Rotational Grazing Modifies Rhipicephalus microplus Infestation in Cattle in the Humid Tropics
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Study Site
2.2. Experimental Design
2.3. Animal Management
2.4. Tick Counts in Cattle
2.5. Climate Data Collection
2.6. Statistical Analysis
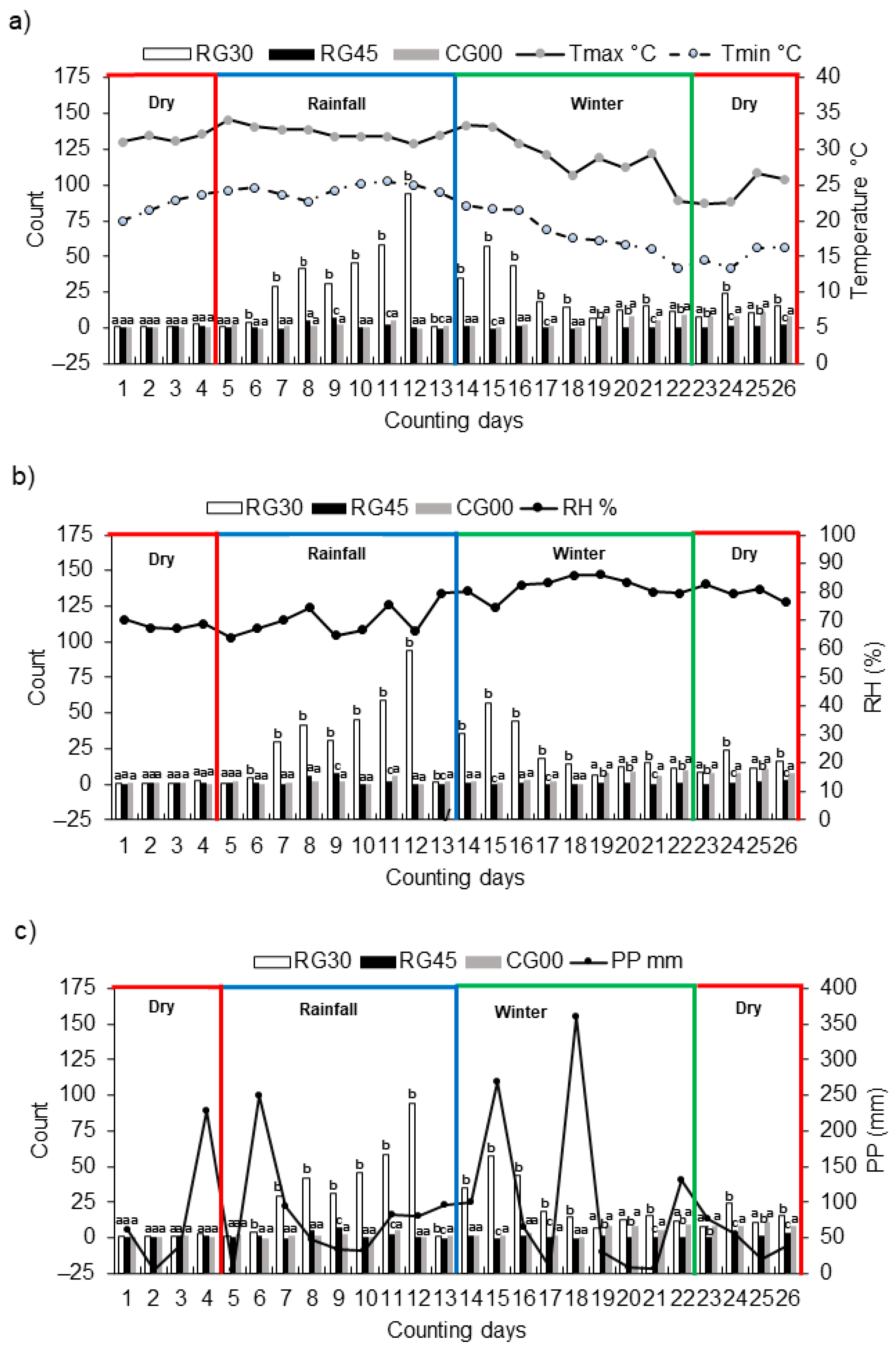
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rochlin, I.; Toledo, A. Emerging tick-borne pathogens of public health importance: A mini-review. J. Med Microbiol. 2020, 69, 781–791. [Google Scholar] [CrossRef]
- De León, A.A.P.; Teel, P.D.; Li, A.; Ponnusamy, L.; Roe, R.M. Advancing Integrated Tick Management to Mitigate Burden of Tick-Borne Diseases. Outlooks Pest Manag. 2014, 25, 382–389. [Google Scholar] [CrossRef]
- Agwunobi, D.O.; Yu, Z.; Liu, J. A retrospective review on ixodid tick resistance against synthetic acaricides: Implications and perspectives for future resistance prevention and mitigation. Pestic. Biochem. Physiol. 2021, 173, 104776. [Google Scholar] [CrossRef]
- Andreotti, R.; Guerrero, F.D.; Soares, M.A.; Barros, J.C.; Miller, R.J.; De Léon, A.P. Acaricide resistance of Rhipicephalus (Boophilus) microplus in State of Mato Grosso do Sul, Brazil. Rev. Bras. Parasitol. Vet 2011, 20, 127–133. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, P. Pasture spelling as a control measure for cattle ticks in southern Queensland. Aust. J. Agric. Res. 1964, 15, 822–840. [Google Scholar] [CrossRef]
- Hüe, T.; Fontfreyde, C. Development of a new approach of pasture management to control Rhipicephalus microplus infestation. Trop. Anim. Health Prod. 2019, 51, 1989–1995. [Google Scholar] [CrossRef]
