Simple Summary
Beluga and sevruga are two highly valuable sturgeon species from the Acipenseride family in Iran. In recent years, research has been focused on commercial rearing of these species. A very important aspect in the sturgeon farming industry is the development of formulated compound diets for promoting growth. However, the ability of fish to digest compound diets is mostly related to the existence of the digestive enzymes in different parts of the gastrointestinal tract. In gastric species, protein digestion is conducted along the gastrointestinal tract by several proteases such as pepsin, trypsin, and chymotrypsin. Trypsin, as an alkaline protease, is able to hydrolyze protein residues and peptides to release free amino acids and small peptides for intestinal absorption; therefore, the activity of trypsin has been widely used as a valuable indicator of digestive capacity in fish. In this work, we aimed to characterize trypsin from beluga and sevruga for the first time. The results of our study show that the physicochemical and biochemical properties of trypsin from beluga and sevruga were in agreement with data reported in bony fish and may be considered a preliminary step to design in vitro tests for the assessment of protein digestibility in these primitive species.
Abstract
This work aimed to determine the physicochemical and biochemical properties of trypsin from beluga Huso huso and sevruga Acipenser stellatus, two highly valuable sturgeon species. According to the results obtained from the methods of casein-zymogram and inhibitory activity staining, the molecular weight of trypsin for sevruga and beluga was 27.5 and 29.5 kDa, respectively. Optimum pH and temperature values for both trypsins were recorded at 8.5 and 55 °C by BAPNA (a specific substrate), respectively. The stability of both trypsins was well-preserved at pH values from 6.0 to 11.0 and temperatures up to 50 °C. TLCK and SBTI, two specific trypsin inhibitors, showed a significant inhibitory effect on the enzymatic activity of both trypsins (p < 0.05). The enzyme activity was significantly increased in the presence of Ca+2 and surfactants and decreased by oxidizing agents, Cu+2, Zn+2, and Co+2 (p < 0.05). However, univalent ions Na+ and K+ did not show any significant effect on the activity of both trypsins (p > 0.05). The results of our study show that the properties of trypsin from beluga and sevruga are in agreement with data reported in bony fish and can contribute to the clear understanding of trypsin activity in these primitive species.
1. Introduction
Beluga (Huso huso) and sevruga (Acipenser stellatus) are among the most important species of sturgeon fish (Acipenseridae) inhabiting the Caspian Sea with a high demand for products such as caviar, meat, skin, and cartilage [1,2]. Today, sturgeons are considered a vulnerable group of fish species for different reasons such as overfishing for production of meat and caviar, water pollution, and destruction of their natural habitats [3,4]. Therefore, in recent years, researchers have focused their studies on restocking and commercial rearing purposes. According to the Iranian Fisheries Organization report, the aquaculture production of sturgeon has increased from 363 t in 2009 to 2516 t in 2020 [5]. A very important aspect in sturgeon farming industry, affecting its production efficiency and long-term sustainability, is the development of formulated compound diets for promoting growth and product quality. However, the ability of fish to digest compound diets is mostly related to the existence of the digestive enzymes in different parts of the gastrointestinal tract [6]. Digestive enzymes reflect the capability of digestion in the organism under study and thus indicate the nutritional status at different stages of growth [7,8]. Herein, analysis of digestive enzymes activity is regarded as a biochemical procedure which may contribute to generate valuable information for understanding the physiology of digestion in fish [9,10]. This important issue can also help to define the requirements of fish for essential nutrients such as proteins, lipids, or carbohydrates [11]. Among macronutrients, dietary proteins are key nutrients for fish growth, since proteins are the building blocks of muscle cells and organs. They occur in a great array of forms, and their nutritional value depends on their amino acid composition. As Moraes and Almeida, 2020 [12] reviewed, the use of dietary proteins depends on a wide array of functional, biochemical, and genetic species-specific characteristics such as the age of organisms; the range of environmental factors (pH, dissolved oxygen, and ammonia levels); the amino acid profile of dietary protein; the digestible usable dietary energy levels; and the presence of antinutritional factors, among others.
Protein digestion is conducted along the gastrointestinal tract by several proteases, with specific actions on the polypeptide chain. In gastric species, pepsin, trypsin, and chymotrypsin are the most important proteolytic enzymes in fish [13,14,15]. As shown by Nolasco-Soria, 2021 [16], trypsin in combination with other alkaline proteases and peptidases such as chymotrypsin, aminopeptidases, and carboxypeptidases complete the acid predigestion conducted in the stomach, hydrolyzing protein residues and peptides to release free amino acids and small peptides for intestinal absorption; therefore, the activity of trypsin has been widely used as a valuable indicator of digestive capacity in fish, as well as a useful biomarker for its nutritional and physiological condition [17]. According to surveys conducted in various species of fish, trypsin participates in activating trypsinogen and other zymogens in the intestine and plays an effective role in protein degradation of the consumed diet in the carnivorous fish up to 40–50% [14,18,19,20,21]. Furthermore, trypsin quantification is essential for the design of in vitro digestibility protocols of feed ingredients and for the formulation of high digestible compound feeds for aquaculture fish species [22].
Hence, a better understanding of the properties of trypsin is necessary to generate valuable information for protein degradation in the fish digestive tract. The characterization of trypsin, especially its physicochemical and biochemical properties, has been thoroughly studied from the intestine of various fish including grass carp (Ctenopharyngodon idellus), spotted goatfish (Pseudupeneus maculatus), grey triggerfish (Balistes capriscus), skipjack tuna (Katsuwonus pelamis), smooth hound (Mustelus mustelus), and Brazilian flounder (Paralichthys orbignyanus) [23,24,25,26,27,28]. The activity of trypsin among several sturgeon species has been mostly studied during larval ontogeny in the members of the genus Acipenser such as A. transmontanus [29], A. fulvescens [30], A. oxyrinchus [31,32], A. baerii [33], A. persicus [34], A. nacarii [35,36], A. stellatus [37], and genus Huso such as H. huso [38]. However, there are no studies evaluating the physicochemical and biochemical characteristics of trypsin in sturgeons; thus, this study attempts to characterize trypsin from beluga and sevruga, as two of the main sturgeon species from the Caspian Sea, for the first time.
2. Materials and Methods
2.1. Fish Samples
Viscera from five specimens of beluga and sevruga (8.0 ± 0.4 kg; 95 ± 8 cm) were obtained from Saei sturgeon rearing center, Sari, Mazandaran, Iran. Fish were fed a commercial diet (crude protein 46%, crude lipid 16%, ash 8.5%, crude fiber 2.5%, and moisture 9%, Mazandaran Animal & Aquatic Feed Company, Semeskandeh Olya, Iran) and kept in fasting condition for 72 h before sampling. Those samples were packed in polyethylene bags, placed in ice with the sample/ice ratio of approximately 1:3 (w/w), and directly transported to the laboratory. Upon arrival, the intestine was separated from the rest of the collected viscera, washed with cold distilled water (4 °C), pooled, and stored at −80 °C for further analysis.
2.2. Preparation of Intestinal Crude Extracts for Trypsin Characterization
The frozen intestine of beluga and sevruga was partially thawed in the refrigerator at 4 °C for 2 h. The samples were then cut into small pieces and homogenized in 50 volumes of 50 mM Tris–HCl buffer (pH 7.5, 10 mM CaCl2, 0.5 M NaCl) by a tissue homogenizer (Heidolph Diax 900, Sigma Co., St. Louis, MO, USA) at 4 °C for 2 min. The homogenate was then filtered with a cheese cloth to separate the floating fat phase and centrifuged for 45 min at 4 °C at 14,000× g by a refrigerated centrifuge (Hettich Benchtop Centrifuge Rotina 420R, Berlin, Germany). The resulting supernatant from each sample was collected, defined as intestinal crude extract (ICE), and then used throughout this study.
2.3. Reagents
EDTA (Ethylenediaminetetraacetic acid), Pepstatin A, PMSF (phenylmethanesulfonyl fluoride), and sodium cholate were obtained from Molekula Co (Gillingham, UK). BAPNA (Nα-benzoyl-DL-arginine-ρ-nitroanilide hydrochloride), ß-mercaptoethanol, BSA (bovine serum albumin), iodoacetic acid, saponin, SBTI (soybean trypsin inhibitor), TLCK (N-ρ-tosyl-L-lysine-chloromethyleketone), and TPCK (N-tosyl-L-phenylalanine chlorom ethyleketone) were purchased from Sigma Chemical Co (St. Louis, MO, USA). Molecular weight marker (PM 2700) was obtained from SMOBIO Technology, Inc. (Hsinchu, Taiwan).
