A Comparative Study on Changes in Protein, Lipid and Meat-Quality Attributes of Camel Meat, Beef and Sheep Meat (Mutton) during Refrigerated Storage
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Meat Samples
2.2. Determination of Meat Quality during Storage
2.2.1. The pH
2.2.2. Drip Loss
2.2.3. Colour
2.2.4. Textural Properties
2.3. Characterisation of Protein Fraction
2.3.1. Protein Analysis by Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.3.2. Determination of Protein Extractability
2.3.3. Determination of Protein Solubility
2.4. Determination of Lipid Oxidation Products
2.4.1. Peroxide Value (PV)
2.4.2. Thiobarbituric Acid Reactive Substances (TBARS)
2.5. Determination of Meat Pigments
2.5.1. Total Haem Pigment Content Analysis
2.5.2. Haemoglobin Content Analysis
2.5.3. Myoglobin Content Analysis
2.6. Statistical Analysis
3. Results
3.1. Changes in pH
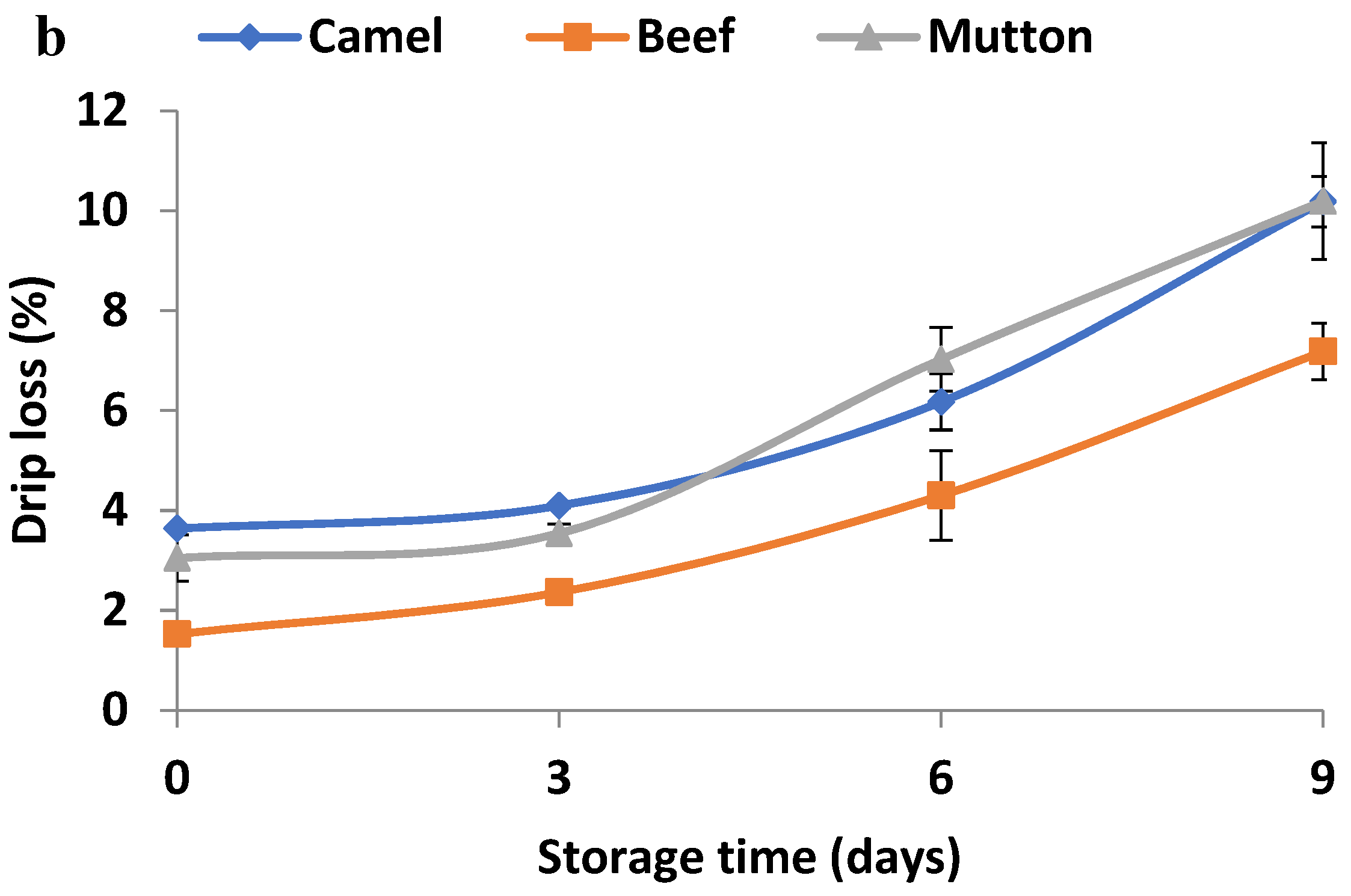
3.2. Changes in Drip Loss
3.3. Changes in Protein Extractability and Solubility
3.4. Changes in Colour Parameters
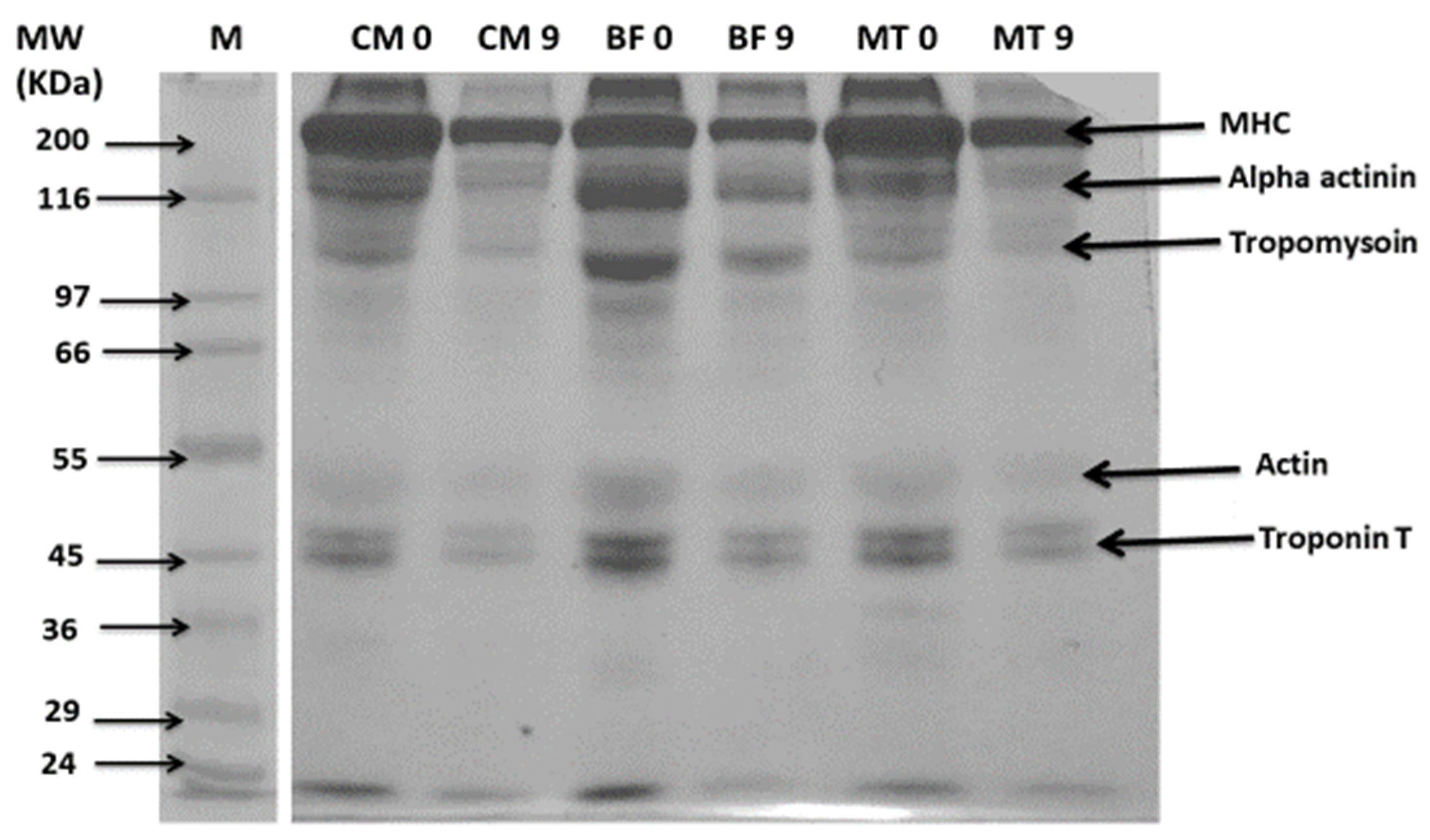
3.5. Changes in Protein Pattern by SDS-PAGE
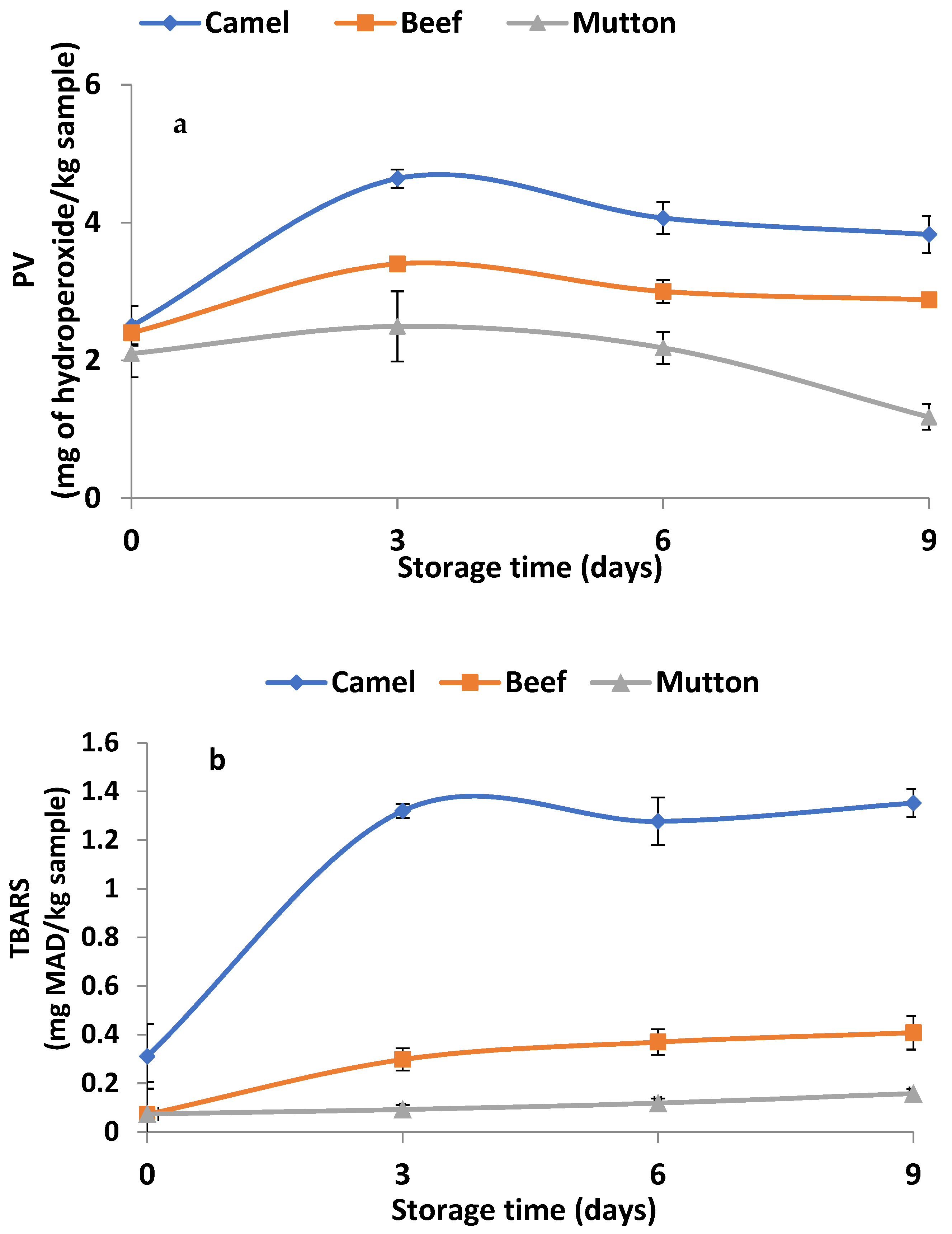
3.6. Changes in Lipid Oxidation Products
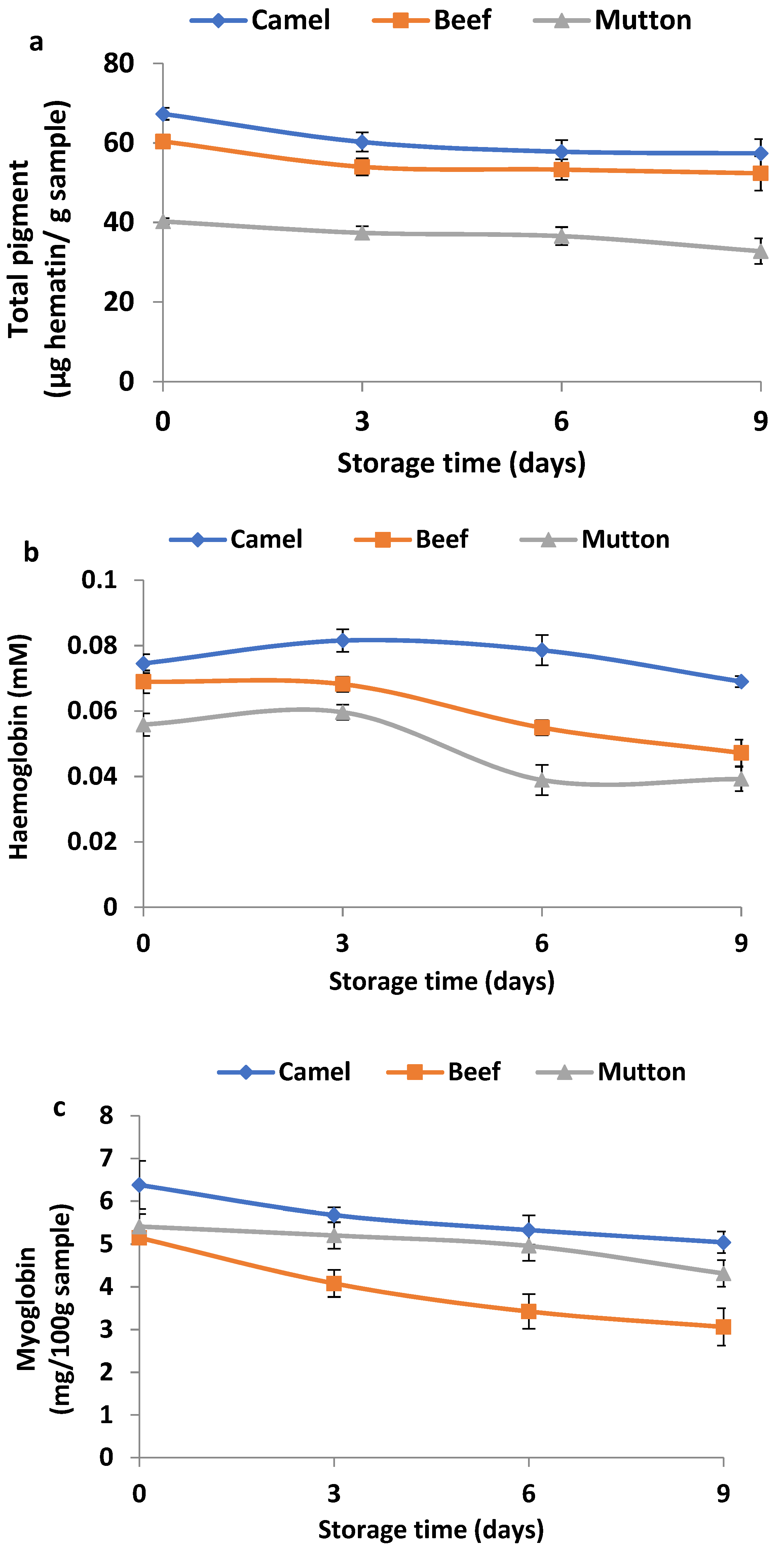
3.7. Changes in Total Pigment, Haemoglobin and Myoglobin
3.8. Changes in Textural Properties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chibuike, C.U.; Ashton, H. Meat proteome as source of functional biopeptides. Food Res. Int. 2013, 54, 1021–1032. [Google Scholar]
- Cabrera, M.C.; Saadoun, A. An overview of the nutritional value of beef and lamb meat from South America. Meat Sci. 2014, 98, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Vannice, G.; Rasmussen, H. Position of the Academy of Nutrition and Dietetics: Dietary fatty acids for healthy adults. J. Acad. Nutr. Diet. 2014, 114, 136–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maqsood, S.; Abushelaibi, S.; Manheem, K.; Kadim, I.T. Characterization of the lipid and protein fraction of fresh camel meat and the associated changes during refrigerated storage. J. Food Comp. Anal. 2015, 41, 212–220. [Google Scholar] [CrossRef]
