Effects of Schisandra chinensis Polysaccharide-Conjugated Selenium Nanoparticles on Intestinal Injury in Mice
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
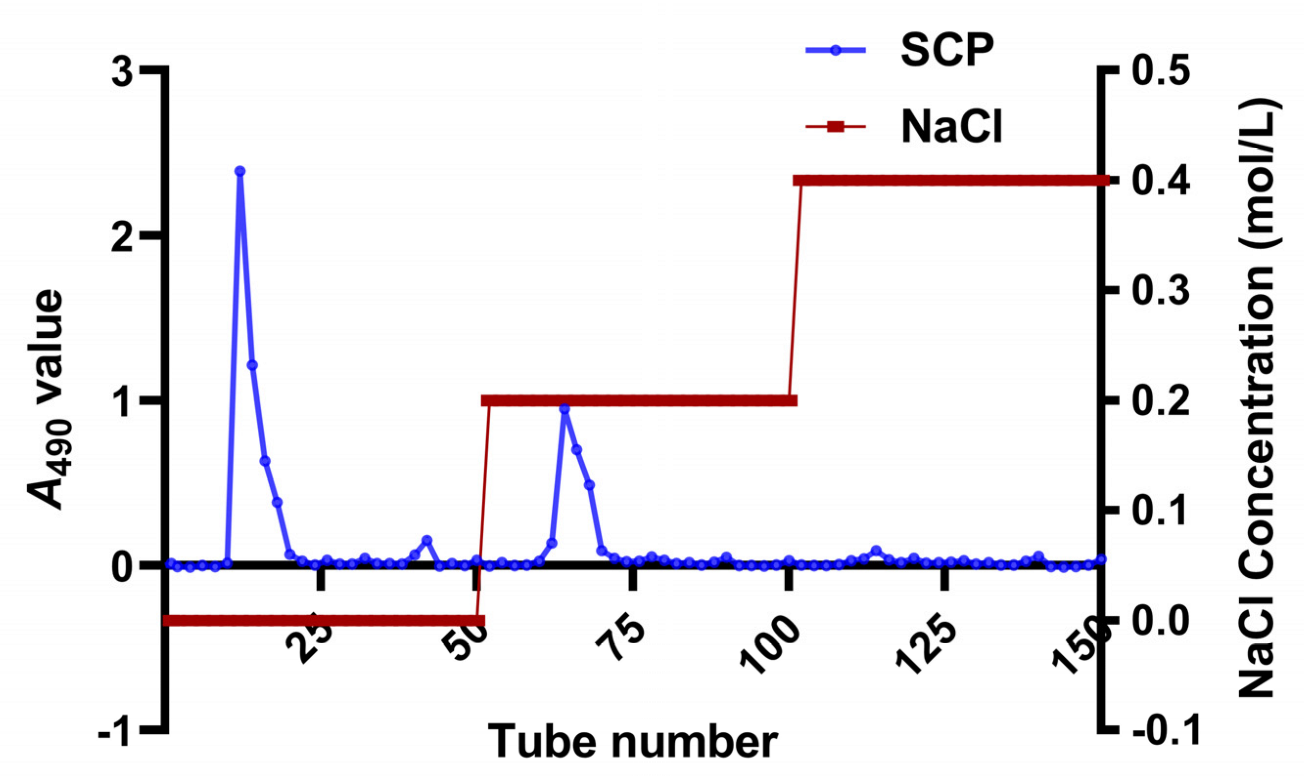
2.2. Isolation and Purification of SCP
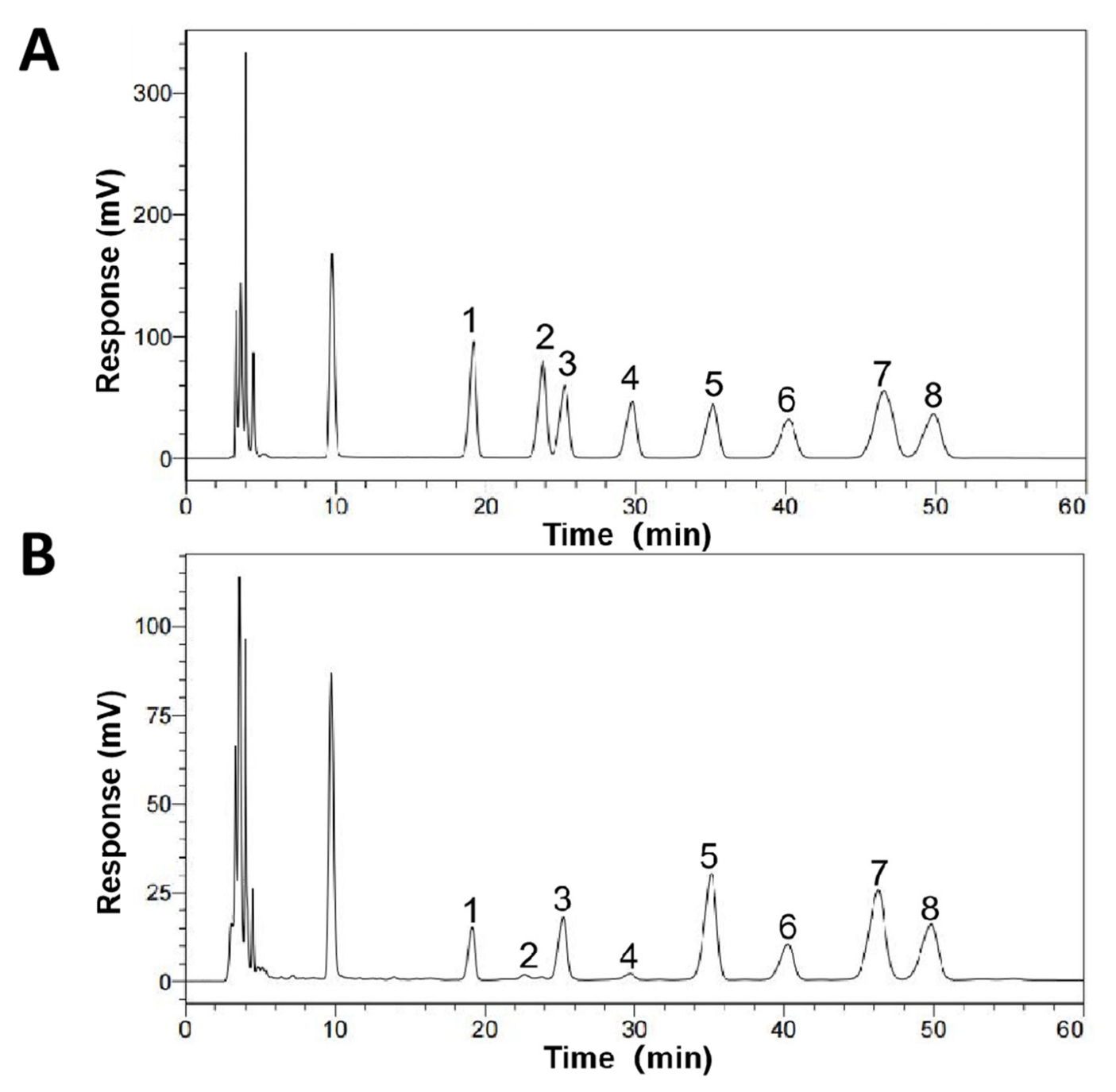
2.3. Monosaccharide Composition Analysis of SCP
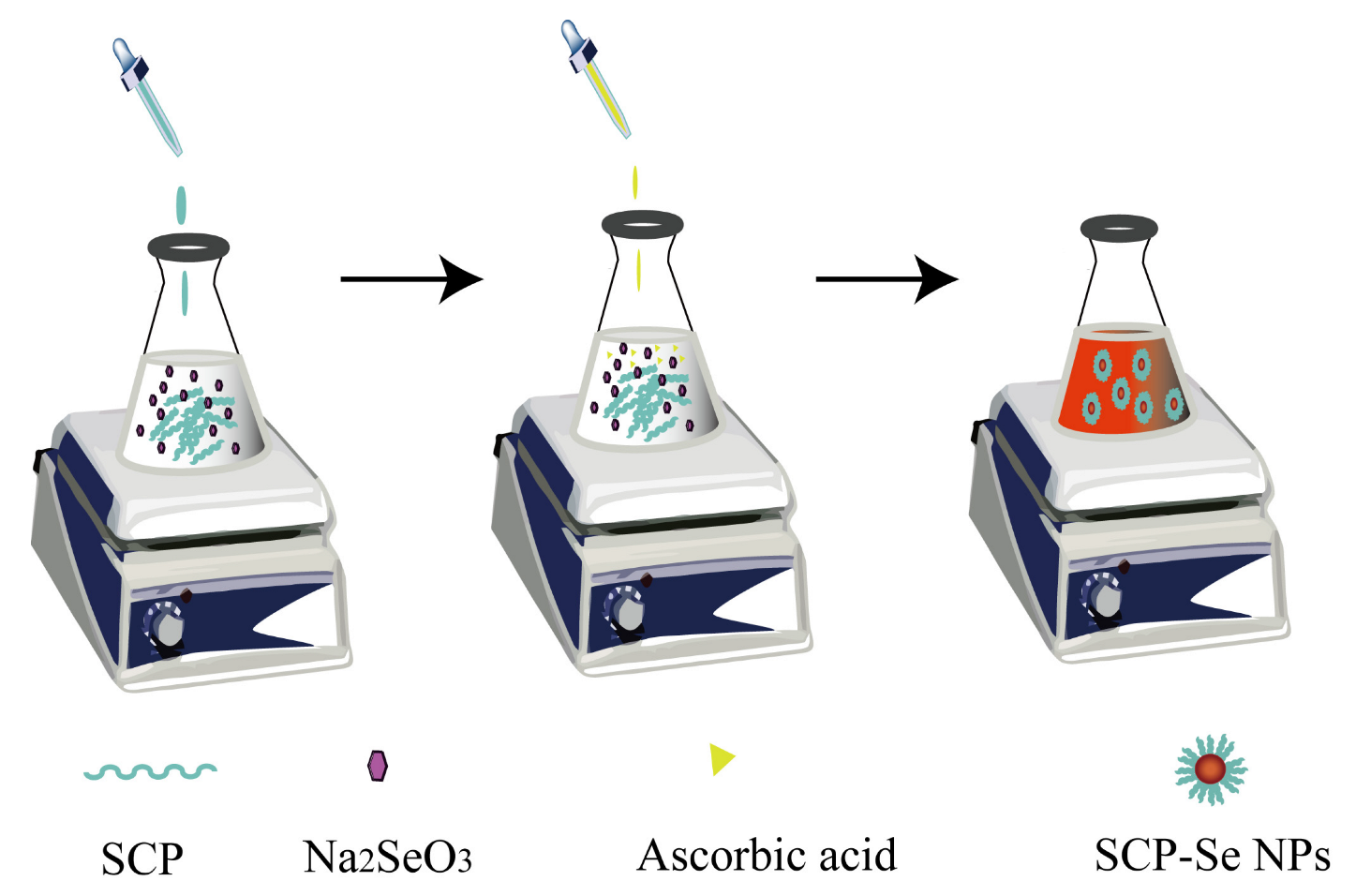
2.4. Preparation of SCP-Se NPs
2.5. Optimization of SCP-Se NPs Synthesis
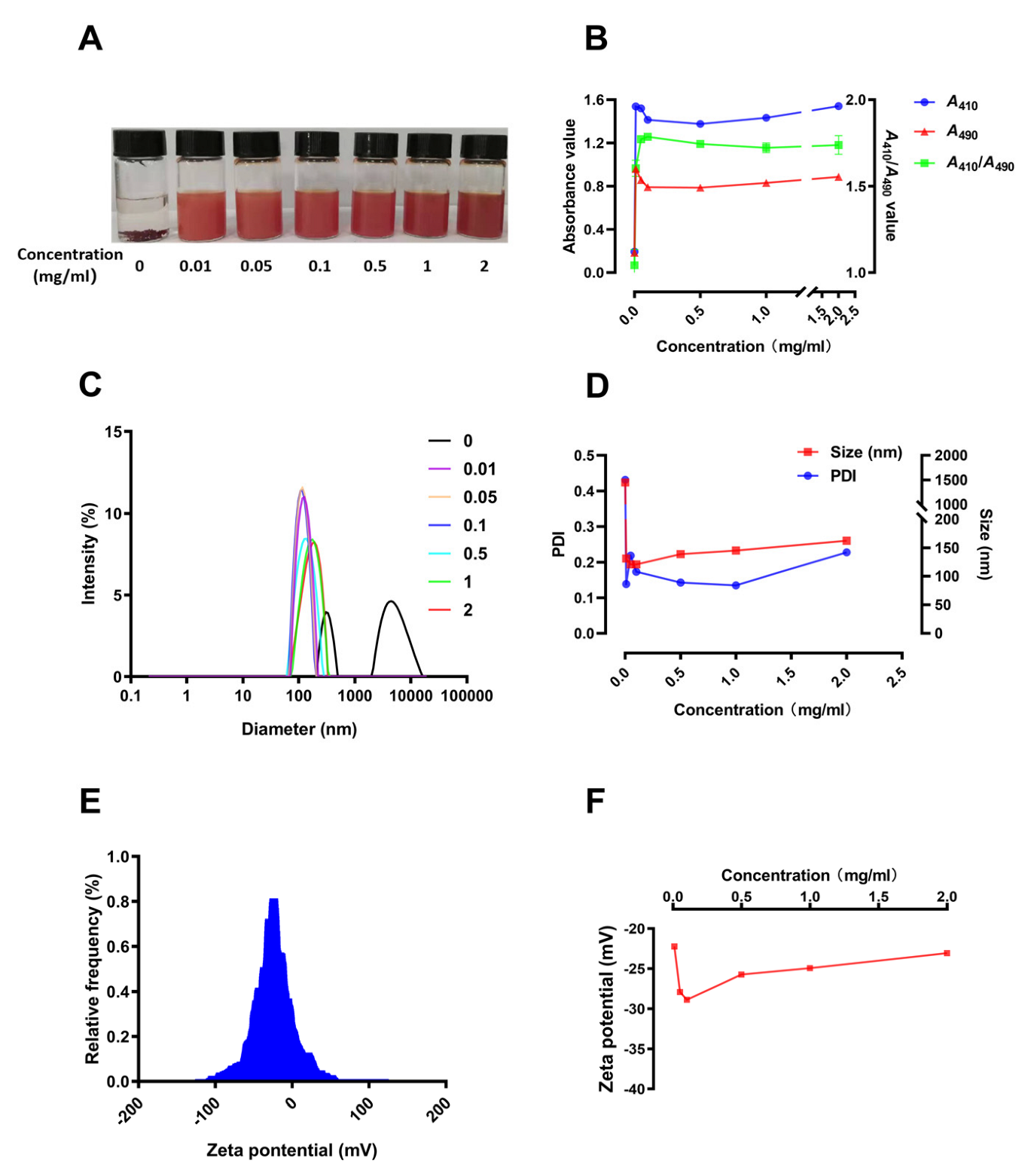
2.6. Characterization of SCP-Se NPs
2.7. Stability of SCP-Se NPs Colloidal Solution at Different Storage Conditions