2.4. Trypsin Assay
To measure the enzyme activity in ICE, BAPNA was used as a substrate at a concentration of 1 mM in 50 mM Tris–HCl, 20 mM CaCl2 (pH 8.5) according to the method of Erlanger et al., 1961 [39]. Each ICE (25 μL) was mixed with the prepared substrate (1250 μL) and incubated at 55 °C for 20 min. The reaction was terminated by adding 30% (v/v) acetic acid (250 μL) to the mixture and followed by measuring the trypsin activity at an absorbance of λ = 410 nm using a spectrophotometer (UV-1601, Shimadzu, Kyoto, Japan). One unit of activity was defined as 1 μmol of ρ-nitroaniline released per min and calculated with the following equation [40]:
where 8800 (cm−1 M−1) is the molar extinction coefficient of ρ-nitroaniline measured at λ = 410 nm.
2.5. Protein Assay
The concentration of protein in both ICEs was determined at λ = 750 nm by using BSA (1 mg mL−1 as a standard) and Folin–Ciocalteau reagent according to the Lowry et al., 1951 [41] method.
2.6. Characterization of Trypsin by SDS-PAGE Electrophoresis
SDS-PAGE electrophoresis was performed to determine the protein pattern in ICEs from both sturgeon species [42]. Each ICE was mixed at 2:1 (v/v) ratio with sample buffer (62.5 mM Tris–HCl pH 6.8, 2% SDS (w/v), 10% (v/v) glycerol, 0.3% (w/v) bromophenol blue and 5% (v/v) ß-mercaptoethanol) and boiled for 10 min. Thereafter, the ICEs (with protein concentration of 15 µg) were loaded onto the gel made of 4% stacking gel and 12% separating gel and the electrophoresis was run at a constant current of 15 mA using a vertical electrophoresis system (Bio-Rad Laboratories, Inc., Hercules, CA, USA). After the run, protein bands present in the gel were stained with 0.1% Coomassie Brilliant Blue (G-250) in methanol (35%) and acetic acid (7.5%) and unstained in methanol (35%) and acetic acid (7.5%).
Casein-zymography was performed after electrophoresis for detection of proteases in both ICEs as described by Garcia-Carreno et al., 1993 [43]. Both ICEs were submitted to native-PAGE electrophoresis in a same manner of SDS-PAGE where samples were not boiled, and SDS and reducing agent were removed. After the run, the gel was immersed in 50 mL of a casein solution (20 mg mL−1 in 50 mM Tris–HCl, pH 7.5) for 1 h at 4 °C with gentle agitation to allow diffusion of the casein into the gel. Thereafter, the gel was transferred to another solution (50 mL) containing casein (20 mg mL−1 in 50 mM Tris–HCl, pH 8.5, 10 mM CaCl2) for 20 min at 55 °C with continuous agitation. The gel was then stained with 0.1% Coomassie Brilliant Blue (R-250) in methanol (35%) and acetic acid (7.5%) and unstained in methanol (35%) and acetic acid (7.5%). The presence of proteolytic activities in both ICEs was indicated by the appearance of clear zones on the blue background of the gel, which meant that casein was digested by the targeted protease in these areas.
To reveal the trypsin present in both ICEs, the inhibitory activity staining was used after the submission of both ICEs to native-PAGE electrophoresis as described by Ahmad and Benjakul [44] with a slight modification. After the run, the gel was immersed in 30 mL of an SBTI solution (1 mg mL−1 in 50 mM Tris–HCl, pH 8.5, 10 mM CaCl2) for 30 min at 4 °C to allow diffusion of SBTI into the gel. Thereafter, the incubation of gel was performed for 40 min at 55 °C and followed by washing in cold distilled water and staining with 0.05% Coomassie Brilliant Blue (R-250) to appear inhibitory zones, indicating the presence of the trypsin in both ICEs. The molecular weight of the trypsin that appeared in both ICEs was estimated using wide-range molecular weight markers (PM2700, SMOBIO, Hsinchu, Taiwan) by calculating the trypsin Rf in comparison with those of protein markers.
2.7. Optimum Temperature and Thermostability
To determine the optimum temperature for trypsin activity, the activity of this alkaline protease was measured in both ICEs at different temperatures, including 10, 25, 35, 45, 50, 55, 60, 65, and 70 °C after 20 min of incubation at pH 8.5, using 1 mM BAPNA as a substrate. For the thermostability test, both ICEs were incubated at the above-mentioned temperatures for 30 min and then cooled in an ice bath for assay of residual activity of the enzyme at pH 8.5 as described by Zamani et al., 2014 [40].
2.8. Optimum pH and Stability
Different buffers in the pH range of 4.0 to 11.0 (50 mM acetic acid–sodium acetate for pHs 4–6; 50 mM Tris–HCl for pHs 7–9 and 50 mM glycine–NaOH for pHs 10–11) were used for determining the optimum pH for trypsin activity. Both ICEs were used, and they were incubated using1 mM BAPNA as a substrate after 20 min of incubation at 55 °C at different pHs. For the pH stability test, the remaining activity of the trypsin from each ICE was measured using 1 mM BAPNA as a substrate at 55 °C after being incubated at the above-mentioned pHs for 30 min [40].
2.9. Effect of Inhibitors
Several protease inhibitors (0.01 mM pepstatin A, 0.05 mM SBTI, 1 mM iodoacetic acid, 2 mM EDTA, 5 mM TLCK, 5 mM TPCK, 5 mM ß-mercaptoethanol, and 10 mM PMSF) were prepared in the relevant solvents and incubated with an equal volume of each ICE at room temperature for 15 min. The remaining activity of the enzyme was then measured by 1 mM BAPNA as a substrate (at 55 °C, pH 8.5) and the percent inhibition was calculated according to the method of Khantaphant and Benjakul, 2010 [45]. The trypsin activity of control was measured in the same manner without the presence of inhibitors and scored to 100%.
2.10. Effect of Metal Ions
To investigate the effect of metal ions (5 mM) on the trypsin activity of both ICEs, univalent (K+, Na+) and divalent (Ca2+, Cu2+, Zn2+ and Co2+) cations were dissolved in 50 mM Tris–HCl (pH 8.5) and then incubated with an equal volume of each ICE for 30 min at room temperature. The residual activity of the enzyme was determined using 1 mM BAPNA as a substrate at 55 °C and pH 8.5 [40]. The enzymatic activity of control was assayed without the presence of metal ions and taken as 100%.
2.11. Effect of Surfactants and Oxidizing Agents
The effect of surfactants (anionic: SDS and sodium cholate; non-ionic: saponin and Triton X-100, all at 1%) and oxidizing agents (sodium perborate at a concentration of 1% and H2O2 at three concentrations of 5%, 10%, and 15%) on the trypsin activity was measured by incubation of the above-mentioned surfactants and oxidizing agents with an equal volume of each ICE for 1 h at 40 °C. The residual activity of the enzyme was then determined at 55 °C and pH 8.5 using 1 mM BAPNA as a substrate. The assessment of control enzymatic activity was conducted in a similar condition in the absence of chemicals and scored to 100% [25].
2.12. Statistical Analysis
This study was conducted on the basis of a completely randomized design, and a one-way ANOVA was used for data analysis using SPSS package 22.0 (SPSS Inc., Chicago, IL, USA). All experimental assessments were performed in triplicate, and data was expressed as the mean ± standard deviation (SD). The comparison of means was carried out by Duncan’s multiple range tests with a statistical significance at p < 0.05.
3. Results and Discussion
Fish proteases such as trypsin have been the main objective of many of studies, but it is difficult to compare the results obtained in different species because the data are affected by the use of many different methodologies, the state of feeding of experimental animals (fed vs. starved fish), and the type of enzyme preparation (intestinal tissues alone vs. intestinal extracts with the intestinal content) [46].
