Trait-Level Resilience in Pet Dogs—Development of the Lincoln Canine Adaptability Resilience Scale (L-CARS)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Demographic Data
2.2. Resilience and Related Items
- Engineering resilience—an individual’s ability to return to normal (‘bounce back’) following adversity;
- Ecological resilience—the capacity to tolerate/withstand disruptions, with the ability to maintain composure during challenges (focus on ‘best efforts’, perseverance);
- Adaptive capacity—the ability to handle and adapt to change, i.e., flexibility in strategies and even enjoyment of change.
2.3. Questionnaire Piloting and Distribution
2.4. Statistical Analysis
2.4.1. Intra-Rater Reliability Assessment
2.4.2. Psychometric Evaluation
3. Results
3.1. Responses
3.2. Demographics
3.3. Intra-Rater Reliability (See Supplementary Material S4 for Full Data)
3.4. Inter-Item Correlations and Principal Component Analysis (PCA) (See Supplementary Material S5 for Supporting Details)
3.5. Comparison of ‘Complete’ and ‘Incomplete’ Datasets
3.6. Construct Validity of Components
3.7. Internal Consistency
3.8. Relationship between Principal Component Scores and Demographics
3.9. Principal Component Scores
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aburn, G.; Gott, M.; Hoare, K. What is resilience? An Integrative Review of the empirical literature. J. Adv. Nurs. 2016, 72, 980–1000. [Google Scholar] [CrossRef] [PubMed]
- Carver, C.S. Resilience and thriving: Issues, models, and linkages. J. Soc. Issues 1998, 54, 245–266. [Google Scholar] [CrossRef]
- Garcia-Dia, M.J.; DiNapoli, J.M.; Garcia-Ona, L.; Jakubowski, R.; O’Flaherty, D. Concept Analysis: Resilience. Arch. Psychiatr. Nurs. 2013, 27, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Windle, G. What is resilience? A review and concept analysis. Rev. Clin. Gerontol. 2011, 21, 152–169. [Google Scholar] [CrossRef]
- Werner, E.E. The children of Kauai—Resiliency and recovery in adolescence and adulthood. J. Adolesc. Health 1992, 13, 262–268. [Google Scholar] [CrossRef]
- Karairmak, O. Establishing the psychometric qualities of the Connor-Davidson Resilience Scale (CD-RISC) using exploratory and confirmatory factor analysis in a trauma survivor sample. Psychiatry Res. 2010, 179, 350–356. [Google Scholar] [CrossRef]
- Edward, K.L. The phenomenon of resilience in crisis care mental health clinicians. Int. J. Ment. Health Nurs. 2005, 14, 142–148. [Google Scholar] [CrossRef]
- Wu, W.W.; Tsai, S.Y.; Liang, S.Y.; Liu, C.Y.; Jou, S.T.; Berry, D.L. The Mediating Role of Resilience on Quality of Life and Cancer Symptom Distress in Adolescent Patients With Cancer. J. Pediatr. Oncol. Nurs. 2015, 32, 304–313. [Google Scholar] [CrossRef]