- Nicaretta, J.E.; dos Santos, J.B.; Couto, L.F.M.; Heller, L.M.; Cruvinel, L.B.; Júnior, R.D.D.M.; Cavalcante, A.S.D.A.; Zapa, D.M.B.; Ferreira, L.L.; Monteiro, C.M.D.O.; et al. Evaluation of rotational grazing as a control strategy for Rhipicephalus microplus in a tropical region. Res. Vet. Sci. 2020, 131, 92–97. [Google Scholar] [CrossRef]
- Teel, P.D.; Grant, W.E.; Marin, S.L.; Stuth, J.W. Simulated Cattle Fever Tick Infestations in Rotational Grazing Systems. J. Range Manag. 1998, 51, 501. [Google Scholar] [CrossRef] [Green Version]
- Cruz, B.C.; Mendes, A.F.D.L.; Maciel, W.G.; dos Santos, I.B.; Gomes, L.V.C.; Felippelli, G.; Teixeira, W.F.P.; Ferreira, L.L.; Soares, V.E.; Lopes, W.D.Z.; et al. Biological parameters for Rhipicephalus microplus in the field and laboratory and estimation of its annual number of generations in a tropical region. Parasitol. Res. 2020, 119, 2421–2430. [Google Scholar] [CrossRef]
- National, Institute of Statistics, Geography and Informatics (INEGI). 2017. Available online: https://www.inegi.org.mx/contenidos/productos/prod_serv/contenidos/espanol/bvinegi/productos/nueva_estruc/anuarios_2017/702825094980.pdf (accessed on 26 May 2022).
- Fernández-Salas, A.; Rodríguez-Vivas, R.; Alonso-Díaz, M. First report of a Rhipicephalus microplus tick population multi-resistant to acaricides and ivermectin in the Mexican tropics. Vet. Parasitol. 2011, 183, 338–342. [Google Scholar] [CrossRef]
- Wharton, R.H.; Utech, K.B.W. The relation between engorgement and dropping of Boophilus microplus (canestrini) (ixodidae) to the assessment of tick numbers on cattle. Aust. J. Èntomol. 1970, 9, 171–182. [Google Scholar] [CrossRef]
- National Meteorological Service (SMN). 2022. Available online: https://smn.conagua.gob.mx/es/climatologia/informacion-climatologica/informacion-estadistica-climatologica (accessed on 5 May 2022).