2.8. Anti-Intestinal Inflammatory Injury Activity of SCP-Se NPs In Vivo
2.8.1. Animal Feeding and Management
2.8.2. Effects of SCP-Se NPs on LPS-Induced Enteritis
2.8.3. Histopathological Analysis of Jejunum
2.8.4. IHC Staining
2.8.5. Inflammatory Cytokine Analysis
2.9. Statistical Analysis
3. Results
3.1. Isolation and Purification of SCP
3.2. Monosaccharide Composition of SCP
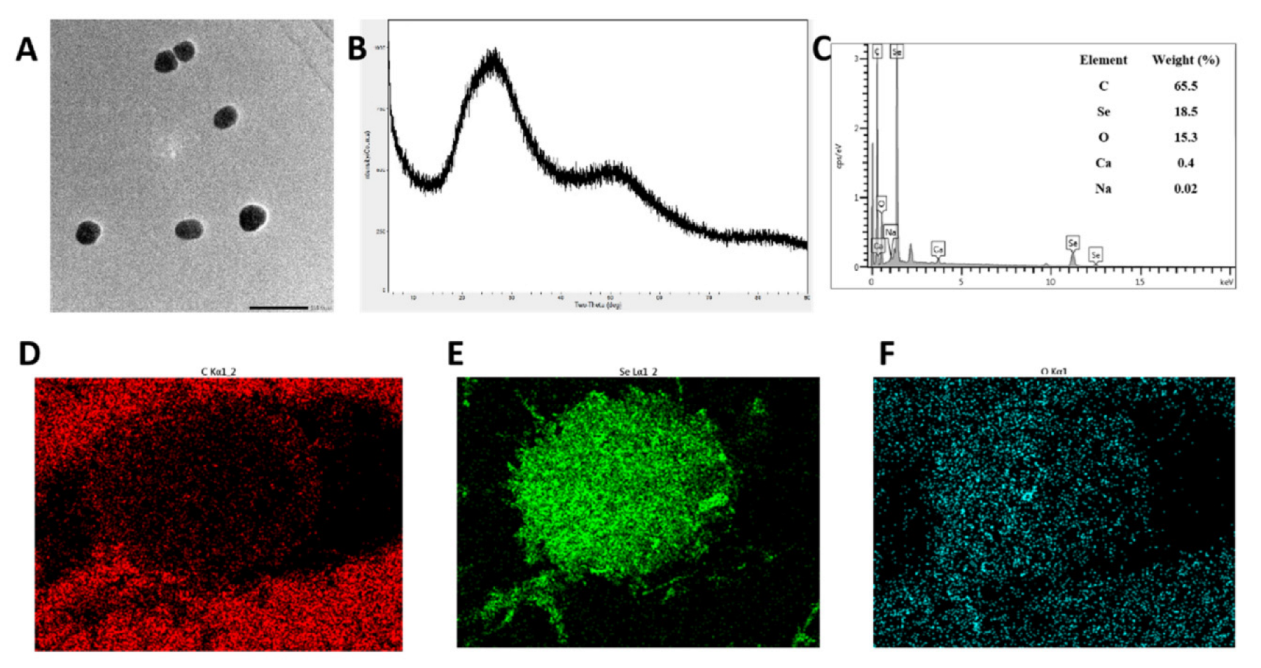
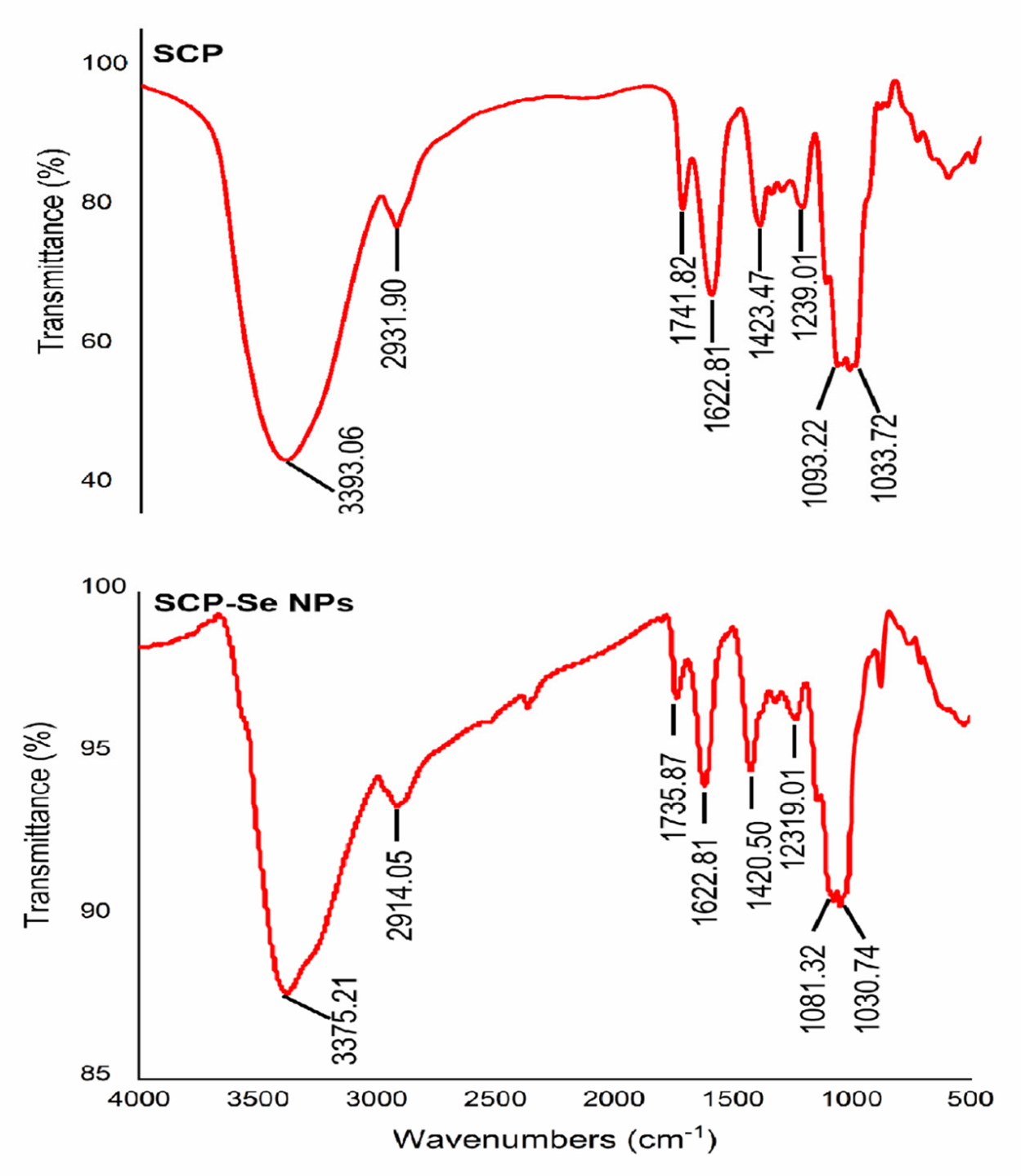
3.3. Preparation and Characterization of SCP-Se NPs
3.4. Characterization of SCP-Se NPs
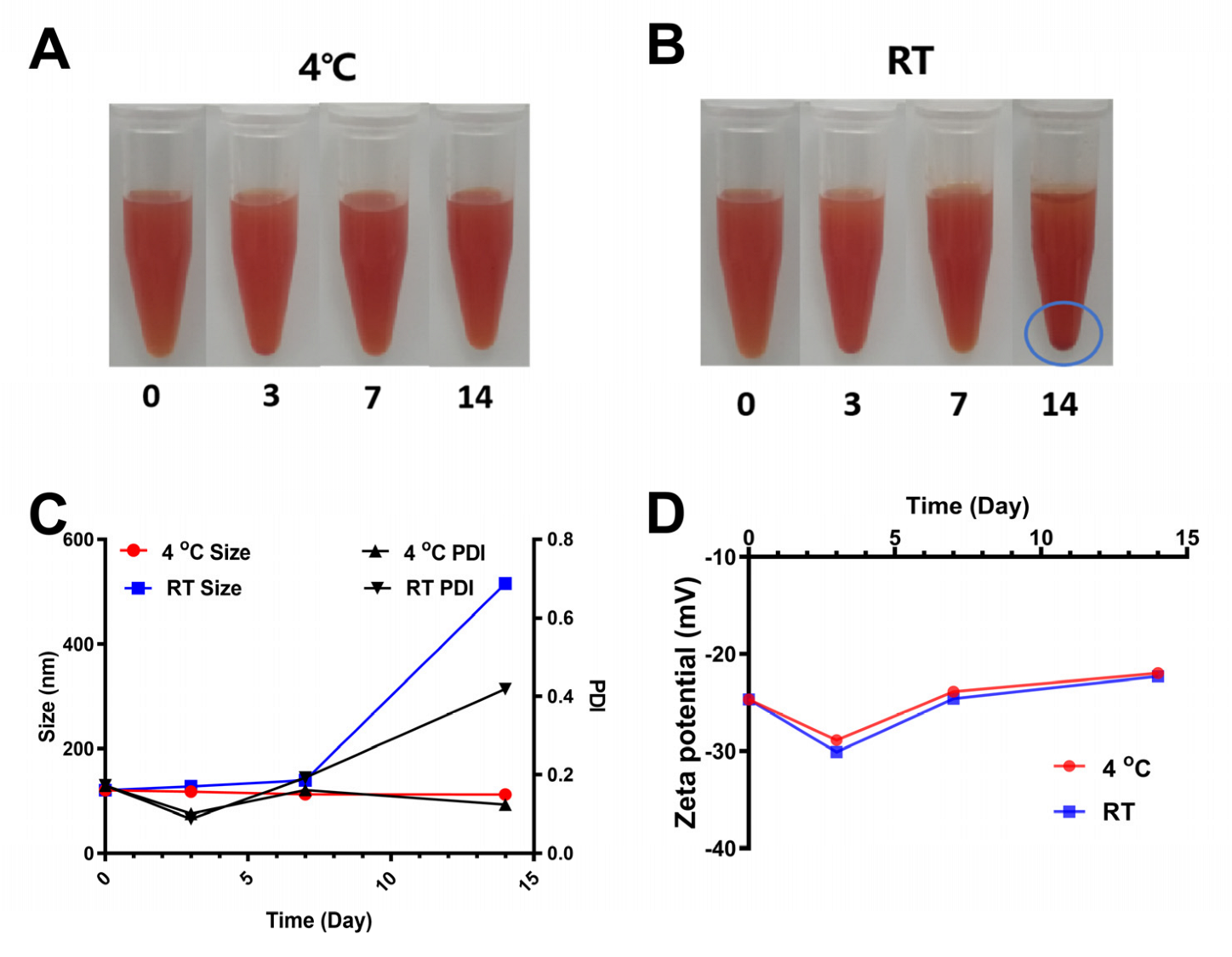
3.5. Stability of SCP-Se NPs at Different Storage Temperatures
