Responses of Urban Bird Assemblages to Land-Sparing and Land-Sharing Development Styles in Two Argentinian Cities
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
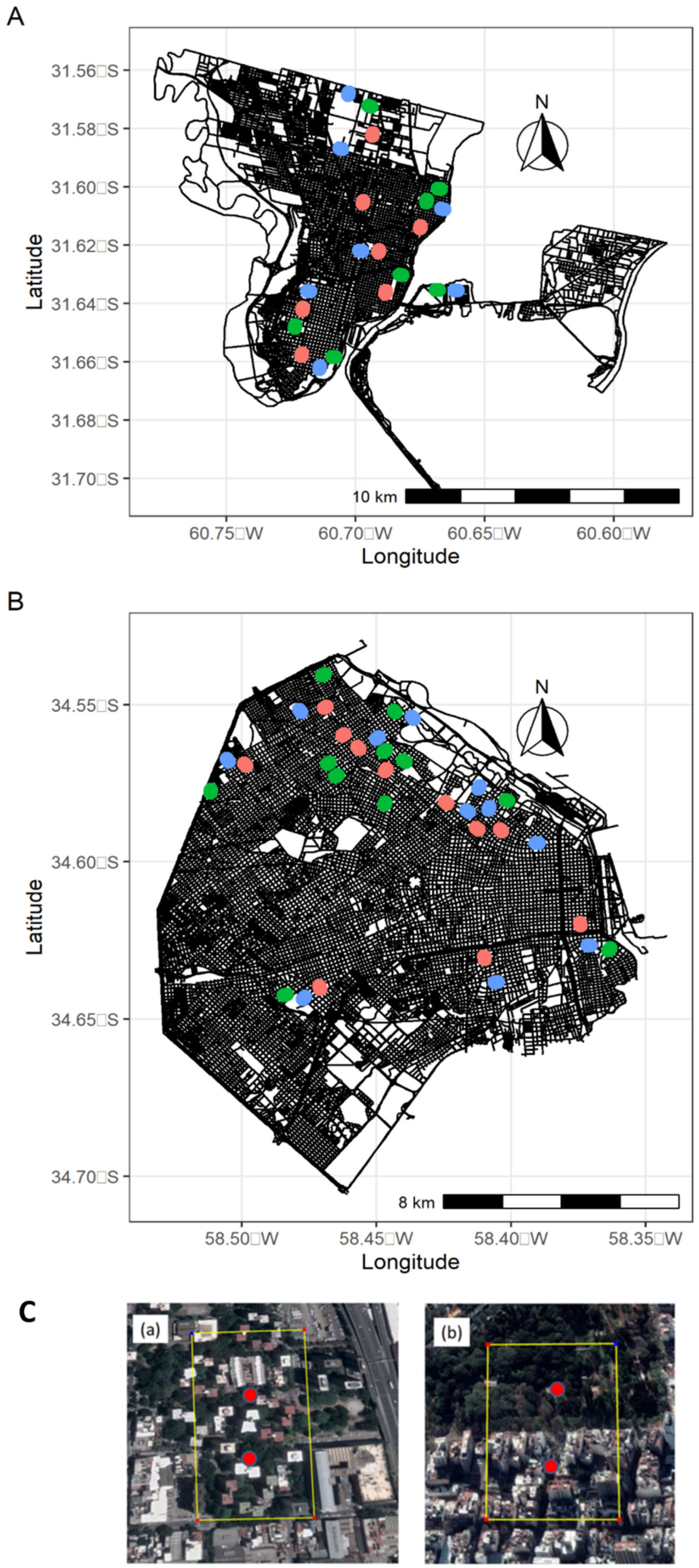
2.1. Study Area and Preliminary Classification of Urban Areas
2.2. Classification of Urban Areas
2.3. Bird Survey
2.4. Environmental Variables
2.5. Data Analysis
2.5.1. Taxonomic Diversity per Urban Development Style
2.5.2. Taxonomic Diversity per Sampling Unit
2.5.3. Taxonomic Composition
3. Results
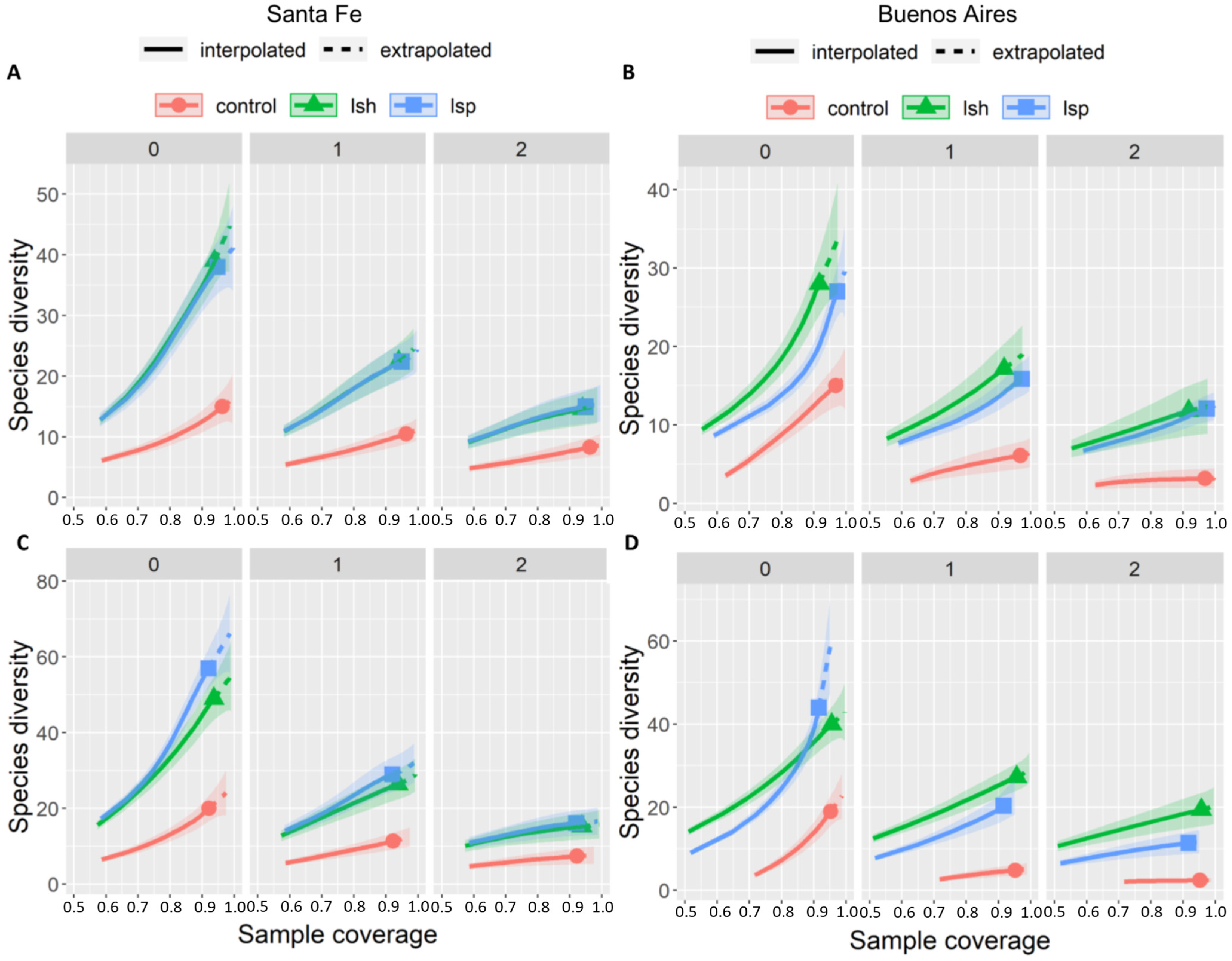
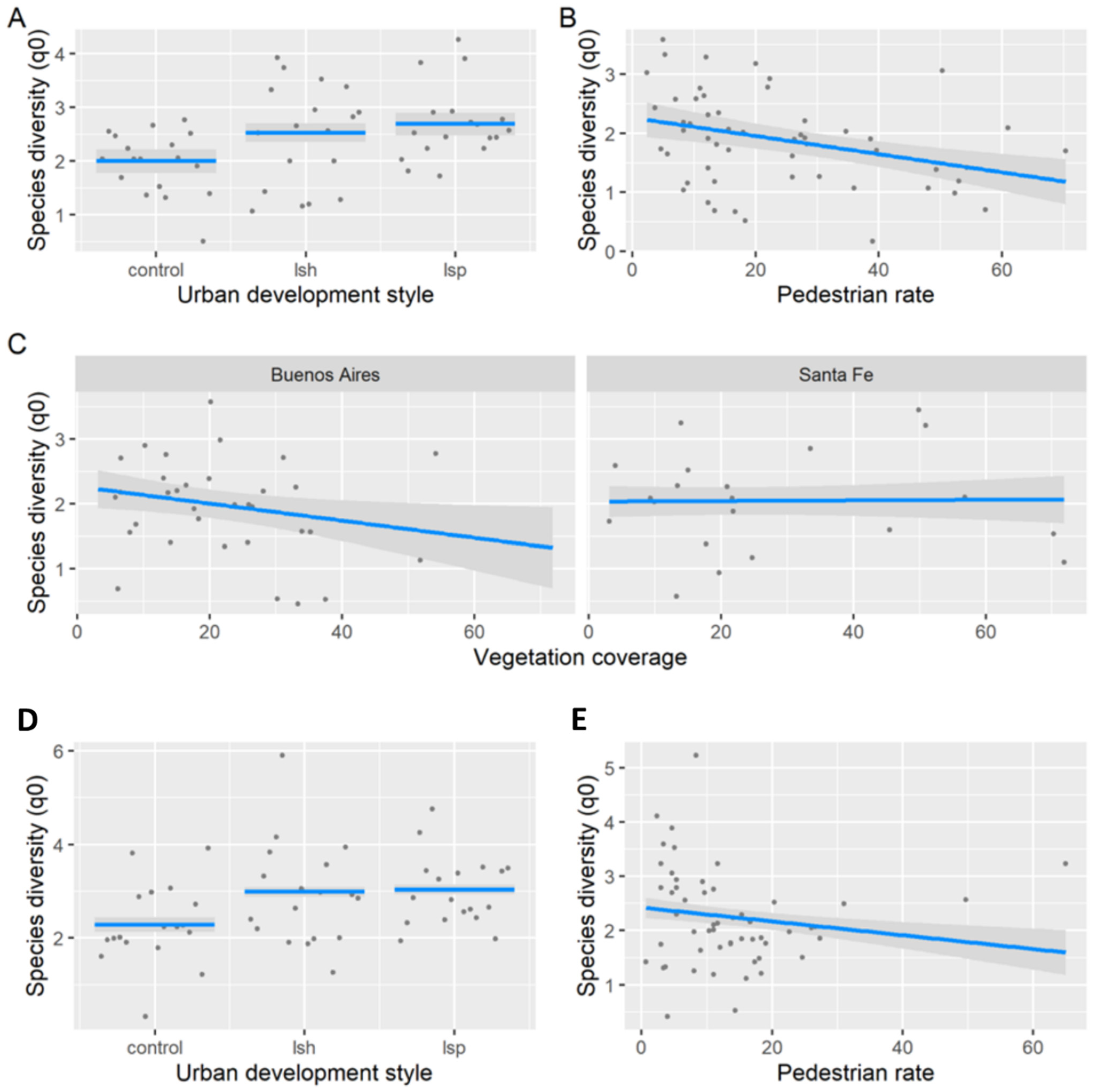
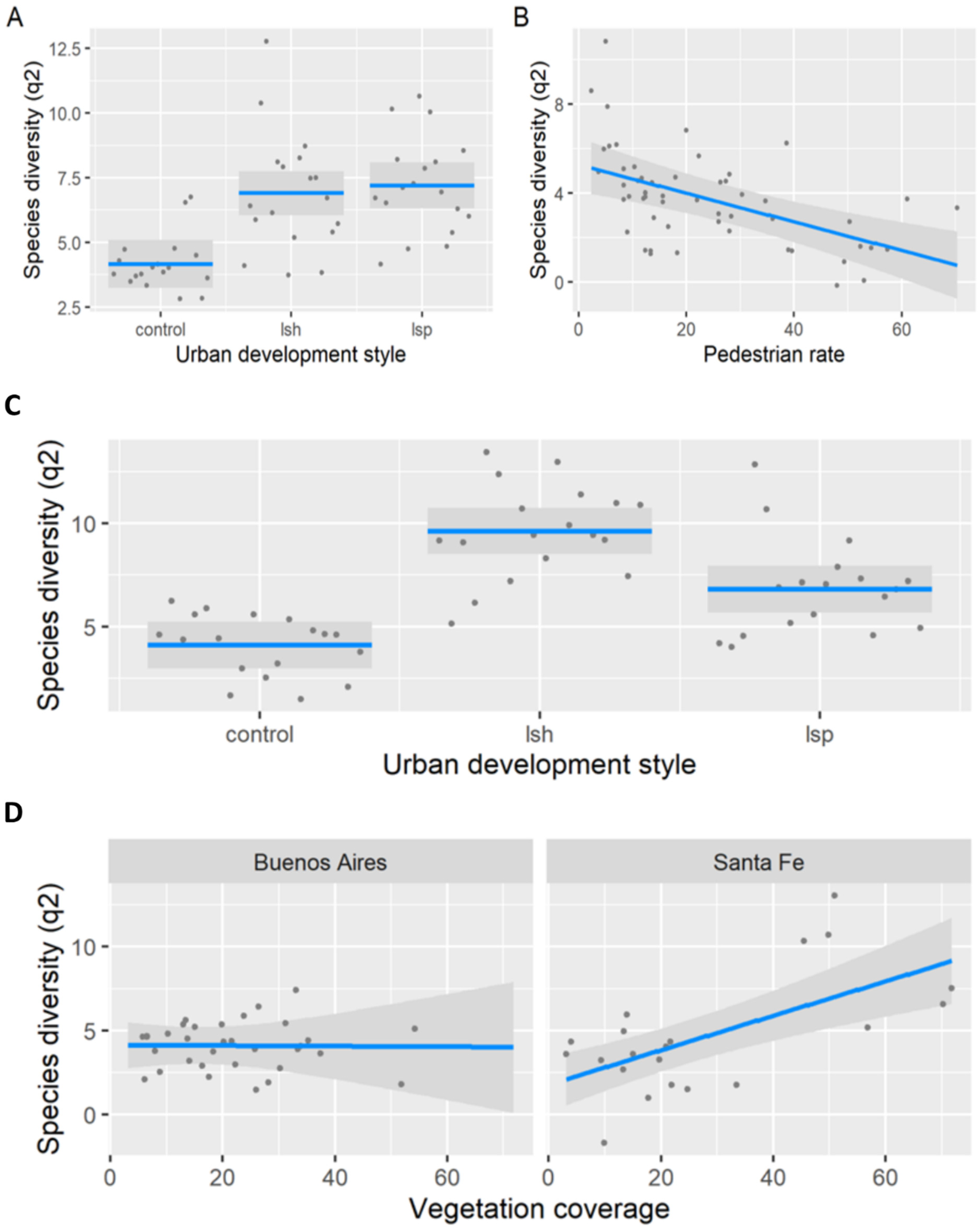
3.1. Taxonomic Diversity per Urban Development Style
3.2. Taxonomic Diversity per Sampling Unit
3.3. Taxonomic Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. World Urbanization Prospects 2018: Highlights; ST/ESA/SER.A/421; Department of Economic and Social Affairs, Population Division: New York, NY, USA, 2019. [Google Scholar]
- Liu, X.; Huang, Y.; Xu, X.; Li, X.; Li, X.; Ciais, P.; Lin, P.; Gong, K.; Ziegler, A.D.; Chen, A.; et al. High-spatiotemporal-resolution mapping of global urban change from 1985 to 2015. Nat. Sustain. 2020, 3, 564–570. [Google Scholar] [CrossRef]
- Grimm, N.B.; Faeth, S.H.; Golubiewski, N.E.; Redman, C.L.; Wu, J.; Bai, X.; Briggs, J.M. Global change and the ecology of cities. Science 2008, 319, 756–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seto, K.C.; Güneralp, B.; Hutyra, L.R. Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. Proc. Natl. Acad. Sci. USA 2012, 109, 16083–16088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pickett, S.T.; Cadenasso, M.L.; Grove, J.M.; Boone, C.G.; Groffman, P.M.; Irwin, E.; Kaushal, S.S.; Marshall, V.; McGrath, B.P.; Nilon, C.H.; et al. Urban ecological systems: Scientific foundations and a decade of progress. J. Environ. Manag. 2011, 92, 331–362. [Google Scholar] [CrossRef]
- Lowry, H.; Lill, A.; Wong, B.B. Behavioural responses of wildlife to urban environments. Biol. Rev. 2013, 88, 537–549. [Google Scholar] [CrossRef]
- Aronson, M.F.J.; La Sorte, F.A.; Nilon, C.H.; Katti, M.; Goddard, M.A.; Lepczyk, C.A.; Warren, P.S.; Williams, N.S.G.; Cilliers, S.; Clarckson, B.; et al. A global analysis of the impacts of urbanization on bird and plant diversity reveals key anthropogenic drivers. Proc. R. Soc. B 2014, 281, 20133330. [Google Scholar] [CrossRef]
- McKinney, M.L. Effects of urbanization on species richness: A review of plants and animals. Urban Ecosyst. 2008, 11, 161–176. [Google Scholar] [CrossRef]