- Tiira, K. Resilience In Dogs? Lessons From Other Species. Vet. Med. 2019, 10, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Davydov, D.M.; Stewart, R.; Ritchie, K.; Chaudieu, I. Resilience and mental health. Clin. Psychol. Rev. 2010, 30, 479–495. [Google Scholar] [CrossRef] [Green Version]
- Hu, T.Q.; Zhang, D.J.; Wang, J.L. A meta-analysis of the trait resilience and mental health. Pers. Individ. Diff. 2015, 76, 18–27. [Google Scholar] [CrossRef]
- Rutter, M. Psychosocial resilience and protective mechanisms. Am. J. Orthopsychiatry 1987, 57, 316–331. [Google Scholar] [CrossRef]
- Oshio, A.; Taku, K.; Hirano, M.; Saeed, G. Resilience and Big Five personality traits: A meta-analysis. Pers. Individ. Diff. 2018, 127, 54–60. [Google Scholar] [CrossRef]
- Connor, K.M.; Davidson, J.R.T. Development of a new resilience scale: The Connor-Davidson Resilience scale (CD-RISC). Depress. Anxiety 2003, 18, 76–82. [Google Scholar] [CrossRef]
- Hekman, J.P.; Karas, A.Z.; Dreschel, N.A. Salivary cortisol concentrations and behavior in a population of healthy dogs hospitalized for elective procedures. Appl. Anim. Behav. Sci. 2012, 141, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Stanford, T.L. Behavior of dogs entering a veterinary clinic. Appl. Anim. Ethol. 1981, 7, 271–279. [Google Scholar] [CrossRef]
- Mariti, C.; Ricci, E.; Mengoli, M.; Zilocchi, M.; Sighieri, C.; Gazzano, A. Survey of travel-related problems in dogs. Vet. Rec. 2012, 170, 542. [Google Scholar] [CrossRef]
- Beerda, B.; Schilder, M.B.H.; van Hooff, J.; de Vries, H.W.; Mol, J.A. Behavioural and hormonal indicators of enduring environmental stress in dogs. Anim. Welf. 2000, 9, 49–62. [Google Scholar] [CrossRef]
- Blackwell, E.J.; Bradshaw, J.W.S.; Casey, R.A. Fear responses to noises in domestic dogs: Prevalence, risk factors and co-occurrence with other fear related behaviour. Appl. Anim. Behav. Sci. 2013, 145, 15–25. [Google Scholar] [CrossRef]
- Meneses, T.; Robinson, J.; Rose, J.; Vernick, J.; Overall, K.L. Review of epidemiological, pathological, genetic, and epigenetic factors that may contribute to the development of separation anxiety in dogs. JAVMA 2021, 259, 1118–1129. [Google Scholar]
- Block, J.; Kremen, A.M. IQ and ego-resiliency: Conceptual and empirical connections and separateness. J. Pers. Soc. Psychol. 1996, 70, 349–361. [Google Scholar] [PubMed]
- Friborg, O.; Hjemdal, O.; Rosenvinge, J.H.; Martinussen, M. A new rating scale for adult resilience: What are the central protective resources behind healthy adjustment? Int. J. Methods Psychiatr. Res. 2003, 12, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Lock, S.; Rees, C.S.; Heritage, B. Development and validation of a brief measure of psychological resilience: The state-trait assessment of resilience scale. Aust. Psychol. 2020, 55, 10–25. [Google Scholar] [CrossRef]