- Al-Ani, F.K. Camel Management and Diseases, 1st ed.; Al-Shraq Printing Press and Dar Ammar Book Publishing: Amman, Jordan, 2004. [Google Scholar]
- Dawood, A.; Alkanhal, M.A. Nutrient composition of Najidi camel meat. Meat Sci. 1995, 39, 71–78. [Google Scholar] [CrossRef]
- Bekhit, A.E.; Farouk, M.M. Meat and Meat Products: Nutritive and Health Value of Camel Meat; Hamilton, CAB International: Oxfordshire, UK, 2013; pp. 205–223. [Google Scholar]
- Soltanizadeh, N.; Kadivar, M.; Keramat, J.; Fazilati, M. Comparison of fresh beef and camel meat proteolysis during cold storage. Meat Sci. 2008, 80, 892–895. [Google Scholar] [CrossRef]
- Kadim, I.T.; Mahgoub, O.; Al-Marzooqi, W.; Al-Zadgali, S.; Annamali, K.; Mansour, M.H. Effects of age on composition and quality of muscle Longissimusthoracis of the Omani Arabian Camel (Camelus dromedarius). Meat Sci. 2006, 73, 619–625. [Google Scholar] [CrossRef]
- Kadim, I.T.; Mahgoub, O.; Purchas, R.W. A review of the growth, and of the carcass and meat quality characteristics of the one-humped camel (Camelus dromedarius). Meat Sci. 2008, 80, 555–569. [Google Scholar] [CrossRef]
- Maqsood, S.; Manheem, K.; Gani, A.; Abushelaibi, A. Degradation of myofibrillar, sarcoplasmic and connective tissue proteins by plant proteolytic enzymes and their impact on camel meat tenderness. J. Food Sci. Technol. 2018, 55, 3427–3438. [Google Scholar] [CrossRef]
- Maqsood, S.; Abushelaibi, S.; Manheem, K.; Rashedi, A.A.; Kadim, I.T. Lipid oxidation, protein degradation, microbial and sensorial quality of camel meat as influenced by phenolic compounds. LWT-Food Sci. Technol. 2015, 63, 953–959. [Google Scholar] [CrossRef]