- Norton, B.A.; Evans, K.L.; Warren, P.H. Urban biodiversity and landscape ecology: Patterns, processes and planning. Curr. Landsc. Ecol. Rep. 2016, 1, 178–192. [Google Scholar] [CrossRef] [Green Version]
- Lin, B.B.; Fuller, R.A. Sharing or sparing? How should we grow the world’s cities? J. Appl. Ecol. 2013, 50, 1161–1168. [Google Scholar] [CrossRef]
- Soga, M.; Yamaura, Y.; Koike, S.; Gaston, K.J. Land sharing vs. land sparing: Does the compact city reconcile urban development and biodiversity conservation? J. Appl. Ecol. 2014, 51, 1378–1386. [Google Scholar] [CrossRef]
- Guida-Johnson, B.; Faggi, A.M.; Zuleta, G.A. Effects of Urban Sprawl on Riparian Vegetation: Is Compact or Dispersed Urbanization Better for Biodiversity? River Res. Appl. 2017, 33, 959–969. [Google Scholar] [CrossRef]
- Caryl, F.M.; Lumsden, L.F.; van der Ree, R.; Wintle, B.A. Functional responses of insectivorous bats to increasing housing density support ‘land-sparing’ rather than ‘land-sharing’ urban growth strategies. J. Appl. Ecol. 2016, 53, 191–201. [Google Scholar] [CrossRef]
- Ibáñez-Álamo, J.D.; Morelli, F.; Benedetti, Y.; Rubio, E.; Jokimäki, J.; Pérez-Contreras, T.; Sprau, P.; Suhonen, J.; Tryjanowski, P.; Kaisanlahti-Jokimäki, M.-L.; et al. Biodiversity within the city: Effects of land sharing and land sparing urban development on avian diversity. Sci. Total. Environ. 2020, 707, 135477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villaseñor, N.R.; Escobar, M.A.H.; Hernández, H.J. Can Aggregated Patterns of Urban Woody Vegetation Cover Promote Greater Species Diversity, Richness and Abundance of Native Birds? Urban For. Urban Green. 2021, 61, 127102. [Google Scholar] [CrossRef]
- Sushinsky, J.R.; Rhodes, J.R.; Possingham, H.P.; Gill, T.K.; Fuller, R.A. How Should We Grow Cities to Minimize Their Biodiversity Impacts? Glob. Change Biol. 2013, 19, 401–410. [Google Scholar] [CrossRef]
- Leveau, L.M. Desde el árbol al bioma: Una solución multiescala para las aves urbanas. Hornero 2022, 37, 13–22. [Google Scholar] [CrossRef]
- Lepczyk, C.A.; La Sorte, F.A.; Aronson, M.F.; Goddard, M.A.; MacGregor-Fors, I.; Nilon, C.H.; Warren, P.S. Global patterns and drivers of urban bird diversity. In Ecology and Conservation of Birds in Urban Environments; Springer International Publishing: New York, NY, USA, 2017; pp. 13–33. [Google Scholar]
