3/calcium [14,16]. The increase of intracellular calcium induced by ATP in BEND cells correlates with the types of receptors expressed at a higher level. Specifically, P2Y2 receptor is expressed at the highest level compared with P2Y1. The P2Y11 receptor is considered an unconventional member of the P2Y family receptors, because it couples with both Gαq and Gαs proteins, and its activation increases IP3 and cAMP, respectively [14,16]; however the expression of the P2Y11 receptor was lower in BEND cells. The P2Y12 receptor is coupled to Gαi protein, and it is activated by ADP.
P2 subtypes receptors induce classical signaling pathways and gene expression; P2Y1 and P2Y2 activate ERK1/2 in different cell types [22,59,60,61], and increase the expression of pro-inflammatory genes such as IL-6 and IL-8 [62]. On the contrary, it has been suggested that the P2Y11 receptor has two types of responses, because it activates the pro-inflammatory NF-κB/ERK1/2 pathway and also activates anti-inflammatory signaling. Activation of P2Y11 receptor inhibited LPS-induced TNF-α production in M2 macrophages, which is proposed to occur through a regulated control of IL-1 receptors [62,63].
4. Conclusions
This study showed that ATP increased IL-8 production, activated intracellular calcium mobilization, and induced ERK1/2 phosphorylation in BEND cells—effects that were partially reduced by a P2Y receptor antagonist. P2Y1 and P2Y2 receptors are expressed at high levels as compared to other subtypes of receptors in BEND cells. These results suggest a key role of P2Y receptors in endometrial inflammatory activation, which could be useful as a therapeutic strategy to regulate uterine inflammation through the modulation of P2Y receptors.
Author Contributions
Methodology, N.G., S.T. and P.A.; Data analysis, P.A., R.A.B. and M.A.H.; Conceptualization, M.A.H. and R.A.B.; Formal analysis, P.A. and M.A.H.; Writing—Original Draft Preparation, M.A.H.; Review and Editing, R.A.B. and M.A.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT) grant number 1200905. The APC was funded by FONDECYT 1200905.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bromfield, J.J.; Santos, J.E.; Block, J.; Williams, R.S.; Sheldon, I.M. Physiology and Endocrinology Symposium: Uterine infection: Linking infection and innate immunity with infertility in the high-producing dairy cow. J. Anim. Sci. 2015, 93, 2021–2033. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, L.C.; Cronin, J.G.; Sheldon, I.M. Mechanisms linking bacterial infections of the bovine endometrium to disease and infertility. Reprod. Biol. 2016, 16, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Peralta, M.B.; Cainelli, S.; Stassi, A.F.; Angeli, E.; Rey, F.; Ortega, H.H.; Salvetti, N.R.; Velazquez, M.M.L. Endometrial expression of members of the IL-1 family: Their involvement in delayed conception of dairy cows. Theriogenology 2023, 195, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Wagener, K.; Drillich, M.; Aurich, C.; Gabler, C. Endometrial Inflammation at the Time of Insemination and Its Effect on Subsequent Fertility of Dairy Cows. Animals 2021, 11, 1858. [Google Scholar] [CrossRef]
- Healy, L.L.; Cronin, J.G.; Sheldon, I.M. Endometrial cells sense and react to tissue damage during infection of the bovine endometrium via interleukin 1. Sci. Rep. 2014, 4, 7060. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Cronin, J.; Goetze, L.; Donofrio, G.; Schuberth, H.J. Defining postpartum uterine disease and the mechanisms of infection and immunity in the female reproductive tract in cattle. Biol. Reprod. 2009, 81, 1025–1032. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Cronin, J.G.; Healey, G.D.; Gabler, C.; Heuwieser, W.; Streyl, D.; Bromfield, J.J.; Miyamoto, A.; Fergani, C.; Dobson, H. Innate immunity and inflammation of the bovine female reproductive tract in health and disease. Reproduction 2014, 148, R41–R51. [Google Scholar] [CrossRef]