- Honikel, K.O. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 1998, 49, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, S.; Benjakul, S. Synergistic effect of tannic acid and modified atmospheric packaging on the prevention of lipid oxidation and quality losses of refrigerated striped catfish slices. Food Chem. 2010, 121, 29–38. [Google Scholar] [CrossRef]
- Yongsawatdigul, J.; Park, J.W. Effects of acid and alkali solubilization on gelation characteristics of rockfish muscle proteins. J. Food Sci. 2004, 69, 499–505. [Google Scholar] [CrossRef]
- Hrynets, Y.; Omana, D.A.; Xu, Y.; Betti, M. Comparative study on the effect of acid-and alkaline-aided extraction on mechanically separated turkey meat (MSTM): Chemical characteristics of recovered proteins. Process. Biochem. 2011, 46, 335–343. [Google Scholar] [CrossRef]
- Richards, M.P.; Hultin, H.O. Effect of pH on lipid oxidation using trout hemolysate as a catalyst: A possible role for deoxyhaemoglobin. J. Agric. Food Chem. 2000, 48, 3141–3147. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar]
- Hornsey, H.C. The colour of cooked cured pork. I. Estimation of the nitric oxide-haem pigments. J. Sci. Food Agric. 1956, 7, 534–540. [Google Scholar] [CrossRef]
- Turhan, S.; Ustun, N.S.; Altunkaynak, T.B. Effect of cooking methods on total and heme iron contents of anchovy (Engraulis encrasicholus). Food Chem. 2004, 88, 169–172. [Google Scholar] [CrossRef]
- Antoni, E.; Brunoni, M. Haemoglobin and Myoglobin in Their Reactions with Ligands; North-Holland Publishing Company: Amsterdam, Holland, 1971; p. 19. [Google Scholar]
- Cho, S.; Lee, W.; Seol, K.; Kim, Y.; Kang, S.M.; Seo, H.; Jung, Y.; Kim, J.; Ba, H.V. Comparison of storage stability, volatile compounds and sensory properties between coarsely and finely-marbled 1+ grade Hanwoo beef lions. Food Sci. Anim. Resour. 2020, 40, 497–511. [Google Scholar] [CrossRef]