- Fuller, R.A.; Irvine, K.N.; Devine-Wright, P.; Warren, P.H.; Gaston, K.J. Psychological benefits of greenspace increase with biodiversity. Biol. Lett. 2007, 3, 390–394. [Google Scholar] [CrossRef] [Green Version]
- Luck, G.W.; Davidson, P.; Boxall, D.; Smallbone, L. Relations between Urban Bird and Plant Communities and Human Well-Being and Connection to Nature. Conserv. Biol. 2011, 25, 816–826. [Google Scholar] [CrossRef]
- Sekercioglu, C. Increasing awareness of avian ecological function. Trends Ecol. Evol. 2006, 21, 464–471. [Google Scholar] [CrossRef]
- Whelan, C.J.; Wenny, D.G.; Marquis, R.J. Ecosystem services provided by birds. Ann. N. Y. Acad. Sci. 2008, 1134, 25–60. [Google Scholar] [CrossRef]
- Leveau, L.M.; Villaseñor, N.R.; Lambertucci, S.A. Ornitología urbana en el Neotrópico: Estado de situación y desafíos. Hornero 2022, 37, 5–11. [Google Scholar] [CrossRef]
- Escobar-Ibáñez, J.F.; MacGregor-Fors, I. What’s New? An Updated Review of Avian Ecology in Urban Latin America. In Avian Ecology in Latin American Cityscapes; MacGregor-Fors, I., Escobar-Ibáñez, J.F., Eds.; Springer: Cham, Switzerland, 2017; pp. 11–31. [Google Scholar]
- Marzluff, J.M. A decadal review of urban ornithology and a prospectus for the future. IBIS 2016, 159, 1–13. [Google Scholar] [CrossRef]
- MacGregor-Fors, I.; Schondube, J.E. Gray vs. green urbanization: Relative importance of urban features for urban bird communities. Basic Appl. Ecol. 2011, 12, 372–381. [Google Scholar] [CrossRef]
- Silva, C.P.; García, C.E.; Estay, S.A.; Barbosa, O. Bird Richness and Abundance in Response to Urban Form in a Latin American City: Valdivia, Chile as a Case Study. PLoS ONE 2015, 10, e0138120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leveau, L.M.; Leveau, C.M.; Villegas, M.; Cursach, J.A.; Suazo, C.G. Bird communities along urbanization gradients: A comparative analysis among three neotropical cities. Ornitol. Neotrop. 2017, 28, 77–87. [Google Scholar] [CrossRef]
- Leveau, L.M.; Ruggiero, A.; Matthews, T.J.; Bellocq, M.I. A global consistent positive effect of urban green area size on bird richness. Avian Res. 2019, 10, 30. [Google Scholar] [CrossRef] [Green Version]
- Melo, M.A.; Campos-Silva, L.A.; Piratelli, A.J. Red clay roof and NDVI drive changes in bird species composition and functional evenness in housing areas of São Paulo megacity, Brazil. Hornero 2022, 37, 87–103. [Google Scholar] [CrossRef]
- De Camargo Barbosa, K.V.; Rodewald, A.D.; Ribeiro, M.C.; Jahn, A.E. Noise level and water distance drive resident and migratory bird species richness within a Neotropical megacity. Landsc. Urban Plan. 2020, 197, 103769. [Google Scholar] [CrossRef]