- Herath, S.; Lilly, S.T.; Santos, N.R.; Gilbert, R.O.; Goetze, L.; Bryant, C.E.; White, J.O.; Cronin, J.; Sheldon, I.M. Expression of genes associated with immunity in the endometrium of cattle with disparate postpartum uterine disease and fertility. Reprod. Biol. Endocrinol. 2009, 7, 55. [Google Scholar] [CrossRef]
- Kasimanickam, R.; Duffield, T.F.; Foster, R.A.; Gartley, C.J.; Leslie, K.E.; Walton, J.S.; Johnson, W.H. Endometrial cytology and ultrasonography for the detection of subclinical endometritis in postpartum dairy cows. Theriogenology 2004, 62, 9–23. [Google Scholar] [CrossRef]
- Fischer, C.; Drillich, M.; Odau, S.; Heuwieser, W.; Einspanier, R.; Gabler, C. Selected pro-inflammatory factor transcripts in bovine endometrial epithelial cells are regulated during the oestrous cycle and elevated in case of subclinical or clinical endometritis. Reprod. Fertil. Dev. 2010, 22, 818–829. [Google Scholar] [CrossRef]
- Gabler, C.; Fischer, C.; Drillich, M.; Einspanier, R.; Heuwieser, W. Time-dependent mRNA expression of selected pro-inflammatory factors in the endometrium of primiparous cows postpartum. Reprod. Biol. Endocrinol. 2010, 8, 152. [Google Scholar] [CrossRef]
- MacKintosh, S.B.; Schuberth, H.J.; Healy, L.L.; Sheldon, I.M. Polarised bovine endometrial epithelial cells vectorially secrete prostaglandins and chemotactic factors under physiological and pathological conditions. Reproduction 2013, 145, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yao, Y.; Sumi, Y.; Li, A.; To, U.K.; Elkhal, A.; Inoue, Y.; Woehrle, T.; Zhang, Q.; Hauser, C.; et al. Purinergic signaling: A fundamental mechanism in neutrophil activation. Sci. Signal. 2010, 3, ra45. [Google Scholar] [CrossRef]
- Faas, M.M.; Saez, T.; de Vos, P. Extracellular ATP and adenosine: The Yin and Yang in immune responses? Mol. Asp. Med. 2017, 55, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Jacob, F.; Perez Novo, C.; Bachert, C.; Van Crombruggen, K. Purinergic signaling in inflammatory cells: P2 receptor expression, functional effects, and modulation of inflammatory responses. Purinergic Signal. 2013, 9, 285–306. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, K.A.; Delicado, E.G.; Gachet, C.; Kennedy, C.; von Kugelgen, I.; Li, B.; Miras-Portugal, M.T.; Novak, I.; Schoneberg, T.; Perez-Sen, R.; et al. Update of P2Y receptor pharmacology: IUPHAR Review 27. Br. J. Pharmacol. 2020, 177, 2413–2433. [Google Scholar] [CrossRef]
- Seo, J.; Osorio, J.S.; Loor, J.J. Purinergic Signal.ing gene network expression in bovine polymorphonuclear neutrophils during the peripartal period. J. Dairy Sci. 2013, 96, 7675–7683. [Google Scholar] [CrossRef]
- Tome, A.R.; Castro, E.; Santos, R.M.; Rosario, L.M. Selective stimulation of catecholamine release from bovine adrenal chromaffin cells by an ionotropic purinergic receptor sensitive to 2-methylthio ATP. BMC Neurosci. 2007, 8, 41. [Google Scholar] [CrossRef]
- Varani, K.; De Mattei, M.; Vincenzi, F.; Gessi, S.; Merighi, S.; Pellati, A.; Ongaro, A.; Caruso, A.; Cadossi, R.; Borea, P.A. Characterization of adenosine receptors in bovine chondrocytes and fibroblast-like synoviocytes exposed to low frequency low energy pulsed electromagnetic fields. Osteoarthr. Cartil. 2008, 16, 292–304. [Google Scholar] [CrossRef]