3.1. Protein Pattern, Casein-Zymographyand Inhibitory Activity
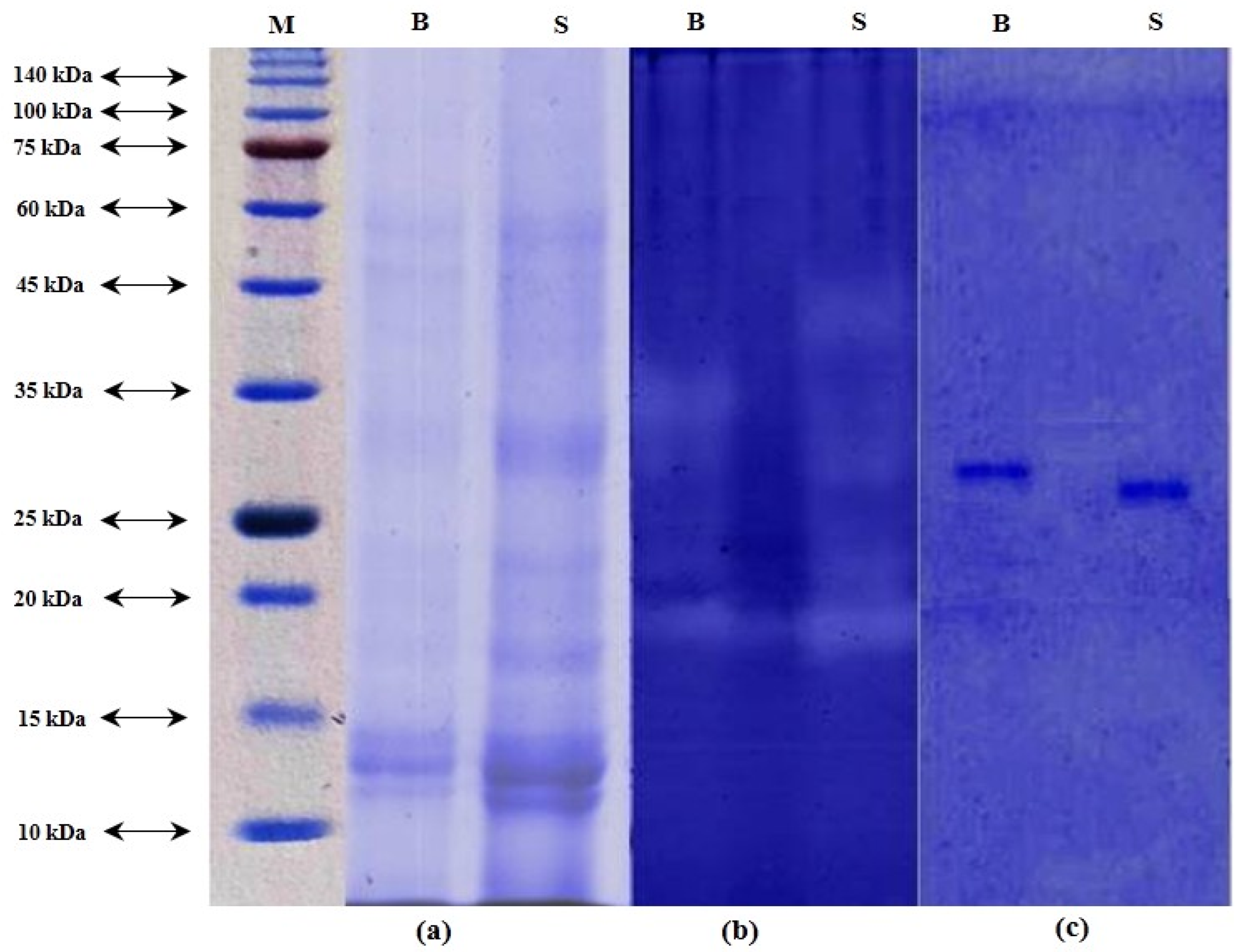
Protein pattern of ICE from beluga and sevruga is depicted in Figure 1a. Based on results obtained from the SDS-PAGE, a number of proteins with different molecular weights were shown in the ICE of both sturgeon species. The major bands of each ICE appeared between molecular weights comprised between 10 and 60 kDa.

Figure 1.
SDS–PAGE (a), zymography (b), and inhibitory activity staining (c) of the intestinal crude extract (ICE) of Huso huso and Acipenser stellatus. (a): Molecular weight marker (M); ICE from Huso huso intestine (B); ICE from Acipenser stellatus intestine (S). Coomassie blue G-250 (0.1%) was used for staining proteins from SDS-PAGE. Zymogram activity and inhibitory activity staining were stained by using 0.1% Coomassie blue R-250.
Casein-zymogram can be used as a highly sensitive and fast assay method for detecting nanograms of protein. The protease activity of both ICEs was demonstrated by casein-zymography as illustrated in Figure 1b. The clear bands, showing the presence of protease, appeared on the gel with different molecular weights. Based on the zymogram pattern, proteases present in ICE from beluga were observed in the range of molecular weights between 19 to 35 kDa, while those in the ICE of sevruga ranged from molecular weights of 19 to 45 kDa.
Inhibitory activity staining for detection of trypsin in ICEs is depicted in Figure 1c. Results showed that a single band for each ICE clearly appeared on the gel with a molecular weight of 27.5 and 29.5 kDa for sevruga and beluga, respectively. In general, trypsins have molecular weights in the range of 20–30 kDa [47]. In particular, different molecular weights for trypsins have been reported in various fish species such as 21.7 kDa for mrigal carp [48], 23.2 kDa for common kilka [40], 23.5 kDa for pirarucu [49], 24 kDa for small red scorpion fish [50], 21 and 24 kDa for liver of albacore tuna [51], 24 kDa for catfish [52], 24.4 kDa for gulf corvina [53], 25 kDa for Monterey sardine [54], 26 kDa for common dolphinfish [55], 27 kDa for zebra blenny [56], 28.8 kDa for sardinelle [57], 29 kDa for Atlantic bonito [58], 38.5 kDa for tambaqui [59], and 42 kDa for skipjack tuna [60]. However, several reasons such as different habitat and climate, autolytic degradation, and genetic variation among fish species may explain why trypsins from various sources have different molecular weights [60,61].
3.2. Optimum Temperature and Thermostability
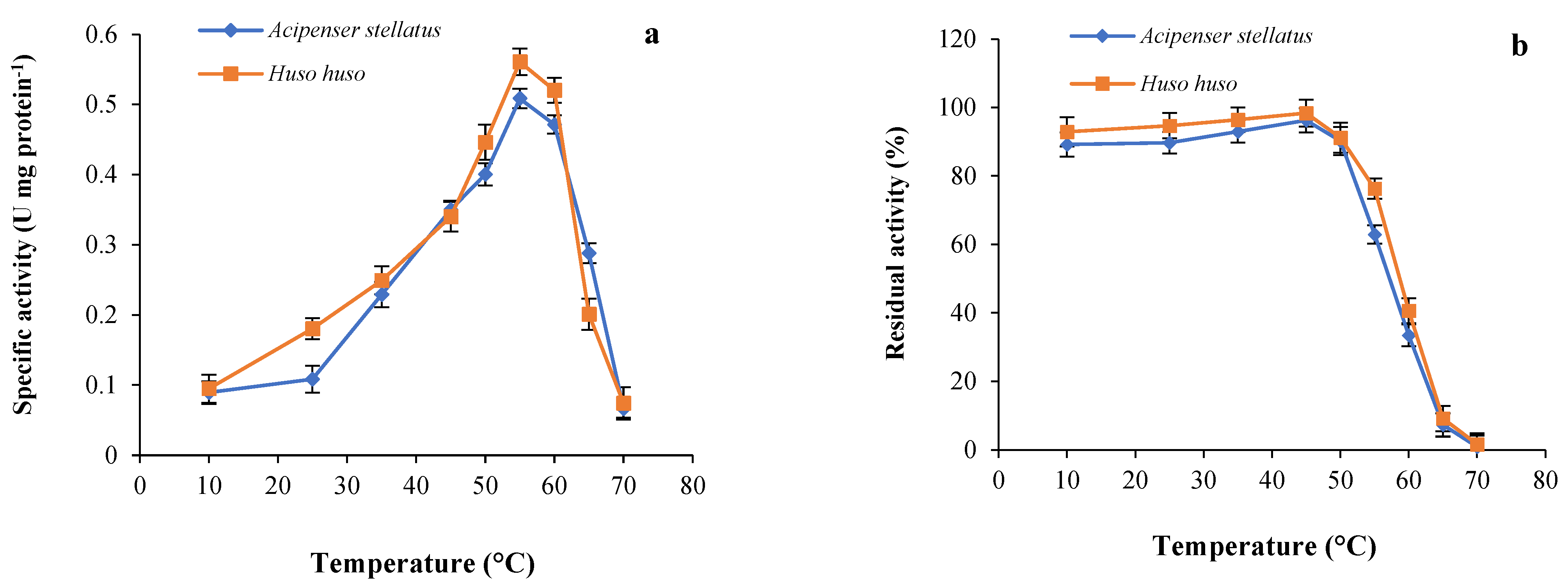
Enzymes are one of the main biological macromolecules and their maximum activity depends on an optimum temperature to make them functional. Figure 2a revealed that optimum temperature of the trypsin in ICE prepared from beluga and sevruga was found to be 55 °C, although 92.70% of the maximum activity of the enzyme was still maintained at 60 °C for both sturgeon species. However, an obvious decrease in the trypsin activity of both ICEs was observed at temperatures above 60 °C, probably due to thermal inactivation of this enzyme caused by protein unfolding [40]. Similar optimum temperature (55 °C) was recorded for trypsins in skipjack tuna [26], gilthead seabream [46], sardinelle [57], and silver mojarra [62]. Optimum temperature of trypsin for both sturgeon species was higher than values reported for cold-water fish such as Atlantic cod (Gadus morhua) [63], grey triggerfish [25], lane snapper (Lutjanus synagris) [64], and Japanese sea bass (L. japonicus) [65] indicating optimum temperatures over a range of 40–45 °C. These differences could be attributed to the temperature of the fish habitat or experimental conditions used in assessments [66]. The optimum temperature for enzyme maximum activity may be interesting for comparative physiological studies, even though such data offer limited information on enzyme activity under normal rearing conditions. Although fish trypsins are mostly unstable at temperatures higher than 40–50 °C, their thermal stability is well known to be at temperatures below 40 °C [67]. Trypsin thermal stability from ICE of beluga and sevruga is displayed in Figure 2b. As can be observed in this figure, the stability of both trypsins was highly maintained up to 50 °C with a remaining activity of 90.2% and 91.7% for sevruga and beluga, respectively. A