- Leveau, L.M.; Leveau, C.M. Street design in suburban areas and its impact on bird communities: Considering different diversity facets over the year. Urban For. Urban Green. 2020, 48, 126578. [Google Scholar] [CrossRef]
- Melo, M.A.; Sanches, P.M.; Filho, D.F.S.; Piratelli, A.J. Influence of habitat type and distance from source area on bird taxonomic and functional diversity in a Neotropical megacity. Urban Ecosyst. 2022, 25, 545–560. [Google Scholar] [CrossRef]
- Leveau, L.M.; Leveau, C.M. Does Urbanization Affect the Seasonal Dynamics of Bird Communities in Urban Parks? Urban Ecosyst. 2016, 19, 631–647. [Google Scholar] [CrossRef]
- Leveau, L.M.; Bocelli, M.L.; Quesada-Acuña, S.G.; González-Lagos, C.; Tapia, P.G.; Dri, G.F.; Delgado, C.A.; Garitano-Zavala, A.; Campos, J.; Benedetti, Y.; et al. Bird diversity-environment relationships in urban parks and cemeteries of the Neotropics during breeding and non-breeding seasons. PeerJ 2022, 10, e14496. [Google Scholar] [CrossRef]
- Hilden, O. Habitat Selection in Birds: A Review. Ann. Zool. Fenn. 1965, 2, 53–75. [Google Scholar]
- Sagario, M.C.; Cueto, V.R. Seasonal Space use and Territory Size of Resident Sparrows in the Central Monte Desert, Argentina. Ardeola 2014, 61, 153–159. [Google Scholar] [CrossRef]
- Oyarzabal, M.; Clavijo, J.; Oakley, L.; Biganzoli, F.; Tognetti, P.; Barberis, I.; Maturo, H.M.; Aragón, R.; Campanello, P.I.; Prado, D.; et al. Unidades de vegetación de la Argentina. Ecol. Austral 2018, 28, 40–63. [Google Scholar] [CrossRef] [Green Version]
- Beninde, J.; Veith, M.; Hochkirch, A. Biodiversity in cities needs space: A meta-analysis of factors determining intra-urban biodiversity variation. Ecol. Lett. 2015, 18, 581–592. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Mason, J.; Moorman, C.; Hess, G.; Sinclair, K. Designing suburban greenways to provide habitat for forest-breeding birds. Landsc. Urban Plan. 2007, 80, 153–164. [Google Scholar] [CrossRef]
- Sieving, K.E.; Willson, M.F.; De Santo, T.L. Defining Corridor Functions for Endemic Birds in Fragmented South-Temperate Rainforest. Conserv. Biol. 2000, 14, 1120–1132. [Google Scholar] [CrossRef]
- Tremblay, M.A.; Clair, C.C.S. Factors affecting the permeability of transportation and riparian corridors to the movements of songbirds in an urban landscape. J. Appl. Ecol. 2009, 46, 1314–1322. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 20 January 2023).