- Ding, S.; Zhu, L.; Tian, Y.; Zhu, T.; Huang, X.; Zhang, X. P2X3 receptor involvement in endometriosis pain via ERK signaling pathway. PLoS ONE 2017, 12, e0184647. [Google Scholar] [CrossRef]
- Arase, T.; Uchida, H.; Kajitani, T.; Ono, M.; Tamaki, K.; Oda, H.; Nishikawa, S.; Kagami, M.; Nagashima, T.; Masuda, H.; et al. The UDP-glucose receptor P2RY14 triggers innate mucosal immunity in the female reproductive tract by inducing IL-8. J. Immunol. 2009, 182, 7074–7084. [Google Scholar] [CrossRef]
- Chang, S.J.; Tzeng, C.R.; Lee, Y.H.; Tai, C.J. Extracellular ATP activates the PLC/PKC/ERK signaling pathway through the P2Y2 purinergic receptor leading to the induction of early growth response 1 expression and the inhibition of viability in human endometrial stromal cells. Cell. Signal. 2008, 20, 1248–1255. [Google Scholar] [CrossRef]
- Li, X.; Qi, X.; Zhou, L.; Catera, D.; Rote, N.S.; Potashkin, J.; Abdul-Karim, F.W.; Gorodeski, G.I. Decreased expression of P2X7 in endometrial epithelial pre-cancerous and cancer cells. Gynecol. Oncol. 2007, 106, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Katzur, A.C.; Koshimizu, T.; Tomic, M.; Schultze-Mosgau, A.; Ortmann, O.; Stojilkovic, S.S. Expression and responsiveness of P2Y2 receptors in human endometrial cancer cell lines. J. Clin. Endocrinol. Metab. 1999, 84, 4085–4091. [Google Scholar] [CrossRef] [PubMed]
- Slater, M.; Murphy, C.R.; Barden, J.A. Purinergic receptor expression in the apical plasma membrane of rat uterine epithelial cells during implantation. Cell Calcium 2002, 31, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, E.; Dittrich-Breiholz, O.; Holtmann, H.; Kracht, M. Multiple control of interleukin-8 gene expression. J. Leukoc. Biol. 2002, 72, 847–855. [Google Scholar] [CrossRef]
- Mena, S.J.; Manosalva, C.; Carretta, M.D.; Teuber, S.; Olmo, I.; Burgos, R.A.; Hidalgo, M.A. Differential free fatty acid receptor-1 (FFAR1/GPR40) signalling is associated with gene expression or gelatinase granule release in bovine neutrophils. Innate Immun. 2016, 22, 479–489. [Google Scholar] [CrossRef]
- Guzeloglu, A.; Michel, F.; Thatcher, W.W. Differential effects of interferon-tau on the prostaglandin synthetic pathway in bovine endometrial cells treated with phorbol ester. J. Dairy Sci. 2004, 87, 2032–2041. [Google Scholar] [CrossRef]
- Valenzuela, P.; Teuber, S.; Manosalva, C.; Alarcon, P.; Figueroa, C.D.; Ratto, M.; Burgos, R.A.; Hidalgo, M.A. Functional expression of the free fatty acids receptor-1 and -4 (FFA1/GPR40 and FFA4/GPR120) in bovine endometrial cells. Vet. Res. Commun. 2019, 43, 179–186. [Google Scholar] [CrossRef]
- Manosalva, C.; Alarcon, P.; Gonzalez, K.; Soto, J.; Igor, K.; Pena, F.; Medina, G.; Burgos, R.A.; Hidalgo, M.A. Free Fatty Acid Receptor 1 Signaling Contributes to Migration, MMP-9 Activity, and Expression of IL-8 Induced by Linoleic Acid in HaCaT Cells. Front. Pharmacol. 2020, 11, 595. [Google Scholar] [CrossRef]
- Manosalva, C.; Quiroga, J.; Teuber, S.; Cardenas, S.; Carretta, M.D.; Moran, G.G.; Alarcon, P.; Hidalgo, M.A.; Burgos, R.A. D-Lactate Increases Cytokine Production in Bovine Fibroblast-Like Synoviocytes via MCT1 Uptake and the MAPK, PI3K/Akt, and NFkappaB Pathways. Animals 2020, 10, 2105. [Google Scholar] [CrossRef] [PubMed]
- Alarcon, P.; Manosalva, C.; Quiroga, J.; Belmar, I.; Alvarez, K.; Diaz, G.; Taubert, A.; Hermosilla, C.; Carretta, M.D.; Burgos, R.A.; et al. Oleic and Linoleic Acids Induce the Release of Neutrophil Extracellular Traps via Pannexin 1-Dependent ATP Release and P2X1 Receptor Activation. Front. Vet. Sci. 2020, 7, 260. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Carretta, M.D.; Conejeros, I.; Hidalgo, M.A.; Burgos, R.A. Propionate induces the release of granules from bovine neutrophils. J. Dairy Sci. 2013, 96, 2507–2520. [Google Scholar] [CrossRef]