gradual decrease in the activity of both trypsins was recorded at 55 °C, whereas enzymatic activity sharply decreased at 60 °C. After heating the ICEs at 70 °C, the relative activities for both trypsins were only about 0.9% and 1.6% of their initial activity for sevruga and beluga, respectively. These results were in accordance with those of sardinelle, common kilka, mrigal carp, and pirarucu, which were exhibited to be stable up to 50 °C [40,48,49,57]. The trypsins from beluga and sevruga showed to be more stable at high temperatures in comparison with those reported for the Monterey sardine, chinook salmon, bluefish, Tunisian barbell, and common dolphinfish that the enzymatic activity was rapidly lost at temperatures above 40 °C [54,55,58,68,69]. In general, thermostability of the trypsin enzyme might vary by some factors such as fish species and experimental conditions [23,70]. From a biological point of view, it is difficult to deduce any advantage for beluga and sevruga in possessing proteases showing different resistances to heating, since the normal temperature of water rarely exceeds 21–24 °C. Nevertheless, from a biotechnological perspective, it may be interesting to have information about active and easily denaturalizable proteases potentially useful in the feed industry [71].

Figure 2.
(a) Optimum temperature: the enzymatic activity of both trypsins was measured at different temperatures at pH 8.5 using BAPNA as a substrate; (b) thermostability: residual activity of both enzymes was determined at 55 °C and pH 8.5 after incubating at different temperatures for 30 min using BAPNA as a substrate.
3.3. Effect of pH on Trypsin Activity and Stability
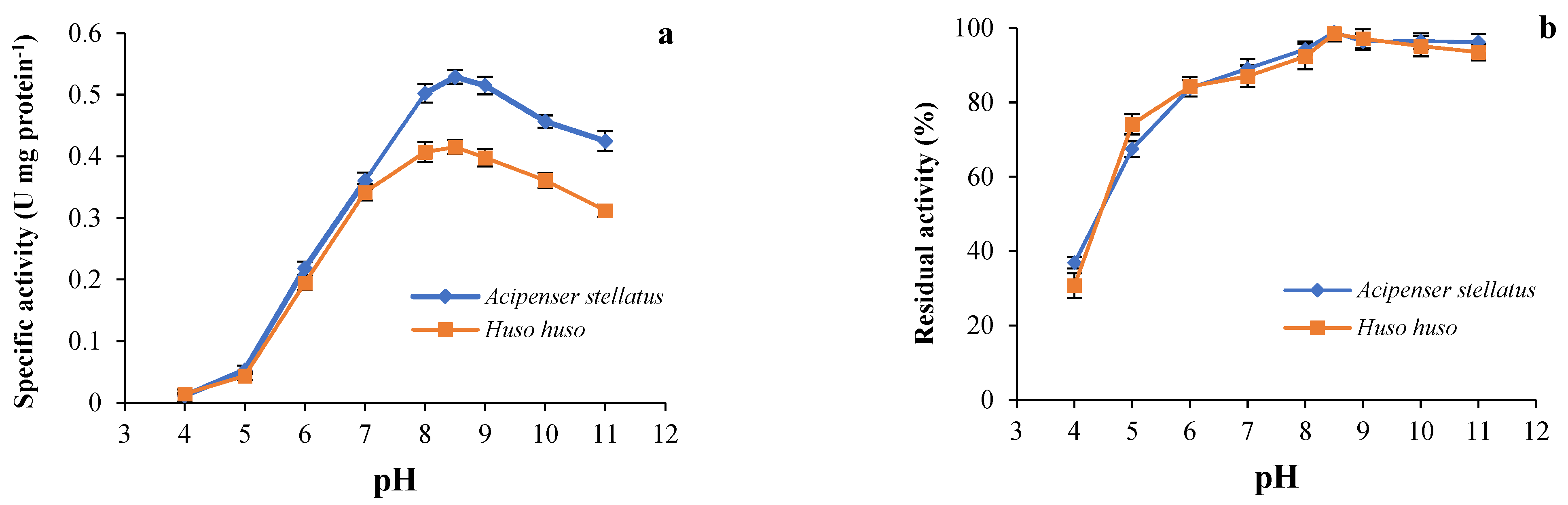
The results observed from the effect of pH on the activity and stability of trypsin from beluga and sevruga are illustrated in Figure 3. Trypsins from both species had a maximal activity at pH 8.5 (Figure 3a). Trypsin activity was dramatically reduced at pH values ranging from 4.0 to 5.0. Our results showed that the stability of trypsin from both sturgeon species was highly preserved at pH values comprised between 5.0 and 11.0 with activity values of 75% for beluga and 80% for sevruga. The high ranges of pH may change the net charge and conformation of an enzyme and inhibit to bind to the substrate properly, resulting in the abrupt loss of enzymatic activity [60,72]. Trypsins are mainly known to have more activity within a range of pH values comprised between 7.5 and 10.5 [46,73]. The optimum pH (8.5) recorded for trypsin in both sturgeon was similar with results reported for trypsins from the brownstripe red snapper viscera and the albacore tuna hepatopancreas [45,51], whereas both trypsins indicated the lower optimum pH than those recorded for the Japanese sea bass, gilthead seabream and common dentex, and the pirarucu [46,49,65]. However, optimum pH may differ depending upon the experimental conditions such as concentration and type of substrate, temperature, and type of metal ions [60]. For instance, Martinez et al., 1988 [74] showed that the trypsin from pyloric caeca of the anchovy had the optimum pHs of 8.0 and 9.5 for the hydrolysis of BAPNA and casein, respectively.

Figure 3.
(a) Optimum pH: the enzymatic activity of both trypsins was determined at different pHs at 55 °C using BAPNA as a substrate; (b) pH stability: residual activity of both enzymes was measured at 55 °C and pH 8.5 after incubating at different pHs for 30 min using BAPNA as substrate.
The effect of pH on the trypsin stability in both ICEs is displayed in Figure 3b. The stability of both enzymes was considerably retained between pH 6.0 to 11.0. Trypsin activity was lost about 63.15% and 69.26% at pH 4.0 in sevruga and beluga, respectively, while the loss of the enzyme activity at pH 5.0 was recorded by 34.13% and 35.84% for sevruga and beluga, respectively. However, trypsin in ICE of sevruga and beluga lost only 1.19% and 1.51% of its activity at pH 8.5, respectively. Similar behavior was reported for trypsins from common kilka [40], common dolphinfish [55], and zebra blenny [56] which retained 80–100% of the activity at pH ranges of neutral and alkaline. The high catalytic activity of the trypsin is observed in alkaline pHs, and its stability at a particular pH may be linked to the net charge of the enzyme at that pH [75].