- Bibby, C.J.; Hill, D.A.; Burgess, N.D.; Mustoe, S. Bird Census Techniques, 2nd ed.; Academic Press: London, UK, 2000. [Google Scholar]
- Ralph, J.C.; Droege, S.; Sauer, J.R. Managing and Monitoring Birds Using Point Counts: Standards and Applications. In Monitoring bird populations by point counts; Ralph, J.C., Sauer, J.R., Droege, S., Eds.; Department of Agriculture, Forest Service, Pacific Southwest Research Station: Albany, CA, USA, 1995; pp. 161–168. [Google Scholar]
- DeGraaf, R.M.; Geis, A.D.; Healy, P.A. Bird population and habitat surveys in urban areas. Landsc. Urban Plan. 1991, 21, 181–188. [Google Scholar] [CrossRef]
- Zanaga, D.; Van De Kerchove, R.; De Keersmaecker, W.; Souverijns, N.; Brockmann, C.; Quast, R.; Wevers, J.; Grosu, A.; Paccini, A.; Vergnaud, S.; et al. ESA WorldCover 10 m 2020 v100. 2021. Available online: https://doi.org/10.5281/zenodo.5571936 (accessed on 20 January 2023). [CrossRef]
- Hijmans, R. raster: Geographic Data Analysis and Modeling. R Package Version 3.6-11. 2022. Available online: https://CRAN.R-project.org/package=raster (accessed on 22 December 2022).
- Bivand, R.; Rundel, C. rgeos: Interface to Geometry Engine—Open Source (‘GEOS’). R Package Version 0.6-1. 2022. Available online: https://CRAN.R-project.org/package=rgeos (accessed on 22 December 2022).
- Hesselbarth, M.H.K.; Sciaini, M.; With, K.A.; Wiegand, K.; Nowosad, J. Landscapemetrics: An open-source R tool to calculate landscape metrics. Ecography 2019, 42, 1648–1657. [Google Scholar] [CrossRef] [Green Version]
- ToolsDev. Sound Meter–Decibel Meter & Noise Meter. 2016. Available online: https://play.google.com/store/apps/details?id=app.tools.soundmeter.decibel.noisedetector&hl=en_US (accessed on 14 February 2023).
- Jost, L.; González-Oreja, J. Midiendo la diversidad biológica: Más allá del índice de Shannon. Acta Zool. Lilloana 2012, 56, 3–14. [Google Scholar]
- Li, D. hillR: Taxonomic, functional, and phylogenetic diversity and similarity through Hill Numbers. J. Open Source Softw. 2018, 3, 1041. [Google Scholar] [CrossRef]
- Chao, A.; Chiu, C.-H.; Jost, L. Unifying Species Diversity, Phylogenetic Diversity, Functional Diversity, and Related Similarity and Differentiation Measures Through Hill Numbers. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 297–324. [Google Scholar] [CrossRef] [Green Version]
- Chao, A.; Jost, L. Coverage-based rarefaction and extrapolation: Standardizing samples by completeness rather than size. Ecology 2012, 93, 2533–2547. [Google Scholar] [CrossRef]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef] [Green Version]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [Green Version]
- Fox, J.; Weisberg, S. An {R} Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019; Available online: https://socialsciences.mcmaster.ca/jfox/Books/Companion/ (accessed on 14 February 2023).
- Lefcheck, J.S. piecewiseSEM: Piecewise structural equation modelling in r for ecology, evolution, and systematics. Methods Ecol. Evol. 2016, 7, 573–579. [Google Scholar] [CrossRef]
- Breheny, P.; Burchett, W. Visualization of Regression Models Using visreg. R J. 2017, 9, 56–71. [Google Scholar] [CrossRef]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. R Package Version 0.4.6. 2022. Available online: https://CRAN.R-project.org/package=DHARMa (accessed on 22 December 2022).
- Legendre, P.; Anderson, M.J. Distance-Based Redundancy Analysis: Testing Multispecies Responses in Multifactorial Ecological Experiments. Ecol. Monogr. 1999, 69, 1–24. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. Vegan: Community Ecology Package. R Package Version 2.6-2. 2022. Available online: https://CRAN.R-project.org/package=vegan (accessed on 22 December 2022).
- Jupke, J.F.; Schäfer, R.B. Should Ecologists Prefer Model- over Distance-Based Multivariate Methods? Ecol. Evol. 2020, 10, 2417–2435. [Google Scholar] [CrossRef] [PubMed]
- Baldaccini, N.E.; Giunchi, D.; Mongini, E.; Ragionieri, L. Foraging Flights of Wild Rock Doves (Columba l. livia): A Spatio-Temporal Analysis. Ital. J. Zool. 2000, 67, 371–377. [Google Scholar] [CrossRef] [Green Version]
- Murton, R.K.; Bucher, E.H.; Nores, M.; Reartes, J. The Ecology of the Eared Dove (Zenaida auriculata) in Argentina. Condor 1974, 76, 80–88. [Google Scholar] [CrossRef]
- Cristaldi, M.A.; Giraudo, A.R.; Arzamendia, V.; Bellini, G.P.; Claus, J. Urbanization impacts on the trophic guild composition of bird communities. J. Nat. Hist. 2017, 51, 2385–2404. [Google Scholar] [CrossRef]
- Curzel, F.; Leveau, L. Bird Taxonomic and Functional Diversity in Three Habitats in Buenos Aires City, Argentina. Birds 2021, 2, 217–229. [Google Scholar] [CrossRef]
- Lucero, M.M.; Brandán, Z.J.; Chani, J.M. Composición y Variación Anual de La Avifauna de Los Tres Grandes Parques Urbanos de San Miguel de Tucumán (Tucumán, Argentina). Acta Zool. Lilloana 2005, 49, 43–48. [Google Scholar]
- Fernandes, F.R.; Cruz, L.D.; Rodrigues, A.A.F. Molt Cycle of the Gray-Breasted Martin (Hirundinidae: Progne chalybea) in a Wintering Area in Maranhão, Brazil. Rev. Bras. Ornitol. 2007, 15, 436–438. [Google Scholar]
- Leveau, L.; Leveau, C. Comunidades de Aves En Un Gradiente Urbano de La Ciudad de Mar Del Plata, Argentina. Hornero 2004, 19, 13–21. [Google Scholar]
- Marreis, Í.T.; Sander, M. Preferência Ocupacional de Ninhos de João-de-Barro (Furnarius rufus, Gmelin) Entre Área Urbanizada e Natural. Biodivers. Pampeana 2006, 4, 29–31. [Google Scholar]