- Pinheiro, R.S.B.; Francisco, C.L.; Lino, D.M.; Borba, H. Meat quality of Santa Ines lamb chilled-then-frozen storage up to 12 months. Meat Sci. 2019, 148, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Rawdkuen, S.; Jaimakreu, M.; Benjakul, S. Physicochemical properties and tenderness of meat samples using proteolytic extract from Calotropis procera latex. Food Chem. 2013, 136, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Kaale, L.D.; Eikevik, T.M.; Rustad, T.; Nordtvedt, T.S. Changes in water holding capacity and drip loss of Atlantic salmon (Salmo salar) muscle during super chilled storage. LWT-Food Sci. Technol. 2014, 55, 528–535. [Google Scholar] [CrossRef] [Green Version]
- Jouki, M.; Khazaei, N. Effects of storage time on some characteristics of packed camel meat in low temperature. Int. J. Anim. Vet. Adv. 2011, 3, 460–464. [Google Scholar]
- Marino, R.; Albenzio, M.; della Malva, A.; Santillo, A.; Loizzo, P.; Sevi, A. Proteolytic pattern of myofibrillar protein and meat tenderness as affected by breed and aging time. Meat Sci. 2013, 95, 281–287. [Google Scholar] [CrossRef]
- Olivan, M.; Martınez, A.; Osoro, K.; Sanudo, C.; Panea, B.; Olleta, J.L.; Campo, M.M.; Oliver, M.À.; Serra, X.; Gil, M.; et al. Effect of muscular hypertrophy on physico-chemical, biochemical and texture traits of meat from yearling bulls. Meat Sci. 2004, 68, 567–575. [Google Scholar] [CrossRef]
- Masniyom, P.; Benjama, O.; Maneesri, J. Extending the shelf-life of refrigerated green mussel (Perna viridis) under modified atmosphere packaging. Songklanakarin J. Sci. Technol. 2011, 33, 171–179. [Google Scholar]
- Gil, M.; Hortos, M.; Sarraga, C. Calpain and cathepsin activities and protein extractability during ageing of longissimus porcine muscle from normal and PSE meat. Food Chem. 1998, 63, 385–390. [Google Scholar] [CrossRef]