3.4. Effect of Inhibitors on Trypsin Activity
The sensitivity of protease enzymes to various inhibitors is a valuable tool for their proper functional characterization [55]. Based on their nature, inhibitors can be classified into two classes: chemical inhibitors and protein inhibitors [76]. A trypsin inhibitor is a type of serine protease inhibitor that reduces the biological activity of trypsin thereby rendering it unavailable to bind with proteins for the digestion process [77,78]. Therefore, it can be considered important to characterize the effect of different inhibitors on the trypsin activity.
Table 1 shows the effect of different inhibitors on the activity of trypsin from beluga and sevruga. As it is shown in this table, a serine protease inhibitor such as PMSF inhibited 39.11% and 36.29% of the trypsin activity in sevruga and beluga ICE samples, respectively. Both enzymes were completely inhibited by trypsin specific inhibitors such as SBTI and TLCK, while a chymotrypsin-specific inhibitor (TPCK) did not show any inhibitory effect on their enzymatic activity (p > 0.05). Furthermore, a metalloproteinase inhibitor (EDTA) and a disulfide bond reducing agent (ß-mercaptoethanol) had a partial inhibitory effect on trypsin activity in both sturgeon species, although the inhibition rates varied between both species. In particular, trypsin from sevruga was inhibited by ß-mercaptoethanol (25.33%) and EDTA (23.55%) more than those in beluga (22.84 and 21.06%, respectively) where no significant difference was shown between both species (p > 0.05). Results from EDTA indicate the high dependence of trypsin activity from both sturgeon species on divalent cations [46]. However, an aspartic proteinase inhibitor (Pepstatin A) and a cysteine proteinase inhibitor (iodoacetic acid) exhibited a negligible inhibitory effect on the trypsin activity of both species. Similar results have been observed in other fish species [40,51,56]. For instance, Khangembam and Chakrabarti, 2015 [48] reported that trypsin activity from the digestive system of mrigal carp was inhibited by SBTI and TLCK. SBTI is a single polypeptide chain that acts as a reversible competitive inhibitor of trypsin and forms a stable, enzymatically inactive complex with trypsin, resulting in reduction of the enzyme availability [79]. TLCK is an irreversible inhibitor of trypsin and trypsin-like serine protease that deactivates these enzymes through the formation of a covalent bond with histidine residue in the catalytic site of the enzyme and blocks the active center of the enzyme for binding to substrate [80]. As reported by several authors [40,49,56], PMSF strongly inhibited the activity of trypsin from viscera of common kilka, pirarucu, and zebra blenny, respectively, whereas TPCK had no effect on the enzyme activity from common kilka [40]. The trypsin activity from the intestine of common dolphinfish was partially inhibited by β-mercaptoethanol and EDTA [54], while the trypsin activity from liver of albacore tuna was not reduced in the presence of pepstatin A and iodoacetic acid [51].

Table 1.
Effect of various inhibitors on the activity of trypsin from Huso huso and Acipenser stellatus.
3.5. Effect of Metal Ions
Metal ions have a key role in the activity regulation of many enzyme-catalyzed reactions [81]. Our results on the effect of metal ions on the trypsin activity in beluga and sevruga are detailed in Table 2. No significant effect on the activity of both enzymes was found in the presence of univalent cations Na+ and K+ (p > 0.05). The enzymatic activity of trypsin in both species was significantly reduced by divalent cations Cu2+, Zn2+ and Co2+, whereas Ca2+ significantly enhanced the activity of both trypsins (p < 0.05). Similar results on the effect of Ca2+ on the trypsin activity were also observed in common kilka, common dolphinfish, and zebra blenny [40,55,56]. The attachment of Ca2+ to the active site of serine proteases such as trypsin not only increases the stability of the enzyme structure, but it also protects the enzyme from self-digestion [66,69,82]. The enzymatic activity of trypsin in common dolphinfish was reduced by 82% and 81% by Zn2+ and Cu2+, respectively [55], while 100% of enzymatic activity of tryspin from zebra blenny was lost in the presence of Zn2+ and Cu2+ [56]. In common kilka [40], no inhibition was observed in the trypsin activity in presence of Na+ and K+. Differences in percent inhibition might be linked to species diversity, environmental adaptations and feeding habits of fish [83].

Table 2.
Effect of various metal ions on activity of trypsin from Huso huso and Acipenser stellatus.
3.6. Effect of Surfactants and Oxidizing Agents
Surfactants are the most widely used groups of compounds today, with wide application in industry and household. These are unique substances that contain hydrophobic and hydrophilic moieties within their molecule and find enormous applications in biology. Oxidizing agents such as sodium perborate and H2O2 are also used in the detergent industry as bleaching agent. Surfactants and oxidizing agents may be harmful or even toxic to aquatic organisms. These compounds can penetrate through tissues and bind to biomolecules, such as enzymes, causing changes in cellular activity [84]. As reviewed by Rubingh, 1996 [85], surfactants can influence the activity of enzymes in two ways. Firstly, by binding to the enzyme, surfactants can influence intrinsic enzyme properties such as the secondary and tertiary structure or flexibility, and thereby, affect its ability to serve as a catalyst. A less direct, but equally important, way in which surfactants affect enzyme activity is by changing the environment in which the enzyme functions. It is well-known that SDS disrupts non-covalent bonds within and between enzymes, denaturing them, and resulting in the loss of their native conformation and function [86], whereas saponin, Triton X-100, and sodium cholate are the non-denaturing surfactants [87,88,89]. Hence, characterizing the effect of these chemical compounds on trypsin activity is of relevance for proper characterizing its activity.
The results on the effect of various surfactants and oxidizing agents on the trypsin activity in sevruga and beluga are shown in Table 3. A significant increase in the activity of both trypsins was observed after incubation for 1 h at 40 °C in the presence of surfactants tested, including saponin, sodium cholate, and Triton X-100 at final concentrations of 1% (p < 0.05). Both trypsins were highly unstable against sodium dodecyl sulfate (SDS), in which trypsins from sevruga and beluga significantly lost about 94% and 97% of their activity in the presence of 0.1% SDS, respectively (p < 0.05). Similar results were found in trypsins of other fish species in the presence of saponin, sodium cholate, Triton X-100 and SDS [40,56]. The obtained results on the effect of oxidizing agents on both trypsins showed that the enzymatic activity was reduced in the presence of sodium perborate (1%) in sevruga and beluga by 22.23% and 24.37%, respectively. The activity of both enzymes was also decreased significantly with an increase in H2O2 concentrations from 5% to 15%, as described in Table 3 (p < 0.05). Trypsin from sevruga showed significantly higher activity than trypsin from beluga in the presence of H2O2 ranging from 5% to 15%, indicating that trypsin from sevruga was more tolerant to H2O2 than trypsin from beluga. The biochemical and structural properties of enzyme can affect its ability as a catalyst in presence of oxidizing agents [85]. These results showed that trypsins from sevruga and beluga were more stable against H2O2 than trypsins from grey triggerfish and zebra blenny [25,56], whereas most proteases have shown to be unstable in the presence of oxidizing agents like hydrogen peroxide [25].

Table 3.
Trypsin activity from Huso huso and Acipenser stellatus in presence of surfactants and oxidizing agents.
4. Conclusions
The results of our study indicated that trypsin from intestine of beluga and sevruga had similar properties to trypsins from bony fish. The enzyme had an optimum temperature of 55 °C and thermal stability was maintained over 90% up to 55 °C. This alkaline protease had an optimum pH of 8.5 and showed to be tolerant in the pH range of 6.0 to 11.0 in both studied sturgeon species. The molecular weight of trypsin for sevruga and beluga was estimated to be 27.5 and 29.5 kDa, respectively, as data from inhibitory activity staining indicated. Both trypsins were inhibited by main specific inhibitors, SBTI and TLCK. Additionally, the enzymatic activity of trypsins was still detected after 1 h in the presence of surfactants and oxidative agents. Information provided in this manuscript related to trypsin activity for beluga and sevruga based on changes in the activity of this alkaline protease based on a tested range of pH and temperature values, and presence of potential inhibitors, ions and cations may be considered a preliminary step to design in vitro tests for the assessment of protein digestibility in these species.
Author Contributions
Conceptualization and methodology, A.Z.; writing—review and editing, A.Z. and E.G.; investigation and writing—original draft, M.K.; data analysis and measurement of physicochemical properties of the enzyme, A.A.K., M.H.N., A.S. and M.E. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Vice-Presidency of Research and Technology from Malayer University, Iran (grant number 84/9-1-310).