- Leveau, L.M. Consistency in bird community assembly over medium-term along rural-urban gradients in Argentina. Ecol. Process. 2021, 10, 34. [Google Scholar] [CrossRef]
- Perepelizin, P.V.; Faggi, A.M. Diversidad de Aves En Tres Barrios de La Ciudad de Buenos Aires, Argentina. Multequina 2009, 18, 71–85. [Google Scholar]
- Jokimäki, J.; Suhonen, J.; Benedetti, Y.; Diaz, M.; Kaisanlahti-Jokimäki, M.-L.; Morelli, F.; Pérez-Contreras, T.; Rubio, E.; Sprau, P.; Tryjanowski, P.; et al. Land-Sharing vs. Land-Sparing Urban Development Modulate Predator–Prey Interactions in Europe. Ecol. Appl. 2020, 30, e02049. [Google Scholar] [CrossRef] [PubMed]
- Leveau, M.L.; Zuria, I. Flocking the City: Avian Demography and Population Dynamics in Urban Latin America. In Avian Ecology in Latin American Cityscapes; Escobar-Ibáñez, J.F., MacGregor-Fors, I., Eds.; Springer: Berlin, Germany, 2017; pp. 57–78. [Google Scholar] [CrossRef]
- Cristaldi, M.A.; Sarquis, J.A.; Leveau, L.M.; Giraudo, A.R. Bird community responses to urbanization in a medium-sized Argentine city: Santo Tomé (Santa Fe Province) as a case study. Hornero 2022, 37, 105–120. [Google Scholar] [CrossRef]
- Rolando, A. On the ecology of home range in birds. Rev. Ecol. Terre Vie Soc. Natl. Prot. Nat. 2002, 57, 53–73, hal-03530065. [Google Scholar] [CrossRef]
- Blumstein, D.T.; Fernández-Juricic, E.; Zollner, P.A.; Garity, S.C. Inter-specific variation in avian responses to human disturbance. J. Appl. Ecol. 2005, 42, 943–953. [Google Scholar] [CrossRef]
- Mikula, P. Pedestrian Density Influences Flight Distances of Urban Birds. Ardea 2014, 102, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Curzel, F.E.; Bellocq, M.I.; Leveau, L.M. Local and landscape features of wooded streets influenced bird taxonomic and functional diversity. Urban For. Urban Green. 2021, 66, 127369. [Google Scholar] [CrossRef]
- Fernández-Juricic, E.; Jokimäki, J. A habitat island approach to conserving birds in urban landscapes: Case studies from southern and northern Europe. Biodivers. Conserv. 2001, 10, 2023–2043. [Google Scholar] [CrossRef]
- Fernández-Juricic, E.; Tellería, J.L. Effects of Human Disturbance on Spatial and Temporal Feeding Patterns of Blackbird Turdus Merula in Urban Parks in Madrid, Spain. Bird Study 2000, 47, 13–21. [Google Scholar] [CrossRef]
- Seas, C.; Quesada-Acuña, S.G.; Barrientos, Z. Efecto de la infraestructura y usuarios de parques urbanos en las poblaciones de la Paloma Columba livia (Columbiformes: Columbidae) en Costa Rica (2014–2020). Hornero 2022, 37, 237–242. [Google Scholar] [CrossRef]
- Haas, A.R.; Kross, S.M.; Kneitel, J.M. Avian community composition, but not richness, differs between urban and exurban parks. J. Urban Ecol. 2020, 6, juaa028. [Google Scholar] [CrossRef]
- Ives, C.D.; Lentini, P.E.; Threlfall, C.G.; Ikin, K.; Shanahan, D.F.; Garrard, G.E.; Bekessy, S.A.; Fuller, R.A.; Mumaw, L.; Rayner, L.; et al. Cities are hotspots for threatened species. Glob. Ecol. Biogeogr. 2016, 25, 117–126. [Google Scholar] [CrossRef]
- Arzamendia, V.; Giraudo, A.R. Influence of large South American rivers of the Plata Basin on distributional patterns of tropical snakes: A panbiogeographical analysis. J. Biogeogr. 2009, 36, 1739–1749. [Google Scholar] [CrossRef]
- Rossetti, M.A.; Giraudo, A.R. Comunidades de aves de bosques fluviales habitados y no habitados por el hombre en el río Paraná medio, Argentina. Hornero 2003, 18, 89–96. [Google Scholar]
- Croci, S.; Butet, A.; Clergeau, P. Does urbanization filter birds on the basis of their biological traits? Condor 2008, 110, 223–240. [Google Scholar] [CrossRef]
- Leveau, L.M. Bird traits in urban–rural gradients: How many functional groups are there? J. Ornithol. 2013, 154, 655–662. [Google Scholar] [CrossRef]
- Leveau, L.M.; Gorleri, F.C.; Roesler, I.; González-Táboas, F. What makes an urban raptor? IBIS 2022, 164, 1213–1226. [Google Scholar] [CrossRef]
- Leveau, L.M. Primary productivity and habitat diversity predict bird species richness and composition along urban-rural gradients of central Argentina. Urban For. Urban Green. 2019, 43, 126349. [Google Scholar] [CrossRef]
- Escobar-Ibáñez, J.F.; Rueda-Hernández, R.; MacGregor-Fors, I. The Greener the Better! Avian Communities Across a Neotropical Gradient of Urbanization Density. Front. Ecol. Evol. 2020, 8, 500791. [Google Scholar] [CrossRef]
- Baxendale, C.; Buzai, G.D. Dinámica de crecimiento urbano y pérdida de suelos productivos en el Gran Buenos Aires (Argentina), 1869–2011. Análisis espacial basado en sistemas de información geográfica. Ser. Geogr. 2011, 17, 77–95. [Google Scholar]
- Yang, G.; Xu, J.; Wang, Y.; Wang, X.; Pei, E.; Yuan, X.; Li, H.; Ding, Y.; Wang, Z. Evaluation of microhabitats for wild birds in a Shanghai urban area park. Urban For. Urban Green. 2015, 14, 246–254. [Google Scholar] [CrossRef]
- Gorosito, C.A.; Cueto, V.R. Do small cities affect bird assemblages? An evaluation from Patagonia. Urban Ecosyst. 2020, 23, 289–300. [Google Scholar] [CrossRef]
- Aronson, M.F.; Lepczyk, C.A.; Evans, K.L.; A Goddard, M.; Lerman, S.B.; MacIvor, J.S.; Nilon, C.H.; Vargo, T. Biodiversity in the city: Key challenges for urban green space management. Front. Ecol. Environ. 2017, 15, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Goddard, M.A.; Dougill, A.J.; Benton, T.G. Scaling up from gardens: Biodiversity conservation in urban environments. Trends Ecol. Evol. 2010, 25, 90–98. [Google Scholar] [CrossRef]