- Holman, B.W.; van de Ven, R.J.; Mao, Y.; Coombs, C.E.; Hopkins, D.L. Using instrumental (CIE and reflectance) measures to predict consumers’ acceptance of beef colour. Meat Sci. 2017, 127, 57–62. [Google Scholar] [CrossRef]
- Khliji, S.; Van de Ven, R.; Lamb, T.A.; Lanza, M.; Hopkins, D.L. Relationship between consumer ranking of lamb colour and objective measures of colour. Meat Sci. 2010, 85, 224–229. [Google Scholar] [CrossRef]
- Maqsood, S.; Benjakul, S. Comparative studies on molecular changes and pro-oxidative activity of haemoglobin from different fish species as influenced by pH. Food Chem. 2011, 124, 875–883. [Google Scholar] [CrossRef]
- Maqsood, S.; Benjakul, S. Effect of bleeding on lipid oxidation retardation and quality retention of seabass (Lates calcarifer) slices during iced storage. Food Chem. 2011, 124, 459–467. [Google Scholar] [CrossRef]
- Gheisari, H.Z.; Aminlari, M.; Shekarforoush, S.S. A comparative study of the biochemical and functional properties of camel and cattle meat during frozen storage. Vet. Arh. 2009, 79, 51–68. [Google Scholar]
- Soyer, A.; Özalp, B.; Dalmış, U.; Bilgin, V. Effects of freezing temperature and duration of frozen storage on lipid and protein oxidation in chicken meat. Food Chem. 2010, 120, 1025–1030. [Google Scholar] [CrossRef]
- Teets, A.S.; Were, L.M. Inhibition of lipid oxidation in refrigerated and frozen salted raw minced chicken breasts with electron beam irradiated almond skin powder. Meat Sci. 2008, 80, 1326–1332. [Google Scholar] [CrossRef]
- Takeungwongtrakul, S.; Benjakul, S.; H-kittikun, A. Lipids from cephalothorax and hepatopancreas of Pacific white shrimp (Litopenaeus vannamei): Compositions and deterioration as affected by iced storage. Food Chem. 2012, 134, 2066–2074. [Google Scholar] [CrossRef]