- Krikun, G.; Trezza, J.; Shaw, J.; Rahman, M.; Guller, S.; Abrahams, V.M.; Lockwood, C.J. Lipopolysaccharide appears to activate human endometrial endothelial cells through TLR-4-dependent and TLR-4-independent mechanisms. Am. J. Reprod. Immunol. 2012, 68, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, N.; Mirahmadian, M.; Jeddi-Tehrani, M.; Rezania, S.; Ghasemi, J.; Kazemnejad, S.; Mirzadegan, E.; Vafaei, S.; Kashanian, M.; Rasoulzadeh, Z.; et al. Lipopolysaccharide- and Lipoteichoic Acid-mediated Pro-inflammatory Cytokine Production and Modulation of TLR2, TLR4 and MyD88 Expression in Human Endometrial Cells. J. Reprod. Infertil. 2015, 16, 72–81. [Google Scholar]
- Turner, M.L.; Cronin, J.G.; Healey, G.D.; Sheldon, I.M. Epithelial and stromal cells of bovine endometrium have roles in innate immunity and initiate inflammatory responses to bacterial lipopeptides in vitro via Toll-like receptors TLR2, TLR1, and TLR6. Endocrinology 2014, 155, 1453–1465. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Purinergic Signal.ling in the reproductive system in health and disease. Purinergic Signal. 2014, 10, 157–187. [Google Scholar] [CrossRef]
- Munoz-Caro, T.; Gibson, A.J.; Conejeros, I.; Werling, D.; Taubert, A.; Hermosilla, C. The Role of TLR2 and TLR4 in Recognition and Uptake of the Apicomplexan Parasite Eimeria bovis and Their Effects on NET Formation. Pathogens 2021, 10, 118. [Google Scholar] [CrossRef]
- Lewandowska-Sabat, A.M.; Hansen, S.F.; Solberg, T.R.; Osteras, O.; Heringstad, B.; Boysen, P.; Olsaker, I. MicroRNA expression profiles of bovine monocyte-derived macrophages infected in vitro with two strains of Streptococcus agalactiae. BMC Genom. 2018, 19, 241. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, G.; Tanaka, S.; Yamaguchi, T. Differential responses between monocytes and monocyte-derived macrophages for lipopolysaccharide stimulation of calves. Cell. Mol. Immunol. 2009, 6, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Aitken, H.; Poyser, N.L.; Hollingsworth, M. The effects of P2Y receptor agonists and adenosine on prostaglandin production by the guinea-pig uterus. Br. J. Pharmacol. 2001, 132, 709–721. [Google Scholar] [CrossRef]
- Abbracchio, M.P.; Burnstock, G.; Boeynaems, J.M.; Barnard, E.A.; Boyer, J.L.; Kennedy, C.; Knight, G.E.; Fumagalli, M.; Gachet, C.; Jacobson, K.A.; et al. International Union of Pharmacology LVIII: Update on the P2Y G protein-coupled nucleotide receptors: From molecular mechanisms and pathophysiology to therapy. Pharmacol. Rev. 2006, 58, 281–341. [Google Scholar] [CrossRef]
- Sun, B.; Yeh, J. Calcium Oscillatory Patterns and Oocyte Activation During Fertilization: A Possible Mechanism for Total Fertilization Failure (TFF) in Human In Vitro Fertilization? Reprod. Sci. 2021, 28, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Galeska, E.; Wrzecinska, M.; Kowalczyk, A.; Araujo, J.P. Reproductive Consequences of Electrolyte Disturbances in Domestic Animals. Biology 2022, 11, 1006. [Google Scholar] [CrossRef]
- Bertan Membrive, C.M.; da Cunha, P.M.; Meirelles, F.V.; Binelli, M. Calcium potentiates the effect of estradiol on PGF2alpha production in the bovine endometrium. J. Anim. Sci. Biotechnol. 2014, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.E. Calcium signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Paik, S.; Kim, J.K.; Silwal, P.; Sasakawa, C.; Jo, E.K. An update on the regulatory mechanisms of NLRP3 inflammasome activation. Cell. Mol. Immunol. 2021, 18, 1141–1160. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, R.; Liu, R.; Song, P.; Lin, P.; Chen, H.; Zhou, D.; Wang, A.; Jin, Y. Autophagy Mediates Escherichia Coli-Induced Cellular Inflammatory Injury by Regulating Calcium Mobilization, Mitochondrial Dysfunction, and Endoplasmic Reticulum Stress. Int. J. Mol. Sci. 2022, 23, 14174. [Google Scholar] [CrossRef]