- Graviola, G.R.; Ribeiro, M.C.; Pena, J.C. Reconciling humans and birds when designing ecological corridors and parks within urban landscapes. AMBIO 2021, 51, 253–268. [Google Scholar] [CrossRef]
- Rohde, C.L.E.; Kendle, A.D. Human well-being, natural landscapes and wildlife in urban areas. A review. Engl. Nat. Sci. 1994, 22. [Google Scholar]
- Sebba, R. The Landscapes of Childhood: The Reflection of Childhood’s Environment in Adult Memories and in Children’s Attitudes. Environ. Behav. 1991, 23, 395–422. [Google Scholar] [CrossRef]
- Aerts, R.; Honnay, O.; Van Nieuwenhuyse, A. Biodiversity and human health: Mechanisms and evidence of the positive health effects of diversity in nature and green spaces. Br. Med Bull. 2018, 127, 5–22. [Google Scholar] [CrossRef] [Green Version]
- Dallimer, M.; Irvine, K.N.; Skinner, A.M.J.; Davies, Z.G.; Rouquette, J.R.; Maltby, L.; Warren, P.H.; Armsworth, P.; Gaston, K.J. Biodiversity and the Feel-Good Factor: Understanding Associations between Self-Reported Human Well-being and Species Richness. Bioscience 2012, 62, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Hedblom, M.; Heyman, E.; Antonsson, H.; Gunnarsson, B. Bird song diversity influences young people’s appreciation of urban landscapes. Urban For. Urban Green. 2014, 13, 469–474. [Google Scholar] [CrossRef]
- Methorst, J.; Rehdanz, K.; Mueller, T.; Hansjürgens, B.; Bonn, A.; Böhning-Gaese, K. The importance of species diversity for human well-being in Europe. Ecol. Econ. 2021, 181, 106917. [Google Scholar] [CrossRef]
- Lerman, S.B.; Narango, D.L.; Avolio, M.L.; Bratt, A.R.; Engebretson, J.M.; Groffman, P.M.; Hall, S.J.; Heffernan, J.B.; Hobbie, S.E.; Larson, K.L.; et al. Residential yard management and landscape cover affect urban bird community diversity across the continental USA. Ecol. Appl. 2021, 31, e02455. [Google Scholar] [CrossRef] [PubMed]


| Scientific Name | Santa Fe City | Buenos Aires City | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Land-Sharing | Land-Sparing | Control | Land-Sharing | Land-Sparing | |||||||
| NBS | BS | NBS | BS | NBS | BS | NBS | BS | NBS | BS | NBS | BS | |
| Columba livia | 12 | 5 | 27 | 32 | 4 | 8 | 50 | 92 | 7 | 12 | 31 | 32 |
| Patagioenas maculosa | 2 | 0 | 3 | 4 | 10 | 5 | 0 | 0 | 0 | 0 | 2 | 0 |
| Patagioenas picazuro | 0 | 0 | 3 | 2 | 3 | 2 | 3 | 5 | 12 | 10 | 32 | 12 |
| Leptotila verreauxi | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Zenaida auriculata | 17 | 24 | 20 | 26 | 26 | 46 | 7 | 12 | 11 | 25 | 17 | 49 |
| Columbina picui | 0 | 1 | 1 | 1 | 16 | 2 | 3 | 2 | 0 | 0 | 0 | 1 |
| Guira guira | 8 | 0 | 3 | 8 | 5 | 8 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tapera naevia | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chlorostilbon lucidus | 0 | 3 | 0 | 2 | 0 | 1 | 0 | 1 | 0 | 3 | 0 | 1 |
| Hylocharis chrysura | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 2 | 2 | 1 | 1 |
| Vanellus chilensis | 0 | 0 | 2 | 3 | 3 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cathartes aura | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rostrhamus sociabilis | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rupornis magnirostris | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Parabuteo unicinctus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 3 | 0 | 1 |
| Athene cunicularia | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Picumnus cirratus | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Melanerpes cactorum | 0 | 0 | 2 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dryobates mixtus | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| Colaptes melanochloros | 0 | 0 | 0 | 2 | 3 | 1 | 0 | 0 | 1 | 2 | 2 | 1 |
| Colaptes campestris | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Caracara plancus | 2 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 2 | 2 | 2 |
| Falco sparverius | 0 | 0 | 1 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Myiopsitta monachus | 4 | 2 | 15 | 7 | 11 | 17 | 2 | 0 | 24 | 7 | 22 | 12 |
| Brotogeris chiriri | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 4 | 21 | 6 |
| Amazona aestiva | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 3 | 2 |
| Pyrrhura frontalis | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 1 | 4 | 14 | 12 | 5 |
| Pyrrhura molinae | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Aratinga nenday | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 5 | 4 |
| Psittacara leucophthalmus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 11 | 0 | 8 |
| Taraba major | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lepidocolaptes angustirostris | 0 | 0 | 2 | 2 | 1 | 1 | 0 | 0 | 2 | 1 | 1 | 1 |
| Furnarius rufus | 5 | 5 | 6 | 5 | 12 | 8 | 2 | 2 | 5 | 9 | 5 | 4 |
| Phacellodomus ruber | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pseudoseisura lophotes | 0 | 0 | 3 | 1 | 3 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Schoeniophylax phryganophilus | 0 | 0 | 2 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Camptostoma obsoletum | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Serpophaga subcristata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 1 | 1 |
| Serpophaga griseicapilla | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pitangus sulphuratus | 3 | 5 | 4 | 6 | 4 | 9 | 3 | 3 | 4 | 5 | 3 | 3 |