Institutional Review Board Statement
This study conformed to the current Iranian law regarding the care and use of laboratory animals and was approved by the Animal Ethical Committee of Malayer University (Code: IR.UMSHA.REC.1397.994).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to express our sincere gratitude to laboratory staffs of Fisheries Department of Natural Resources and Environment Faculty of Malayer University for helpful assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kalbassi, M.R.; Abdollahzadeh, E.; Salari-Joo, H. A review on aquaculture development in Iran. Ecopersia 2013, 1, 159–178. [Google Scholar]
- Vasilyeva, L.M.; Elhetawy, A.I.G.; Sudakova, N.V.; Astafyeva, S.S. History, current status and prospects of sturgeon aquaculture in Russia. Aquac. Res. 2019, 50, 979–993. [Google Scholar] [CrossRef]
- Aghilinejhad, S.M.; Gorgin, S.; van Uhm, D.; Joolaie, R.; Ghorbani, R.; Paighambari, S.Y.; Mohammadi, J.; Jalali, A. What are the drivers of the occurrence of illegal fishing and conservation barriers of sturgeons in the Caspian Sea? Aquat. Conserv. Mar. Freshw. Ecosyst. 2018, 28, 690–701. [Google Scholar] [CrossRef]
- Mirrasooli, E.; Ghorbani, R.; Gorgin, S.; Aghilinejhad, S.M.; Jalali, A. Factors associated with illegal fishing and fisher attitudes toward sturgeon conservation in the southern Caspian Sea. Mar. Policy 2019, 100, 107–115. [Google Scholar] [CrossRef]
- Iranian Fisheries Organization Statistical Yearbook. Budget and Planning Deputy; Iranian Fisheries Organization Statistical Yearbook: Tehran, Iran, 2022; p. 64. (In Persian) [Google Scholar]
- Furne, M.; García-Gallego, M.; Hidalgo, M.C.; Morales, A.E.; Domezain, A.; Domezain, J.; Sanz, A. Effect of starvation and refeeding on digestive enzyme activities in sturgeon (Acipenser naccarii) and trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. 2008, 149A, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Kolkovski, S. Digestive enzymes in fish larvae and juveniles-Implications and applications to formulated diets. Aquaculture 2001, 200, 181–201. [Google Scholar] [CrossRef]
- Yufera, M.; Darias, M.J. The onset of exogenous feeding in marine fish larvae. Aquaculture 2007, 268, 53–63. [Google Scholar] [CrossRef]
- Twining, S.S.; Alexander, P.A.; Huibregste, K.; Glick, D.M. A pepsinogen from rainbow trout. Comp. Biochem. Physiol. 1983, 75B, 109–112. [Google Scholar] [CrossRef]
- Navarro-Guillen, C.; Yufera, M.; Perera, E. Biochemical features and modulation of digestive enzymes by environmental temperature in the greater amberjack, Seriola dumerili. Front. Mar. Sci. 2022, 28, 1391. [Google Scholar] [CrossRef]
- Bolasina, S.; Perez, A.; Yamashita, Y. Digestive enzymes activity during ontogenetic development and effect of starvation in Japanese flounder, Paralichthys olivaceus. Aquaculture 2006, 252, 503–515. [Google Scholar] [CrossRef]
- Moraes, G.; de Almeida, L.C. Nutrition and functional aspects of digestion in fish. In Biology and Physiology of Freshwater Neotropical Fish; Academic Press: Cambridge, MA, USA, 2020; pp. 251–271. [Google Scholar]
- Glass, H.J.; MacDonald, N.L.; Moran, R.M.; Stark, J.R. Digestion of protein in different marine species. Comp. Biochem. Physiol. 1989, 94B, 607–611. [Google Scholar] [CrossRef]
- Eshel, A.; Lindner, P.; Smirnoff, P.; Newton, S.; Harpaz, S. Comparative study of proteolytic enzymes in the digestive tracts of the European sea bass and hybrid striped bass reared in freshwater. Comp. Biochem. Physiol. 1993, 106A, 627–634. [Google Scholar] [CrossRef]
- Rungruangsak-Torrissen, K.; Moss, R.; Andresen, L.H.; Berg, A.; Waagbo, R. Different expressions of trypsin and chymotrypsin in relation to growth in Atlantic salmon (Salmo salar L.). Fish Physiol. Biochem. 2006, 32, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Nolasco-Soria, H. Improving and standardizing protocols for alkaline protease quantification in fish. Rev. Aquac. 2021, 13, 43–65. [Google Scholar] [CrossRef]
- Nazdar, N.; Imani, A.; Noori, F.; Sarvi Moghanlou, K. Effect of silymarin supplementation on nickel oxide nanoparticle toxicity to rainbow trout (Oncorhynchus mykiss) fingerlings: Pancreas tissue histopathology and alkaline protease activity. Iran. J. Sci. Technol. Trans. A Sci. 2018, 42, 353–361. [Google Scholar] [CrossRef]
- Krogdahl, A.; Lea, T.B.; Olli, J.J. Soybean proteinase inhibitors affect intestinal trypsin activities and amino acid digestibilities in rainbow trout (Oncorhyncus mykiss). Comp. Biochem. Physiol. 1994, 107A, 215–219. [Google Scholar] [CrossRef]
- Haard, N.F.; Dimes, L.E.; Arndt, R.E.; Dong, F.M. Estimation of protein digestibility: IV. Digestive proteinases from the pyloric caeca of coho salmon (Oncorhynchus kisutch) fed diets containing soybean meal. Comp. Biochem. Physiol. 1996, 115B, 533–540. [Google Scholar] [CrossRef]
- Chong, A.S.C.; Hashim, R.; Chow-Yang, L.; Ali, A.B. Partial characterization and activities of proteases from the digestive tract of discus fish, Symphysodon aeguifasciata. Aquaculture 2002, 203, 321–333. [Google Scholar] [CrossRef]
- Hedstrom, L. Serine protease mechanism and specificity. Chem. Rev. 2002, 102, 4501–4524. [Google Scholar] [CrossRef]
- Gilannejad, N.; Martínez-Rodríguez, G.; Yúfera, M.; Moyano, F.J. Estimating the effect of different factors on the digestive bioaccessibility of protein by the Senegalese sole (Solea senegalensis); combination of response surface methodology and in vitro assays. Aquaculture 2017, 477, 28–34. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Wang, Z.; Xu, S.Y.; Xu, L.N. Two trypsin isoforms from the intestine of the grass carp (Ctenopharyngodon idellus). J. Comp. Physiol. 2007, 177B, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.A.; Amaral, I.P.; Santo, A.R.E.; Carvalho, J.R.L.B.; Bezerra, R.S. Trypsin-like enzyme from intestine and pyloric caeca of spotted goatfish (Pseudupeneus maculatus). Food Chem. 2007, 100, 1429–1434. [Google Scholar] [CrossRef]
- Jellouli, K.; Bougatef, A.; Daassi, D.; Balti, R.; Barkia, A.; Nasri, M. New alkaline trypsin from the intestine of grey triggerfish (Balistes capriscus) with high activity at low temperature: Purification and characterisation. Food Chem. 2009, 116, 644–650. [Google Scholar] [CrossRef]
- Klomklao, S.; Kishimura, H.; Nonami, Y.; Benjakul, S. Biochemical properties of two isoforms of trypsin purified from the intestine of skipjack tuna (Katsuwonus pelamis). Food Chem. 2009, 115, 155–162. [Google Scholar] [CrossRef]
- Bougatef, A.; Balti, R.; Nasri, R.; Jellouli, K.; Souissi, N.; Nasri, M. Biochemical properties of anionic trypsin acting at high concentration of NaCl purified from the intestine of a carnivorous fish: Smooth hound (Mustelus mustelus). J. Agric. Food Chem. 2010, 58, 5763–5769. [Google Scholar] [CrossRef] [PubMed]
- Candiotto, F.B.; Freitas-Junior, A.C.V.; Neri, R.C.A.; Bezerra, R.S.; Rodrigues, R.V.; Sampaio, L.A.; Tesser, M.B. Characterization of digestive enzymes from captive Brazilian flounder Paralichthys orbignyanus. Braz. J. Biol. 2017, 78, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Buddington, R.K.; Doroshov, S.I. Feeding trials with hatchery produced white sturgeon juveniles (Acipenser transmontanus). Aquaculture 1984, 36, 237–243. [Google Scholar] [CrossRef]