- Smith, B.W.; Dalen, J.; Wiggins, K.; Tooley, E.; Christopher, P.; Bernard, J. The brief resilience scale: Assessing the ability to bounce back. Int. J. Behav. Med. 2008, 15, 194–200. [Google Scholar] [PubMed]
- Wagnild, G.M.; Young, H.M. Development and psychometric evaluation of the Resilience Scale. J. Nurs. Meas. 1993, 1, 165–178. [Google Scholar]
- Luthar, S.S.; Cicchetti, D.; Becker, B. The construct of resilience: A critical evaluation and guidelines for future work. Child Dev. 2000, 71, 543–562. [Google Scholar]
- Maltby, J.; Day, L.; Hall, S. Refining Trait Resilience: Identifying Engineering, Ecological, and Adaptive Facets from Extant Measures of Resilience. PLoS ONE 2015, 10, e0131826. [Google Scholar] [CrossRef] [Green Version]
- Masten, A.S. Ordinary magic—Resilience processes in development. Am. Psychol. 2001, 56, 227–238. [Google Scholar] [CrossRef]
- Southwick, S.M.; Bonanno, G.A.; Masten, A.S.; Panter-Brick, C.; Yehuda, R. Resilience definitions, theory, and challenges: Interdisciplinary perspectives. Eur. J. Psychotraumatol. 2014, 5, 25338. [Google Scholar] [CrossRef] [Green Version]
- Mills, D.S. Clinical Animal Behaviour: Paradigms, Problems and Practice. Animals 2022, 12, 3103. [Google Scholar] [CrossRef]
- Sheppard, G.; Mills, D.S. The Development of a Psychometric Scale for the Evaluation of the Emotional Predispositions of Pet Dogs. Int. J. Comp. Psychol. 2002, 15, 201–222. [Google Scholar] [CrossRef]
- Wright, H.F.; Mills, D.S.; Pollux, P.M.J. Development and Validation of a Psychometric Tool for Assessing Impulsivity in the Domestic Dog (Canis familiaris). Int. J. Comp. Psychol. 2011, 24, 210–225. [Google Scholar] [CrossRef]
- McPeake, K.J.; Collins, L.M.; Zulch, H.; Mills, D.S. The Canine Frustration Questionnaire-Development of a New Psychometric Tool for Measuring Frustration in Domestic Dogs (Canis familiaris). Front. Vet. Sci. 2019, 6, 152. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Madrigal, C.; de la Fuente, J.; Garcia-Manglano, J.; Martinez-Vicente, J.M.; Peralta-Sanchez, F.J.; Amate-Romera, J. The Role of Gender and Age in the Emotional Well-Being Outcomes of Young Adults. Int. J. Environ. Res. Public Health 2021, 18, 522. [Google Scholar] [CrossRef]
- Mills, D.S.; Demontigny-Bedard, I.; Gruen, M.; Klinck, M.P.; McPeake, K.J.; Barcelos, A.M.; Hewison, L.; Van Haevermaet, H.; Denenberg, S.; Hauser, H.; et al. Pain and Problem Behavior in Cats and Dogs. Animals 2020, 10, 318. [Google Scholar] [CrossRef] [Green Version]
- DeVellis, R.F.; Thorpe, C.T. Scale Development: Theory and Applications, 5th ed.; SAGE Publications, Inc.: Thousand Oaks, CA, USA, 2021. [Google Scholar]
- Cro, S.; Morris, T.P.; Kenward, M.G.; Carpenter, J.R. Sensitivity analysis for clinical trials with missing continuous outcome data using controlled multiple imputation: A practical guide. Stat. Med. 2020, 39, 2815–2842. [Google Scholar] [CrossRef]