| Machetornis rixosa | 1 | 1 | 2 | 2 | 1 | 3 | 0 | 0 | 1 | 3 | 1 | 4 |
| Myiodynastes maculatus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Tyrannus melancholicus | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 0 |
| Tyrannus savana | 0 | 0 | 0 | 2 | 0 | 3 | 0 | 0 | 0 | 1 | 0 | 1 |
| Sublegatus modestus | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cyclarhis gujanensis | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Progne tapera | 0 | 0 | 0 | 5 | 0 | 4 | 0 | 0 | 0 | 1 | 0 | 1 |
| Progne chalybea | 0 | 4 | 0 | 6 | 0 | 7 | 0 | 5 | 0 | 6 | 0 | 4 |
| Tachycineta leucorrhoa | 0 | 1 | 3 | 2 | 0 | 4 | 0 | 1 | 5 | 5 | 0 | 3 |
| Troglodytes aedon | 1 | 3 | 3 | 3 | 2 | 2 | 2 | 2 | 3 | 4 | 2 | 4 |
| Polioptila dumicola | 0 | 0 | 3 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 1 |
| Turdus rufiventris | 1 | 0 | 2 | 2 | 2 | 2 | 3 | 4 | 11 | 7 | 12 | 8 |
| Turdus amaurochalinus | 0 | 0 | 5 | 1 | 2 | 1 | 0 | 0 | 0 | 1 | 0 | 1 |
| Mimus saturninus | 4 | 2 | 5 | 2 | 3 | 0 | 0 | 1 | 4 | 2 | 2 | 3 |
| Mimus triurus | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Sturnus vulgaris | 0 | 0 | 4 | 2 | 0 | 2 | 0 | 1 | 0 | 3 | 7 | 16 |
| Passer domesticus | 13 | 18 | 15 | 9 | 11 | 12 | 6 | 5 | 2 | 5 | 12 | 13 |
| Spinus magellanicus | 0 | 1 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 3 | 2 | 1 |
| Zonotrichia capensis | 2 | 2 | 3 | 2 | 2 | 3 | 1 | 1 | 1 | 2 | 1 | 1 |
| Icterus pyrrhopterus | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Molothrus rufoaxillaris | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 2 | 0 | 3 |
| Molothrus bonariensis | 5 | 7 | 5 | 5 | 21 | 15 | 1 | 0 | 1 | 4 | 12 | 1 |
| Agelaioides badius | 0 | 0 | 4 | 4 | 4 | 0 | 0 | 0 | 7 | 3 | 7 | 4 |
| Geothlypis aequinoctialis | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Setophaga pitiayumi | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 |
| Piranga flava | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 2 | 0 | 0 |
| Sicalis flaveola | 0 | 1 | 1 | 3 | 3 | 2 | 0 | 0 | 2 | 1 | 0 | 2 |
| Sicalis luteola | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Saltator coerulescens | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Paroaria coronata | 0 | 2 | 0 | 4 | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 1 |
| Paroaria capitata | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Thraupis sayaca | 0 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 2 |
| Response Variable | Predictor | Estimate | Standard Error | z Test/ t-test | p |
|---|---|---|---|---|---|
| (a) Non-breeding season | |||||
| Species richness | Intercept | 3.23 | 0.23 | 13.6 | <0.001 |
| Landscape_control | −0.69 | 0.12 | −5.66 | <0.001 | |
| Landscape_land-sharing | −0.16 | 0.1 | −1.63 | 0.1 | |
| Pedestrians | −0.01 | 0.004 | −3.77 | <0.001 | |
| Vegetation | −0.01 | 0.01 | −2.26 | 0.02 | |
| City_SantaFe | −0.24 | 0.2 | −1.17 | 0.24 | |
| Vegetation:City_SantaFe | 0.01 | 0.01 | 2.23 | 0.026 | |
| Shannon diversity | Intercept | 12.39 | 1.55 | 7.97 | <0.001 |
| Landscape_control | −4.29 | 0.79 | −5.4 | <0.001 | |
| Landscape_land-sharing | −0.6 | 0.75 | −0.81 | 0.42 | |
| Pedestrians | −0.09 | 0.03 | −3.55 | <0.001 | |
| Vegetation | −0.07 | 0.04 | −1.87 | 0.07 | |
| City_SantaFe | −2.19 | 1.3 | −1.68 | 0.1 | |
| Vegetation:City_SantaFe | 0.11 | 0.04 | 2.66 | 0.01 | |
| Simpson diversity | Intercept | 8.32 | 0.59 | 14.07 | <0.001 |
| Landscape_control | −3.03 | 0.62 | −4.93 | <0.001 | |
| Landscape_land-sharing | −0.29 | 0.63 | −0.47 | 0.64 | |
| Pedestrians | −0.06 | 0.02 | −4.17 | <0.001 | |
| (b) Breeding season | |||||
| Species richness | Intercept | 3.17 | 0.07 | 44.97 | <0.001 |
| Landscape_control | −0.75 | 0.09 | −7.92 | <0.001 | |
| Landscape_land-sharing | −5 | 0.07 | −0.66 | 0.51 | |
| Pedestrians | −0.01 | 0.003 | −3.24 | 0.001 | |
| Shannon diversity | Intercept | 8.63 | 1.36 | 6.36 | <0.001 |
| Landscape_control | −2.21 | 1.52 | −1.45 | 0.15 | |
| Landscape_land-sharing | 5.25 | 1.87 | 2.8 | 0.007 | |
| Vegetation | 0.04 | 0.05 | 0.9 | 0.37 | |
| City_SantaFe | −2.02 | 1.36 | −1.48 | 0.14 | |
| Landscape_control:Vegetation | −0.13 | 0.06 | −2.07 | 0.04 | |
| Landscape_land-sharing:Vegetation | −0.09 | 0.06 | −1.6 | 0.11 | |
| Vegetation:City_SantaFe | 0.13 | 0.05 | 2.67 | 0.01 | |
| Simpson diversity | Intercept | 6.84 | 1.02 | 6.69 | <0.001 |
| Landscape_control | −2.71 | 0.79 | −3.42 | 0.001 | |
| Landscape_land-sharing | 2.82 | 0.71 | 3.99 | <0.001 | |
| Vegetation | −0.002 | 0.03 | −0.05 | 0.96 | |
| City_SantaFe | −2.35 | 1.11 | −2.12 | 0.04 | |
| Vegetation:City_SantaFe | 0.1 | 0.04 | 2.67 | 0.01 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cristaldi, M.A.; Godoy, I.N.; Leveau, L.M. Responses of Urban Bird Assemblages to Land-Sparing and Land-Sharing Development Styles in Two Argentinian Cities. Animals 2023, 13, 894. https://doi.org/10.3390/ani13050894
Cristaldi MA, Godoy IN, Leveau LM. Responses of Urban Bird Assemblages to Land-Sparing and Land-Sharing Development Styles in Two Argentinian Cities. Animals. 2023; 13(5):894. https://doi.org/10.3390/ani13050894
Chicago/Turabian StyleCristaldi, Maximiliano A., Ianina N. Godoy, and Lucas M. Leveau. 2023. "Responses of Urban Bird Assemblages to Land-Sparing and Land-Sharing Development Styles in Two Argentinian Cities" Animals 13, no. 5: 894. https://doi.org/10.3390/ani13050894