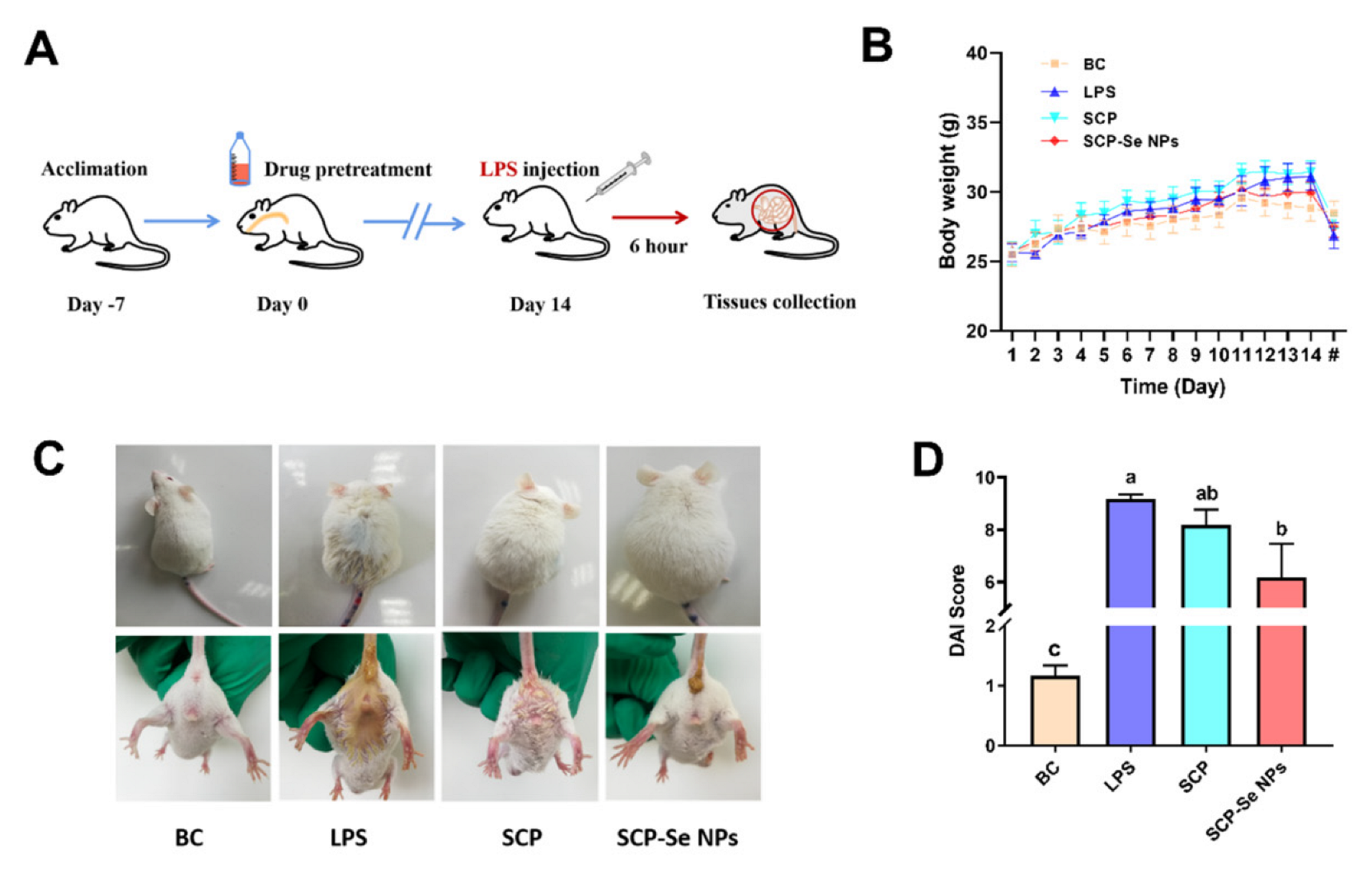
3.6. Effects of SCP-Se NPs on LPS-Induced Enteritis in Mice
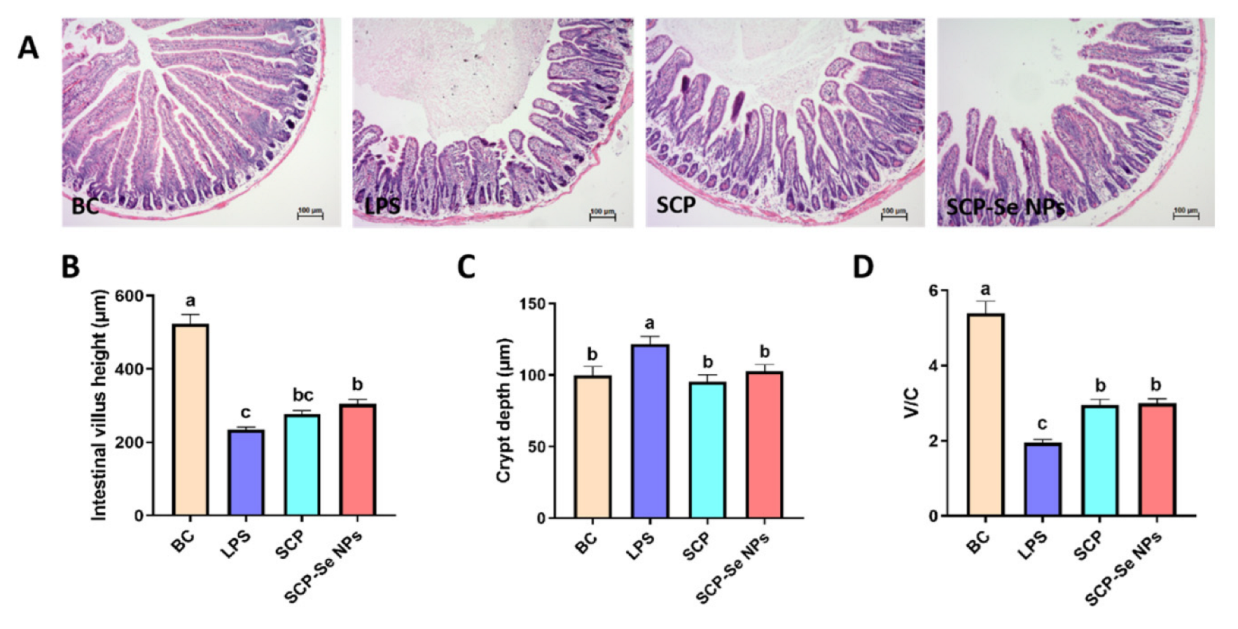
3.7. Effects of SCP-Se NPs on LPS-Induced Intestinal Injuries in Mice
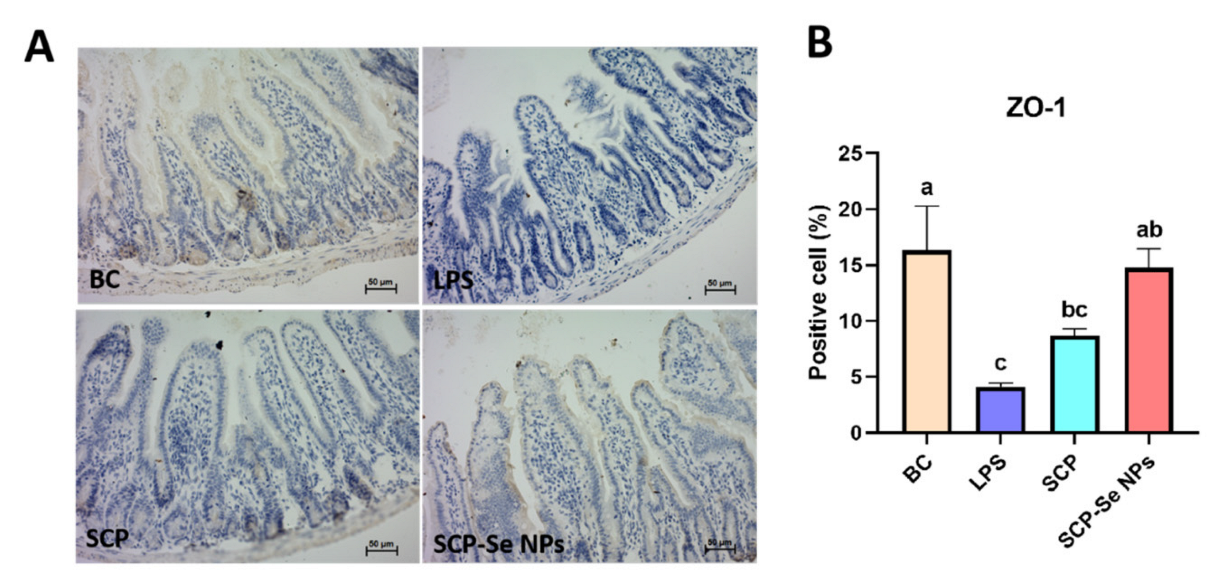
3.8. Influence of SCP-Se NPs on Jejunum ZO-1 Expression
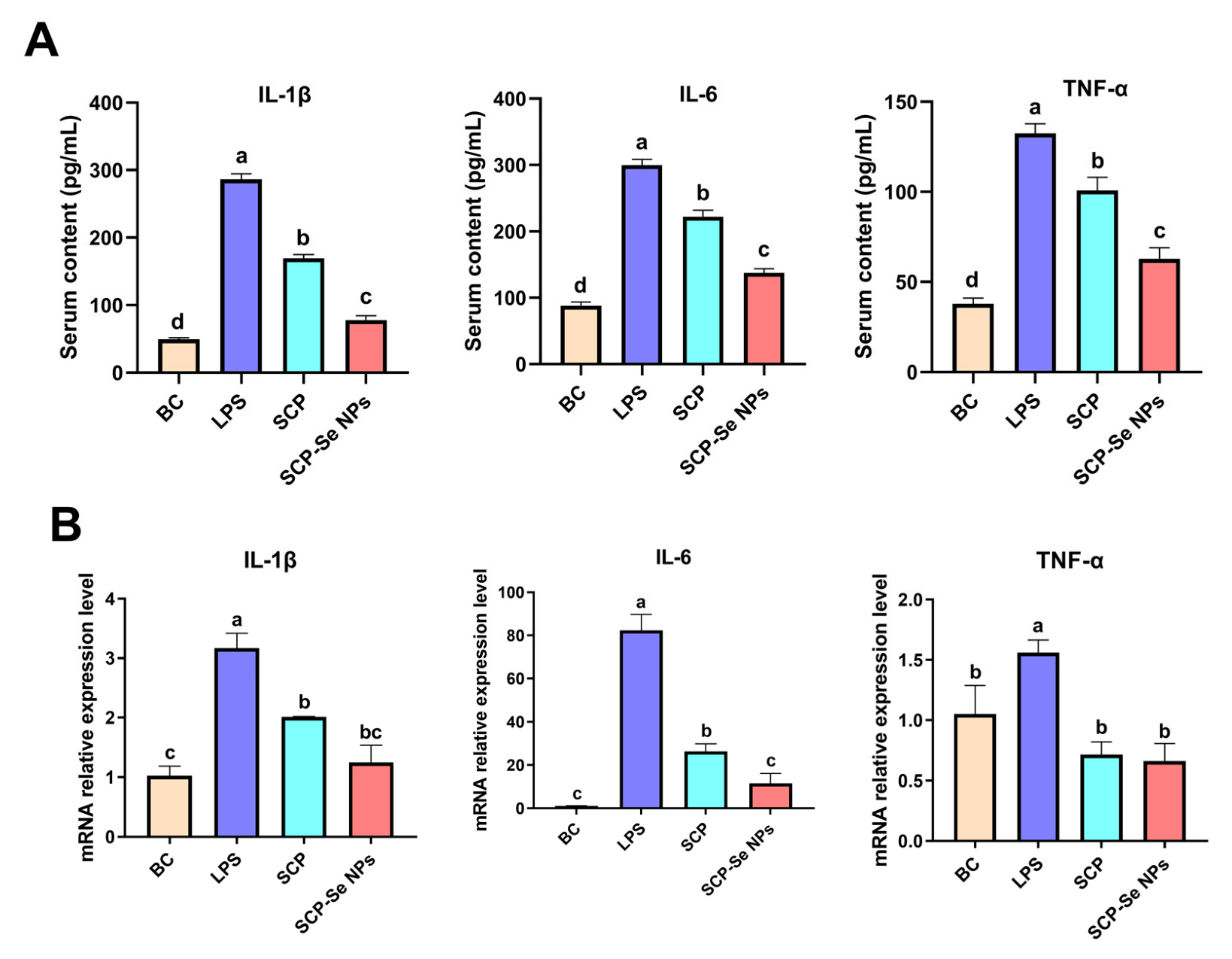
3.9. Effects of SCP-Se NPs on the Expression of Inflammatory Cytokines
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Madsen, K.; Spiller, R.; Greenwood-Van, M.B.; Van Meerveld, B.G.; Verne, G.N. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol. Motil. 2012, 24, 503–512. [Google Scholar] [CrossRef]