- Mendoza-Martínez, N.; Alonso-Díaz, M.A.; Merino, O.; Fernández-Salas, A.; Lagunes-Quintanilla, R. Protective efficacy of the peptide Subolesin antigen against the cattle tick Rhipicephalus microplus under natural infestation. Vet. Parasitol. 2021, 299, 109577. [Google Scholar] [CrossRef] [PubMed]
- Nava, S.; Mastropaolo, M.; Guglielmone, A.A.; Mangold, A.J. Effect of deforestation and introduction of exotic grasses as livestock forage on the population dynamics of the cattle tick Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) in northern Argentina. Res. Vet. Sci. 2013, 95, 1046–1054. [Google Scholar] [CrossRef]
- Labruna, M.B.; Verissimo, C.J. 2001. Observations of infestation by Boophilus microplus (Acari: Ixodidae) in cattle kept in pasture rotation, under high animal density. Ar. Inst. Biol. 2001, 68, 115–120. [Google Scholar]
- de Barros, M.N.D.; Riet-Correa, F.; Azevedo, S.S.; Labruna, M.B. Off-host development and survival of Rhipicephalus (Boophilus) microplus in the Brazilian semiarid. Vet. Parasitol. Reg. Stud. Rep. 2017, 9, 17–24. [Google Scholar] [CrossRef]
- Canevari, J.T.; Mangold, A.J.; Guglielmone, A.A.; Nava, S. Population dynamics of the cattle tick Rhipicephalus(Boophilus) microplus in a subtropical subhumid region of Argentina for use in the design of control strategies. Med. Vet. Èntomol. 2016, 31, 6–14. [Google Scholar] [CrossRef]
- Nieto, N.C.; Holmes, E.A.; Foley, J.E. Survival rates of immature Ixodes pacificus (Acari: Ixodidae) ticks estimated using field-placed enclosures. J. Vector Ecol. 2010, 35, 43–49. [Google Scholar] [CrossRef]
- Morel, N.; Signorini, M.L.; Mangold, A.J.; Guglielmone, A.A.; Nava, S. Strategic control of Rhipicephalus (Boophilus) microplus infestation on beef cattle grazed in Panicum maximum grasses in a subtropical semi-arid region of Argentina. Prev. Vet. Med. 2017, 144, 179–183. [Google Scholar] [CrossRef]
- Mastropaolo, M.; Mangold, A.J.; Guglielmone, A.A.; Nava, S. Non-parasitic life cycle of the cattle tick Rhipicephalus (Boophilus) microplus in Panicum maximum pastures in northern Argentina. Res. Vet. Sci. 2017, 115, 138–145. [Google Scholar] [CrossRef]
- Alonso-Díaz, M.A.; Lopez-Silva, B.J.; Leme, A.C.; Magalhães-Labarthe, L.; Rodríguez-Vivas, R.I. Infestación natural de hem-bras de Boophilus microplus Canestrini, 1887 (Acari: Ixodidae) en dos genotipos de bovinos en el trópico húmedo de Veracruz, México. Vet Méx 2007, 38, 503–509. [Google Scholar]
- Estrada-Peña, A.; Mallón, A.R.; Bermúdez, S.; de la Fuente, J.; Domingos, A.; García, M.P.E.; Labruna, M.B.; Merino, O.; Mosqueda, J.; Nava, S.; et al. One Health Approach to Identify Research Needs on Rhipicephalus microplus Ticks in the Americas. Pathogens 2022, 11, 1180. [Google Scholar] [CrossRef] [PubMed]
- Nicaretta, J.E.; Zapa, D.M.B.; Couto, L.F.M.; Heller, L.M.; Cavalcante, A.S.D.A.; Cruvinel, L.B.; Júnior, R.D.D.M.; Ferreira, L.L.; Nascimento, R.M.D.; Soares, V.E.; et al. Rhipicephalus microplus seasonal dynamic in a Cerrado biome, Brazil: An update data considering the global warming. Vet. Parasitol. 2021, 296, 109506. [Google Scholar] [CrossRef] [PubMed]
| Date | Sampling Number | CG00 | RG30 | RG45 | |||
|---|---|---|---|---|---|---|---|
| Means 1 | SD | Means | SD | Means | SD | ||