- Buddington, R.K. Digestive secretions of lake sturgeon, Acipenser fulvescens, during early development. J. Fish Biol. 1985, 26, 715–723. [Google Scholar] [CrossRef]
- Dabrowski, K.; Kaushik, S.J.; Fauconneau, B. Rearing of sturgeon (Acipenser baeri Brandt) larvae: I. Feeding trial. Aquaculture 1985, 47, 185–192. [Google Scholar] [CrossRef]
- Ostaszewska, T.; Kolman, R.; Kamaszewski, M.; Wiszniewski, G.; Adamek, D.; Duda, A. Morphological changes in digestive tract of Atlantic sturgeon (Acipenser oxyrinchus) during organogenesis. Int. Aquat. Res. 2011, 3, 101–105. [Google Scholar]
- Bardi, R.W.; Chapman, F.A.; Barrows, F.T. Feeding trials with hatchery-produced Gulf of Mexico sturgeon larvae. Prog. Fish Cult. 1998, 60, 25–31. [Google Scholar] [CrossRef]
- Babaei, S.S.; Abedian Kenari, A.; Rajabmohammad Nazari, R.M.; Gisbert, E. Developmental changes of digestive enzymes in Persian sturgeon (Acipenser persicus) during larval ontogeny. Aquaculture 2011, 318, 138–144. [Google Scholar] [CrossRef]
- Camacho, S.; Carmona, R.; Llorente, J.I.; Sanz, A.; Garcia-Gallego, M.; Domezain, A.; Dominguez, N.; Ostos-Garrido, M.V. Stomach development in the sturgeon Acipenser naccarii: Histoenzymatic and ultrastructural analysis. J. Appl. Ichthyol. 2011, 27, 693–700. [Google Scholar] [CrossRef]
- Sanz, A.; Llorente, J.I.; Furne, M.; Ostos-Garrido, M.V.; Carmona, R.; Domezain, A.; Hidalgo, M.C. Digestive enzymes during ontogeny of the sturgeon Acipenser naccarii: Intestine and pancreas development. J. Appl. Ichthyol. 2011, 27, 1139–1146. [Google Scholar] [CrossRef]
- Ghasemi, N.; Imani, A.; Noori, F.; Shahrooz, R. Ontogeny of digestive tract of stellate sturgeon (Acipenser stellatus) from hatching to juvenile stage: Digestive enzymes activity, stomach and proximal intestine. Aquaculture 2020, 519, 734751. [Google Scholar] [CrossRef]
- Asgari, R.; Rafiee, G.; Eagderi, S.; Noori, F.; Agh, N.; Poorbagher, H.; Gisbert, E. Ontogeny of the digestive enzyme activities in hatchery produced beluga (Huso huso). Aquaculture 2013, 416, 33–40. [Google Scholar] [CrossRef]
- Erlanger, B.F.; Kokowski, N.; Cohen, W. The preparation and properties of two new chromogenic substrates of trypsin. Arch. Biochem. Biophys. 1961, 95, 271–278. [Google Scholar] [CrossRef]
- Zamani, A.; Rezaei, M.; Madani, R.; Habibi Rezaie, M. Trypsin enzyme from viscera of common kilka (Clupeonella cultriventris caspia): Purification, characterization, and its compatibility with oxidants and surfactants. J. Aquat. Food Prod. Technol. 2014, 23, 237–252. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during assembly of the head bacteriophague T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Garcia-Carreno, F.L.; Dimes, L.E.; Haard, N.F. Substrate-gel electrophoresis for composition and molecular weight of proteinases or proteinaceous proteinase inhibitors. Anal. Biochem. 1993, 214, 65–69. [Google Scholar] [CrossRef]
- Ahmad, M.; Benjakul, S. Impact of legume seed extracts on degradation and functional properties of gelatin from unicorn leatherjacket skin. Process. Biochem. 2011, 46, 2021–2029. [Google Scholar] [CrossRef]
- Khantaphant, S.; Benjakul, S. Purification and characterization of trypsin from the pyloric caeca of brownstripe red snapper (Lutjanus vitta). Food Chem. 2010, 120, 658–664. [Google Scholar] [CrossRef]
- Alarcón, F.J.; Díaz, M.; Moyano, F.J. Characterization and functional properties of digestive proteases in two sparids; gilthead seabream (Sparus aurata) and common dentex (Dentex dentex). Fish Physiol. Biochem. 1998, 19, 257–267. [Google Scholar] [CrossRef]
- Gendry, P.; Launay, J.F. Pancreatic anionic trypsin: Evidence for the existence of a 30 kDa form. Comp. Biochem. Physiol. 1992, 102B, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Khangembam, B.K.; Chakrabarti, R. Trypsin from the digestive system of carp Cirrhinus mrigala: Purification, characterization and its potential application. Food Chem. 2015, 175, 386–394. [Google Scholar] [CrossRef] [PubMed]
- De Freitas-Junior, A.C.V.; da Costa, H.M.S.; Marcuschi, M.; Icimoto, M.Y.; Machado, M.F.; Machado, M.F.; Ferreira, J.C.; de Oliveira, V.M.; Buarque, D.S.; Bezerra, R.S. Substrate specificity, physicochemical and kinetic properties of a trypsin from the giant Amazonian fish pirarucu (Arapaima gigas). Biocatal. Agric. Biotechnol. 2021, 35, 102073. [Google Scholar] [CrossRef]
- Aissaoui, N.; Marzouki, M.N.; Abidi, F. Purification and biochemical characterization of a novel intestinal protease from Scorpaena notata. Int. J. Food Prop. 2017, 20, 2151–2165. [Google Scholar] [CrossRef]
- Klomklao, S.; Benjakul, S. Two trypsin isoforms from albacore tuna (Thunnus alalunga) liver: Purification and physicochemical and biochemical characterization. Int. J. Biol. Macromol. 2018, 107, 1864–1870. [Google Scholar] [CrossRef]
- Dos Santos, C.W.V.; da Costa Marques, M.E.; de AraujoTenorio, H.; de Miranda, E.C.; Pereira, H.J.V. Purification and characterization of trypsin from Luphiosilurus alexandri pyloric cecum. Biochem. Biophys. Rep. 2016, 8, 29–33. [Google Scholar] [CrossRef]
- Gonzalez-Felix, M.L.; De La Ree-Rodriguez, C.; Perez-Velazquez, M. Partial characterization, quantification and optimum activity of trypsin and lipase from the sciaenids Cynoscion othonopterus, Cynoscion parvipinnis and Cynoscion xanthulus. Arch. Biol. Sci. 2020, 72, 81–93. [Google Scholar] [CrossRef]
- Castillo-Yanez, F.J.; Pacheco-Aguilar, R.; Garcia-Carreno, F.L.; Del Toro, M.A.N. Isolation and characterization of trypsin from pyloric caeca of Monterey sardine (Sardinops sagax caerulea). Comp. Biochem. Physiol. 2005, 140B, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, D.M.R.C.; dos Santos, C.W.V.; de Souza, C.B.; de Albuquerque, F.S.; dos Santos Oliveira, J.M.; Pereira, H.J.V. Trypsin purified from Coryphaena hippurus (common dolphinfish): Purification, characterization, and application in commercial detergents. Biocatal. Agric. Biotechnol. 2020, 25, 101584. [Google Scholar] [CrossRef]
- Ktari, N.; Ben Khaled, H.; Nasri, R.; Jellouli, K.; Ghorbel, S.; Nasri, M. Trypsin from zebra blenny (Salaria basilisca) viscera: Purification, characterisation and potential application as a detergent additive. Food Chem. 2012, 130, 467–474. [Google Scholar] [CrossRef]
- Ben Khaled, H.; Jellouli, K.; Souissi, N.; Ghorbel, S.; Nasri, M. Purification and characterisation of three trypsin isoforms from viscera of sardinelle (Sardinella aurita). Fish Physiol. Biochem. 2011, 37, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Klomklao, S.; Benjakul, S.; Visessanguan, W.; Kishimura, H.; Simpson, B.K. 29 kDa trypsin from the pyloric ceca of Atlantic bonito (Sarda sarda): Recovery and characterization. J. Agric. Food Chem. 2007, 55, 4548–4553. [Google Scholar] [CrossRef]
- Bezerra, R.S.; Santos, J.F.; Paiva, P.M.; Correia, M.T.S.; Coelho, L.C.B.B.; Vieira, V.L.L.; Carvalho, J.R.L.B. Partial purification and characterization of a thermostable trypsin from pyloric caeca of tambaqui (Colossoma macropomum). J. Food Biochem. 2001, 25, 199–210. [Google Scholar] [CrossRef]