- Sales, K.J.; Maldonado-Perez, D.; Grant, V.; Catalano, R.D.; Wilson, M.R.; Brown, P.; Williams, A.R.; Anderson, R.A.; Thompson, E.A.; Jabbour, H.N. Prostaglandin F(2alpha)-F-prostanoid receptor regulates CXCL8 expression in endometrial adenocarcinoma cells via the calcium-calcineurin-NFAT pathway. Biochim. Biophys. Acta 2009, 1793, 1917–1928. [Google Scholar] [CrossRef]
- Gorodeski, G.I. Regulation of transcervical permeability by two distinct P2 purinergic receptor mechanisms. Am. J. Physiol. Cell Physiol. 2002, 282, C75–C83. [Google Scholar] [CrossRef]
- Sophocleous, R.A.; Miles, N.A.; Ooi, L.; Sluyter, R. P2Y(2) and P2X4 Receptors Mediate Ca(2+) Mobilization in DH82 Canine Macrophage Cells. Int. J. Mol. Sci. 2020, 21, 8572. [Google Scholar] [CrossRef] [PubMed]
- Nakano, H.; Shimada, A.; Imai, K.; Takahashi, T.; Hashizume, K. ATP-evoked increase in intracellular calcium via the P2Y receptor in proliferating bovine trophoblast cells. Cell Tissue Res. 2003, 313, 227–236. [Google Scholar] [CrossRef]
- Huang, T.; Zhou, J.; Wang, J. Calcium and calcium-related proteins in endometrial cancer: Opportunities for pharmacological intervention. Int. J. Biol. Sci. 2022, 18, 1065–1078. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Zhao, L.; Zhang, L.; Zhang, G.; Wang, J.; Wei, L. Nongenomic effect of estrogen on the MAPK signaling pathway and calcium influx in endometrial carcinoma cells. J. Cell. Biochem. 2009, 106, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.J.; Wang, T.Y.; Lee, Y.H.; Tai, C.J. Extracellular ATP activates nuclear translocation of ERK1/2 leading to the induction of matrix metalloproteinases expression in human endometrial stromal cells. J. Endocrinol. 2007, 193, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Cronin, J.G.; Turner, M.L.; Goetze, L.; Bryant, C.E.; Sheldon, I.M. Toll-like receptor 4 and MYD88-dependent signaling mechanisms of the innate immune system are essential for the response to lipopolysaccharide by epithelial and stromal cells of the bovine endometrium. Biol. Reprod. 2012, 86, 51. [Google Scholar] [CrossRef]
- Waldo, G.L.; Harden, T.K. Agonist binding and Gq-stimulating activities of the purified human P2Y1 receptor. Mol. Pharmacol. 2004, 65, 426–436. [Google Scholar] [CrossRef]
- Wang, M.J.; Yang, B.R.; Jing, X.Y.; Wang, Y.Z.; Kang, L.; Ren, K.; Kang, L. P2Y1R and P2Y2R: Potential molecular triggers in muscle regeneration. Purinergic Signal. 2022, 1–9. [Google Scholar] [CrossRef]
- Gao, Z.G.; Jacobson, K.A. Distinct Signaling Patterns of Allosteric Antagonism at the P2Y(1) Receptor. Mol. Pharmacol. 2017, 92, 613–626. [Google Scholar] [CrossRef]
- Miras-Portugal, M.T.; Queipo, M.J.; Gil-Redondo, J.C.; Ortega, F.; Gomez-Villafuertes, R.; Gualix, J.; Delicado, E.G.; Perez-Sen, R. P2 receptor interaction and signalling cascades in neuroprotection. Brain Res. Bull. 2019, 151, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Klaver, D.; Thurnher, M. Control of Macrophage Inflammation by P2Y Purinergic Receptors. Cells 2021, 10, 1098. [Google Scholar] [CrossRef] [PubMed]
- Gruenbacher, G.; Gander, H.; Dobler, G.; Rahm, A.; Klaver, D.; Thurnher, M. The human G protein-coupled ATP receptor P2Y(11) is a target for anti-inflammatory strategies. Br. J. Pharmacol. 2021, 178, 1541–1555. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).