- Maqsood, S.; Benjakul, S. Comparative studies of four different phenolic compounds on In vitro antioxidative activity and the preventive effect on lipid oxidation of fish oil emulsion and fish mince. Food Chem. 2010, 119, 123–132. [Google Scholar] [CrossRef]
- Chaijan, M.; Benjakul, S.; Wonnop, V.; Cameron, F. Changes of pigments and colour in sardine (Sardinella gibbosa) and mackerel (Rastrelliger kanagurta) muscle during iced storage. Food Chem. 2005, 93, 607–617. [Google Scholar] [CrossRef]
- Luciano, G.; Monahan, F.J.; Vasta, V.; Pennisi, P.; Bella, M.; Priolo, A. Lipid and colour stability of meat from lambs fed fresh herbage or concentrate. Meat Sci. 2009, 82, 193–199. [Google Scholar] [CrossRef]
- McKenna, D.R.; Mies, P.D.; Baird, B.E.; Pfeiffer, K.D.; Ellebracht, J.W.; Savell, J.W. Biochemical and physical factors affecting discoloration characteristics of 19 bovine muscles. Meat Sci. 2005, 70, 665–682. [Google Scholar] [CrossRef]
- Deng, S.; Han, Y.; Gao, T.; Ye, K.; Liu, J. Effect of temperature fluctuation during frozen storage on beef quality. J. Food Process Preserv. 2020, 45, e15043. [Google Scholar] [CrossRef]
- Lawrie, R.A.; Ledward, D.A. Lawrie’s Meat Science; Woodhead Publishing Ltd.: Cambridge, UK, 2006. [Google Scholar]
- Asghar, A.; Pearson, A.M. Influence of ante-and postmortem treatments upon muscle composition and meat quality. Adv. Food Res. 1980, 26, 53–213. [Google Scholar]


| Parameters | Sample | Storage Time (Days) | |||
|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | ||
| Protein extractability (Water-soluble protein) (mg of protein/g of sample) | CM | 3.13 ± 0.01 bB | 3.51 ± 0.01 bA | 4.00 ± 0.00 bA | 4.04 ± 0.05 bA |
| BF | 3.44 ± 0.01 aC | 3.48 ± 0.02 bC | 4.58 ± 0.01 aB | 4.86 ± 0.01 aA | |
| MT | 3.41 ± 0.02 aC | 4.35 ± 0.02 aB | 4.58 ± 0.01 aAB | 4.76 ± 0.01 aA | |