- Klomklao, S.; Benjakul, S.; Visessanguan, W.; Kishimura, H.; Simpson, B.K. Proteolytic degradation of sardine (Sardinella gibbosa) proteins by trypsin from skipjack tuna (Katsuwonus pelamis) spleen. Food Chem. 2006, 98, 14–22. [Google Scholar] [CrossRef]
- Lu, B.J.; Zhou, L.G.; Cai, Q.F.; Hara, K.; Maeda, A.; Su, W.J.; Cao, M.J. Purification and characterisation of trypsins from the pyloric caeca of Mandarin fish (Siniperca chuatsi). Food Chem. 2008, 110, 352–360. [Google Scholar] [CrossRef]
- Silva, J.F.; Esposito, T.S.; Marcuschi, M.; Ribeiro, K.; Cavalli, R.O.; Oliveira, V.; Bezerra, R.S. Purification and partial characterisation of a trypsin from the processing waste of the silver mojarra (Diapterus rhombeus). Food Chem. 2011, 129, 777–782. [Google Scholar] [CrossRef]
- Stefansson, B.; Sandholt, G.B.; Gudmundsdottir, Á. Elucidation of different cold-adapted Atlantic cod (Gadus morhua) trypsin X isoenzymes. Biochim. Biophys. Acta-Proteins Proteom. 2017, 1865, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Esposito, T.S.; Marcuschi, M.; Amaral, I.P.; Carvalho, L.B., Jr.; Bezerra, R.S. Trypsin from the processing waste of the lane snapper (Lutjanus synagris) and its compatibility with oxidants, surfactants and commercial detergents. J. Agric. Food Chem. 2010, 58, 6433–6439. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.F.; Jiang, Y.K.; Zhou, L.G.; Sun, L.C.; Li, G.M.; Osatomi, K.; Cao, M.J. Biochemical characterization of trypsins from the hepatopancreas of Japanese sea bass (Lateolabrax japonicus). Comp. Biochem. Physiol. 2011, 159B, 183–189. [Google Scholar] [CrossRef]
- Kishimura, H.; Klomklao, S.; Benjakul, S.; Chun, B.S. Characteristics of trypsin from the pyloric ceca of walleye pollock (Theragra chalcogramma). Food Chem. 2008, 106, 194–199. [Google Scholar] [CrossRef]
- Klomklao, S.; Kishimura, H.; Benjakul, S. Anionic trypsin from the pyloric ceca of Pacific saury (Cololabis saira): Purification and biochemical characteristics. J. Aquat. Food. Prod. Technol. 2014, 23, 186–200. [Google Scholar] [CrossRef]
- Kurtovic, I.; Marshall, S.N.; Simpson, B.K. Isolation and characterization of a trypsin fraction from the pyloric ceca of chinook salmon (Oncorhynchus tshawytscha). Comp. Biochem. Physiol. 2006, 143B, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Sila, A.; Nasri, R.; Jridi, M.; Balti, R.; Nasri, M.; Bougatef, A. Characterisation of trypsin purified from the viscera of Tunisian barbel (Barbus callensis) and its application for recovery of carotenoproteins from shrimp wastes. Food Chem. 2012, 132, 1287–1295. [Google Scholar] [CrossRef]
- Kanno, G.; Kishimura, H.; Ando, S.; Nalinanon, S.; Klomklao, S.; Benjakul, S.; Chun, B.S.; Saeki, H. Structural properties of trypsin from cold-adapted fish, arabesque greenling (Pleurogrammus azonus). Eur. Food Res. Technol. 2011, 232, 381–388. [Google Scholar] [CrossRef]
- Haard, N.F. A review of proteolytic enzymes from marine organisms and their application in the food industry. J. Aquatic Food Product. Technol. 1992, 1, 17–35. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Y.; Tong, B.; Chen, X.; Chen, J. Purification and biochemical characterization of a thermostable and acid-stable alpha-amylase from Bacillus licheniformis B4-423. Int. J. Biol. Macromol. 2018, 109, 329–337. [Google Scholar] [CrossRef]
- Simpson, B.K. Digestive proteinases from marine animals. In Seafood Enzymes: Utilization and Influence on Postharvest Seafood Quality; Haard, N.F., Simpson, B.K., Eds.; Marcel Dekker: New York, NY, USA, 2000; pp. 531–540. [Google Scholar]
- Martinez, A.; Olsen, R.L.; Serra, J.L. Purification and characterization of two trypsin like enzymes from the digestive tract of anchovy Engraulis encrasicholus. Comp. Biochem. Physiol. 1988, 91B, 677–684. [Google Scholar] [CrossRef]
- Mamimin, C.; O-thong, S.; Nitipan, S. Application of metagenomics technique in gene encoding thermostable enzyme discovery from hot spring. Thaksin Univ. J. 2016, 19, 96–105. [Google Scholar]
- Cristina Oliveira de Lima, V.; Piuvezam, G.; Leal Lima Maciel, B.; Heloneida de Araújo Morais, A. Trypsin inhibitors: Promising candidate satietogenic proteins as complementary treatment for obesity and metabolic disorders? J. Enzyme. Inhib. Med. Chem. 2019, 34, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Farady, C.J.; Craik, C.S. Mechanisms of macromolecular protease inhibitors. Chembiochem 2010, 11, 2341–2346. [Google Scholar] [CrossRef] [PubMed]
- Avilés-Gaxiola, S.; Chuck-Hernández, C.; Serna Saldívar, S.O. Inactivation methods of trypsin inhibitor in legumes: A review. J. Food Sci. 2018, 83, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Senphan, T.; Benjakul, S.; Kishimura, H. Purification and characterization of trypsin from hepatopancreas of Pacific white shrimp. J. Food Biochem. 2015, 39, 388–397. [Google Scholar] [CrossRef]
- Choi, S.M.; Oh, E.S.; Kim, D.S.; Pyeun, J.H.; Cho, D.M.; Ahn, C.B.; Kim, H.R. Comparative Biochemical Properties of Proteinases from the Hepatopancreas of Shrimp.-I. Purification of Protease from the Hepatopancreas of Penaeus japonicus. Fish Aquatic. Sci. 1998, 1, 201–208. [Google Scholar]
- Page, M.J.; Di Cera, E. Role of Na+ and K+ in enzyme function. Physiol. Rev. 2006, 86, 1049–1092. [Google Scholar] [CrossRef]
- Fu, X.Y.; Xue, C.H.; Miao, B.C.; Li, Z.J.; Gao, X.; Yang, W.G. Characterization of proteases from the digestive tract of sea cucumber (Stichopus japonicus): High alkaline protease activity. Aquaculture 2005, 246, 321–329. [Google Scholar] [CrossRef]
- Bougatef, A. Trypsins from fish processing waste: Characteristics and biotechnological applications—Comprehensive review. J. Clean. Prod. 2013, 57, 257–265. [Google Scholar] [CrossRef]
- Mandal, R.; Mandal, D.; Mishra, N.; Bahadur, A. Effect of surfactants on phosphatase level of fresh water fish Labeo rohita. J. Environ. Biol. 2010, 31, 395–398. [Google Scholar] [PubMed]
- Rubingh, D.N. The influence of surfactants on enzyme activity. Curr. Opin. Colloid Interface Sci. 1996, 1, 598–603. [Google Scholar] [CrossRef]
- Jelińska, A.; Zagożdżon, A.; Górecki, M.; Wisniewska, A.; Frelek, J.; Holyst, R. Denaturation of proteins by surfactants studied by the Taylor dispersion analysis. PLoS ONE 2017, 12, e0175838. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Blankschtein, D. Role of the bile salt surfactant sodium cholate in enhancing the aqueous dispersion stability of single-walled carbon nanotubes: A molecular dynamics simulation study. J. Phys. Chem. 2010, 114B, 15616–15625. [Google Scholar] [CrossRef] [PubMed]
- Parra, J.G.; Iza, P.; Dominguez, H.; Schott, E.; Zarate, X. Effect of Triton X-100 surfactant on the interfacial activity of ionic surfactants SDS, CTAB and SDBS at the air/water interface: A study using molecular dynamic simulations. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125284. [Google Scholar] [CrossRef]
- Rai, S.; Acharya-Siwakoti, E.; Kafle, A.; Devkota, H.P.; Bhattarai, A. Plant-derived saponins: A review of their surfactant properties and applications. Science 2021, 3, 44. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).