| 02/04/2021 | 1 | 1.60 a | 1.58 | 1.40 a | 1.65 | 1.00 a | 1.41 |
| 17/04/2021 | 2 | 1.40 a | 1.65 | 1.40 a | 2.12 | 1.60 a | 2.07 |
| 01/05/2021 | 3 | 2.00 a | 2.49 | 2.20 a | 3.19 | 2.80 a | 3.68 |
| 18/05/2021 | 4 | 0.80 a | 1.69 | 6.20 a | 10.73 | 2.60 a | 4.01 |
| 01/06/2021 | 5 | 5.00 a | 4.74 | 2.20 a | 3.19 | 1.40 a | 2.50 |
| 15/06/2021 | 6 | 0.00 a | 0.00 | 8.60 b | 10.50 | 2.00 a | 5.66 |
| 29/06/2021 | 7 | 3.00 a | 3.16 | 59.20 b | 47.27 | 0.00 c | 0.00 |
| 13/07/2021 | 8 | 3.40 a | 3.53 | 83.80 b | 59.45 | 11.20 a | 14.58 |
| 27/07/2021 | 9 | 4.80 a | 3.16 | 61.80 b | 40.09 | 15.00 c | 7.62 |
| 11/08/2021 | 10 | 0.40 a | 0.84 | 90.80 b | 77.00 | 1.00 a | 1.94 |
| 24/08/2021 | 11 | 11.20 a | 8.12 | 117.20 b | 94.07 | 4.40 c | 6.24 |
| 08/09/2021 | 12 | 0.20 a | 0.63 | 188.40 b | 159.84 | 0.60 a | 1.35 |
| 20/09/2021 | 13 | 3.80 a | 5.29 | 162.40 b | 138.17 | 0.00 c | 0.00 |
| 05/10/2021 | 14 | 3.40 a | 3.41 | 70.80 b | 63.01 | 2.20 a | 2.90 |
| 18/10/2021 | 15 | 2.00 a | 3.27 | 115.00 b | 81.83 | 0.00 c | 0.00 |
| 03/11/2021 | 16 | 6.00 a | 6.18 | 88.00 b | 82.84 | 3.20 a | 4.54 |
| 16/11/2021 | 17 | 3.40 a | 3.66 | 36.60 b | 17.39 | 0.40 c | 0.84 |
| 30/11/2021 | 18 | 0.60 a | 1.90 | 29.00 b | 15.03 | 0.00 a | 0.00 |
| 14/12/2021 | 19 | 16.40 a | 13.16 | 13.00 a | 5.10 | 2.20 b | 2.57 |
| 28/12/2021 | 20 | 17.00 a | 5.52 | 25.00 a | 29.55 | 1.60 b | 2.27 |
| 11/01/2022 | 21 | 11.20 a | 7.19 | 31.00 b | 17.64 | 3.20 c | 3.16 |
| 25/01/2022 | 22 | 19.20 a | 14.37 | 23.00 a | 11.21 | 0.80 b | 1.93 |
| 08/02/2022 | 23 | 16.40 a | 11.46 | 16.20 a | 13.45 | 1.80 b | 2.57 |
| 22/02/2022 | 24 | 16.80 a | 9.53 | 48.00 b | 36.49 | 0.80 c | 1.40 |
| 09/03/2022 | 25 | 21.80 a | 5.37 | 22.20 a | 10.17 | 2.80 b | 3.43 |
| 23/03/2022 | 26 | 16.40 a | 8.04 | 31.80 b | 21.63 | 3.40 c | 2.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-González, G.; Pinos-Rodríguez, J.M.; Alonso-Díaz, M.Á.; Romero-Salas, D.; Vicente-Martínez, J.G.; Fernández-Salas, A.; Jarillo-Rodríguez, J.; Castillo-Gallegos, E. Rotational Grazing Modifies Rhipicephalus microplus Infestation in Cattle in the Humid Tropics. Animals 2023, 13, 915. https://doi.org/10.3390/ani13050915
Cruz-González G, Pinos-Rodríguez JM, Alonso-Díaz MÁ, Romero-Salas D, Vicente-Martínez JG, Fernández-Salas A, Jarillo-Rodríguez J, Castillo-Gallegos E. Rotational Grazing Modifies Rhipicephalus microplus Infestation in Cattle in the Humid Tropics. Animals. 2023; 13(5):915. https://doi.org/10.3390/ani13050915
Chicago/Turabian StyleCruz-González, Gabriel, Juan Manuel Pinos-Rodríguez, Miguel Ángel Alonso-Díaz, Dora Romero-Salas, Jorge Genaro Vicente-Martínez, Agustin Fernández-Salas, Jesús Jarillo-Rodríguez, and Epigmenio Castillo-Gallegos. 2023. "Rotational Grazing Modifies Rhipicephalus microplus Infestation in Cattle in the Humid Tropics" Animals 13, no. 5: 915. https://doi.org/10.3390/ani13050915
APA StyleCruz-González, G., Pinos-Rodríguez, J. M., Alonso-Díaz, M. Á., Romero-Salas, D., Vicente-Martínez, J. G., Fernández-Salas, A., Jarillo-Rodríguez, J., & Castillo-Gallegos, E. (2023). Rotational Grazing Modifies Rhipicephalus microplus Infestation in Cattle in the Humid Tropics. Animals, 13(5), 915. https://doi.org/10.3390/ani13050915