| Protein extractability (Salt-soluble protein) (mg of protein/g of sample) | CM | 1.35 ± 0.02 bC | 1.67 ± 0.02 bB | 2.02 ± 0.01 aA | 2.06 ± 0.0 cA |
| BF | 1.40 ± 0.02 bC | 1.87 ± 0.01 bB | 1.98 ± 0.01 aB | 2.59 ± 0.02 bA | |
| MT | 1.96 ± 0.01 aC | 2.18 ± 0.02 aB | 2.18 ± 0.01 aB | 3.01 ± 0.02 aA | |
| Sarcoplasmic protein solubility (mg of protein/g of sample) | CM | 3.83 ± 0.02 bC | 3.97 ± 0.06 bC | 4.56 ± 0.01 cB | 5.06 ± 0.02 cA |
| BF | 3.78 ± 0.01 bB | 3.95 ± 0.02 bB | 5.19 ± 0.01 bA | 5.36 ± 0.02 bA | |
| MT | 4.96 ± 0.02 aD | 5.21 ± 0.02 aC | 5.42 ± 0.01 aB | 5.81 ± 0.16 aA | |
| Total protein solubility (mg of protein/g of sample) | CM | 9.86 ± 0.11 bB | 11.88 ± 0.05 aA | 12.03 ± 0.09 bA | 12.62 ± 0.02 bA |
| BF | 10.17 ± 0.05 bB | 11.59 ± 0.07 aB | 13.12 ± 0.02 aA | 13.61 ± 0.03 aA | |
| MT | 11.12 ± 0.03 aC | 11.69 ± 0.07 aBC | 12.52 ± 0.01 abAB | 12.79 ± 0.02 abA | |
| L* | CM | 41.69 ± 0.24 cA | 40.91 ± 0.38 cA | 35.61 ± 0.66 bB | 34.55 ± 0.73 bB |
| BF | 45.58 ± 0.26 bA | 43.88 ± 0.08 bB | 42.16 ± 0.20 aC | 39.67 ± 0.13 aD | |
| MT | 46.84 ± 0.05 aA | 46.27 ± 0.03 aA | 43.38 ± 0.22 aB | 41.31 ± 0.12 aC | |
| a* | CM | 21.67 ± 0.18 aA | 18.13 ± 0.39 aB | 12.43 ± 0.22 aC | 17.74 ± 0.20 aB |
| BF | 18.74 ± 0.32 bA | 15.98 ± 0.13 bB | 14.85 ± 1.83 bD | 14.73 ± 0.15 bC | |
| MT | 13.84 ± 0.28 cA | 11.43 ± 0.09 cC | 10.18 ± 0.12 bD | 12.59 ± 0.12 cB | |
| b* | CM | 17.10 ± 0.22 aA | 16.34 ± 0.13 aA | 11.05 ± 0.26 cB | 9.945 ± 0.14 cC |
| BF | 16.97 ± 0.24 aA | 16.40 ± 0.07 aA | 14.74 ± 0.16 aB | 14.56 ± 0.13 aB | |
| MT | 15.82 ± 0.19 bA | 15.27 ± 0.03 bA | 13.08 ± 0.07 bB | 12.32 ± 0.15 bC | |
| Storage Time | Samples | Hardness (N) | Cohesiveness | Springiness (mm) | Gumminess (g) | Chewiness (mJ) | Shear Force (N) |
|---|---|---|---|---|---|---|---|
| Day 0 | CM | 37.26 ± 0.08 a | 0.85 ± 0.08 a | 6.99 ± 0.08 a | 1906.3 ± 5.06 a | 98.12 ± 0.08 a | 58.05 ± 3.04 a |