- Tulkens, J.; Vergauwen, G.; Van Deun, J.; Geeurickx, E.; Dhondt, B.; Lippens, L.; De Scheerder, M.A.; Miinalainen, I.; Rappu, P.; De Geest, B.G.; et al. Increased levels of systemic LPS-positive bacterial extracellular vesicles in patients with intestinal barrier dysfunction. Gut 2020, 69, 191–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caradonna, L.; Amati, L.; Magrone, T.; Pellegrino, N.M.; Jirillo, E.; Caccavo, D. Enteric bacteria, lipopolysaccharides and related cytokines in inflammatory bowel disease: Biological and clinical significance. J. Endotoxin Res. 2000, 6, 205–214. [Google Scholar]
- Rahimi, R.; Mozaffari, S.; Abdollahi, M. On the use of herbal medicines in management of inflammatory bowel diseases: A systematic review of animal and human studies. Dig. Dis. Sci. 2009, 54, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Seyed, R.H.; Homa, D. Herbal plants and their derivatives as growth and health promoters in animal nutrition. Vet. Res. Commun. 2011, 35, 169–180. [Google Scholar]
- Fu, J.; Li, J.X.; Sun, Y.Z.; Liu, S.; Song, F.R.; Liu, Z.Y. In-depth investigation of the mechanisms of Schisandra chinensis polysaccharide mitigating Alzheimer’s disease rat via gut microbiota and feces metabolomics. Int. J. Biol. Macromol. 