- Bro, R.; Smilde, A.K. Principal component analysis. Anal. Methods 2014, 6, 2812–2831. [Google Scholar] [CrossRef] [Green Version]
- Budaev, S.V. Using Principal Components and Factor Analysis in Animal Behaviour Research: Caveats and Guidelines. Ethology 2010, 116, 472–480. [Google Scholar] [CrossRef]
- Ricolfi, L.; Testa, S. Factorial Invariance and Orthogonal Rotation. Multivariate Behav. Res. 2021, 56, 787–804. [Google Scholar] [CrossRef]
- Cattell, R.B. The Scree Test For The Number Of Factors. Multivariate Behav. Res. 1966, 1, 245–276. [Google Scholar] [CrossRef]
- Kaiser, H.F. The varimax criterion for analytic rotation in factor analysis. Psychometrika 1958, 23, 187–200. [Google Scholar] [CrossRef]
- Kaiser, H.F.; Rice, J. Little Jiffy, Mark 4. Educ. Psychol. Meas. 1974, 34, 111–117. [Google Scholar] [CrossRef]
- Bartlett, M.S. Properties of sufficiency and statistical tests. Proc. Math. Phys. Eng. Sci. 1937, 160, 268–282. [Google Scholar]
- Notari, L.; Kirton, R.; Mills, D.S. Psycho-Behavioural Changes in Dogs Treated with Corticosteroids: A Clinical Behaviour Perspective. Animals 2022, 12, 592. [Google Scholar] [CrossRef] [PubMed]
- Mason, G.; Rushen, J. Stereotypic Animal Behaviour: Fundamentals and Applications To Welfare, 2nd ed.; CABI: Wallingford, UK, 2006. [Google Scholar]
- Burn, C.C. A Vicious Cycle: A Cross-Sectional Study of Canine Tail-Chasing and Human Responses to It, Using a Free Video-Sharing Website. PLoS ONE 2011, 6, e26553. [Google Scholar] [CrossRef] [PubMed]
- Oken, B.S.; Chamine, I.; Wakeland, W. A systems approach to stress, stressors and resilience in humans. Behav. Brain Res. 2015, 282, 144–154. [Google Scholar] [CrossRef]
- Tami, G.; Gallagher, A. Description of the behaviour of domestic dog (Canis familiaris) by experienced and inexperienced people. Appl. Anim. Behav. Sci. 2009, 120, 159–169. [Google Scholar] [CrossRef]
- McPeake, K.J.; Collins, L.M.; Zulch, H.; Mills, D.S. Behavioural and Physiological Correlates of the Canine Frustration Questionnaire. Animals 2021, 11, 3364. [Google Scholar] [CrossRef]
- Wright, H.F.; Mills, D.S.; Pollux, P.M.J. Behavioural and physiological correlates of impulsivity in the domestic dog (Canis familiaris). Physiol. Behav. 2012, 105, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Mills, D.S.; Dube, M.B.; Zulch, H. Stress and Pheromonatherapy in Small Animal Clinical Behaviour, 1st ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2013. [Google Scholar]
- Masood, A.; Masud, Y.; Mazahir, S. Gender differences in resilience and psychological distress of patients with burns. Burns 2016, 42, 300–306. [Google Scholar] [CrossRef]