| BF | 30.49 ± 0.06 b | 0.89 ± 0.06 a | 6.97 ± 0.12 a | 1701.8 ± 5.92 b | 72.97 ± 0.05 b | 49.22 ± 1.66 b | |
| MT | 27.06 ± 0.07 d | 0.83 ± 0.04 ab | 6.91 ± 0.10 a | 1182.4 ± 3.72 c | 51.63 ± 0.07 d | 45.40 ± 1.17 c | |
| Day 9 | CM | 28.59 ± 0.05 c | 0.72 ± 0.04 c | 5.88 ± 0.09 b | 945.1 ± 3.23 e | 52.69 ± 0.06 d | 51.48 ± 1.96 b |
| BF | 28.43 ± 0.04 c | 0.76 ± 0.03 bc | 5.84 ± 0.07 b | 992.7 ± 3.46 d | 47.48 ± 0.06 c | 41.58 ± 1.96 cd | |
| MT | 26.25 ± 0.03 e | 0.72 ± 0.02 c | 5.78 ± 0.03 b | 1178.3 ± 2.45 f | 46.54 ± 0.04 c | 38.73 ± 1.96 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manheem, K.; Adiamo, O.; Roobab, U.; Mohteshamuddin, K.; Hassan, H.M.; Nirmal, N.P.; Maqsood, S. A Comparative Study on Changes in Protein, Lipid and Meat-Quality Attributes of Camel Meat, Beef and Sheep Meat (Mutton) during Refrigerated Storage. Animals 2023, 13, 904. https://doi.org/10.3390/ani13050904
Manheem K, Adiamo O, Roobab U, Mohteshamuddin K, Hassan HM, Nirmal NP, Maqsood S. A Comparative Study on Changes in Protein, Lipid and Meat-Quality Attributes of Camel Meat, Beef and Sheep Meat (Mutton) during Refrigerated Storage. Animals. 2023; 13(5):904. https://doi.org/10.3390/ani13050904
Chicago/Turabian StyleManheem, Kusaimah, Oladipupo Adiamo, Ume Roobab, Khaja Mohteshamuddin, Hassan. M. Hassan, Nilesh. P. Nirmal, and Sajid Maqsood. 2023. "A Comparative Study on Changes in Protein, Lipid and Meat-Quality Attributes of Camel Meat, Beef and Sheep Meat (Mutton) during Refrigerated Storage" Animals 13, no. 5: 904. https://doi.org/10.3390/ani13050904
APA StyleManheem, K., Adiamo, O., Roobab, U., Mohteshamuddin, K., Hassan, H. M., Nirmal, N. P., & Maqsood, S. (2023). A Comparative Study on Changes in Protein, Lipid and Meat-Quality Attributes of Camel Meat, Beef and Sheep Meat (Mutton) during Refrigerated Storage. Animals, 13(5), 904. https://doi.org/10.3390/ani13050904