- Portnoy, G.A.; Relyea, M.R.; Decker, S.; Shamaskin-Garroway, A.; Driscoll, M.; Brandt, C.A.; Haskell, S.G. Understanding Gender Differences in Resilience Among Veterans: Trauma History and Social Ecology. J. Trauma. Stress 2018, 31, 845–855. [Google Scholar] [CrossRef]
- Isaacs, A.J. Gender Differences in Resilience of Academic Deans. J. Res. Educ. 2014, 24, 112–119. [Google Scholar]
- Sun, J.; Stewart, D. Age and Gender Effects on Resilience in Children and Adolescents. Int. J. Ment. Health Promot. 2007, 9, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Leipold, B.; Munz, M.; Michele-Malkowsky, A. Coping and Resilience in the Transition to Adulthood. Emerg. Adulthood 2019, 7, 12–20. [Google Scholar] [CrossRef]
- Davies, M. Geriatric screening in first opinion practice—Results from 45 dogs. J. Small Anim. Pract. 2012, 53, 507–513. [Google Scholar] [CrossRef]
- Hielm-Bjorkman, A.K.; Kapatkin, A.S.; Rita, H.J. Reliability and validity of a visual analogue scale used by owners to measure chronic pain attributable to osteoarthritis in their dogs. Am. J. Vet. Res. 2011, 72, 601–607. [Google Scholar] [CrossRef]
- Packer, R.M.A.; Hendricks, A.; Burn, C.C. Do dog owners perceive the clinical signs related to conformational inherited disorders as ‘normal’ for the breed? A potential constraint to improving canine welfare. Anim. Welf. 2012, 21, 81–93. [Google Scholar] [CrossRef] [Green Version]
| Problem Behaviour Category | Number | Percentage (%) |
|---|---|---|
| “Unfriendly or aggressive behaviour towards dogs” | 407 | 37.5 |
| “Fears or phobias e.g., noise reactivity/fear, fear of car travel” | 355 | 32.7 |
| “Separation related behaviour problem” | 237 | 21.9 |
| “General hyperactivity/overexcitement” | 233 | 20.6 |
| “Unfriendly or aggressive behaviour towards people” | 188 | 17.3 |
| “Repetitive behaviour including self-mutilation (e.g., compulsive licking/chewing), tail-chasing or shadow chasing” | 108 | 10.0 |
| “Other problem behaviour not listed” | 96 | 8.9 |
| PC | Item Number | Item (R) = Reverse Scored | Variance Explained (%) |
|---|---|---|---|
| PC1 Adaptability/ behavioural flexibility | 4 | If something frightened my dog, he/she would be nervous to return to that location for a long time afterwards (R) | 42.61 |
| 5 | If my dog were to have a bad experience with another individual (dog or person), he/she would forget about it quickly and not hold onto it | ||
| 6 | If something were to startle or frighten my dog, he/she would remain on edge for some time afterwards (R) | ||
| 8 | My dog does not get upset easily | ||
| 9 | My dog generally takes stressful situations in their stride | ||
| 11 | My dog will sometimes seem out of sorts for no apparent reason (R) | ||
| 12 | If another dog has a negative reaction to something, my dog is likely to become upset too (R) | ||
| 14 | My dog enjoys anything that is new or unusual, e.g., objects, animals or anything they have not seen before | ||
| 15 | I would regard my dog to be very adaptable, i.e., able to fit into any situation | ||
| 18 | If something unexpected were to happen and my life circumstances were to change, I know my dog could cope | ||
| 19 | I believe my dog to be a resilient individual, i.e., able to cope with, ‘bounce back’ from and/or adapt to, adversity or change | ||
| PC2 Perseverance | 7 | My dog always tries his/her hardest even when the task is difficult | 12.61 |
| 10 | My dog will persevere even when they do not succeed in something straight away, e.g., when learning a new trick, when trying to solve a puzzle, etc. | ||
| 17 | My dog enjoys challenges, e.g., learning new tricks, finding hidden items, solving a difficult task |
| PC1 Adaptability/Behavioural Flexibility | PC2 Perseverance | |||||
|---|---|---|---|---|---|---|
| Demographic Variable | df | F | p | df | F | p |
| Country | 2 | 3.179 | 0.084 | 2 | 1.338 | 0.526 |
| Age | 4 | 1.645 | 0.322 | 4 | 2.083 | 0.162 |
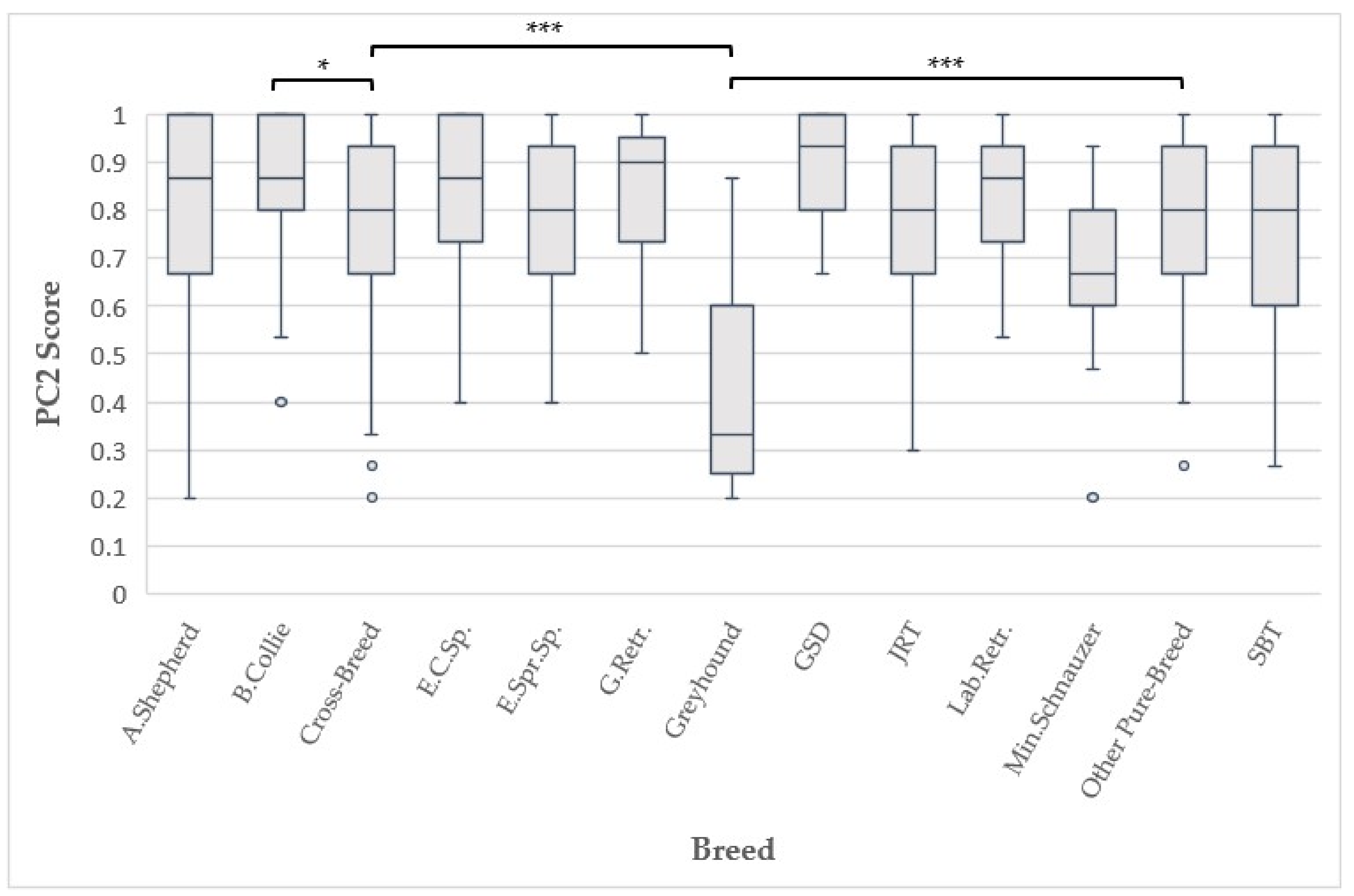
| Breed | 12 | 1.605 | 0.170 | 12 | 8.269 | <0.001 *** |
| Sex | 1 | 0.263 | 1.000 | 1 | 0.918 | 0.677 |
| Neuter Status | 1 | 1.819 | 0.356 | 1 | 1.290 | 0.513 |
| Pre-existing medical condition | 1 | 1.595 | 0.414 | 1 | 0.298 | 1.000 |
| Takes prescription medication | 1 | 2.931 | 0.175 | 1 | 2.912 | 0.177 |
| Takes supplements or nutraceuticals | 1 | 0.312 | 1.000 | 1 | 1.828 | 0.353 |
| Uses pheromone products | 1 | 5.660 | 0.035 * | 1 | 0.087 | 1.000 |
| Problem Behaviour Category: | ||||||
| • Aggressive to dogs | 1 | 75.109 | <0.001 *** | 1 | 0.030 | 1.000 |
| • Fears/phobias | 1 | 243.870 | <0.001 *** | 1 | 9.749 | 0.026 * |
| • Separation related behaviour problem | 1 | 31.687 | <0.001 *** | 1 | 3.071 | 1.000 |
| • General hyperactivity/overexcitement | 1 | 1.995 | 1.000 | 1 | 5.037 | 0.350 |
| • Aggressive to people | 1 | 59.557 | <0.001 *** | 1 | 3.156 | 1.000 |
| • Repetitive behaviour | 1 | 2.930 | 1.000 | 1 | 8.366 | 0.055 |
| • Other problem behaviour | 1 | 32.975 | <0.001 *** | 1 | 3.102 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mackay, E.L.M.; Zulch, H.; Mills, D.S. Trait-Level Resilience in Pet Dogs—Development of the Lincoln Canine Adaptability Resilience Scale (L-CARS). Animals 2023, 13, 859. https://doi.org/10.3390/ani13050859
Mackay ELM, Zulch H, Mills DS. Trait-Level Resilience in Pet Dogs—Development of the Lincoln Canine Adaptability Resilience Scale (L-CARS). Animals. 2023; 13(5):859. https://doi.org/10.3390/ani13050859
Chicago/Turabian StyleMackay, Eilidh L. M., Helen Zulch, and Daniel S. Mills. 2023. "Trait-Level Resilience in Pet Dogs—Development of the Lincoln Canine Adaptability Resilience Scale (L-CARS)" Animals 13, no. 5: 859. https://doi.org/10.3390/ani13050859