2023, 232, 123488. [Google Scholar] [CrossRef]
- Xu, M.J.; Yan, T.X.; Gong, G.W.; Wu, B.; He, B.S.; Du, Y.Y.; Xiao, F.; Jia, Y. Purification, structural characterization, and cognitive improvement activity of a polysaccharides from Schisandra chinensis. Int. J. Biol. Macromol. 2020, 163, 497–507. [Google Scholar] [CrossRef]
- Qi, Y.L.; Chen, L.X.; Gao, K.; Shao, Z.J.; Huo, X.H.; Hua, M.; Liu, S.X.; Sun, Y.S.; Li, S.S. Effects of Schisandra chinensis polysaccharides on rats with antibiotic-associated diarrhea. Int. J. Biol. Macromol. 2019, 124, 627–634. [Google Scholar] [CrossRef]
- Su, L.L.; Mao, C.Q.; Wang, X.C.; Li, L.; Tong, H.J.; Mao, J.; Ji, D.; Lu, T.L.; Hao, M.; Huang, Z.Y.; et al. The Anti-colitis Effect of Schisandra chinensis Polysaccharide Is Associated With the Regulation of the Composition and Metabolism of Gut Microbiota. Front. Cell. Infect. Microbiol. 2020, 10, 519479. [Google Scholar] [CrossRef]
- Tatiana, M.G.; Sergey, V.K.; Ivan, F.G.; Andrey, V.K.; Anna, V.G.; Marina, I.S.; Dmiytiy, V.N.; Alireza, S.; Alexander, A.M. Biomedical evaluation of antioxidant properties of lamb meat enriched with iodine and selenium. Open Life Sci. 2022, 17, 180–188. [Google Scholar]
- El-Ghazaly, M.A.; Fadel, N.; Rashed, E.; El-Batal, A.; Kenawy, S.A. Anti-inflammatory effect of selenium nanoparticles on the inflammation induced in irradiated rats. Can. J. Physiol. Pharmacol. 2017, 95, 101–110. [Google Scholar] [CrossRef]
- Zoidis, E.; Seremelis, I.; Kontopoulos, N.; Danezis, G.P. Selenium-Dependent Antioxidant Enzymes: Actions and Properties of Selenoproteins. Antioxidants 2018, 7, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahram, S.; Raveendra, R.K.; Alexander, Y.; Adrianna, L.; Khaled, T.; Tamiru, N.A.; Neda, B.; Wanderly, M.Q.; Trevor, K.S.; Shayan, S. Dietary selenium supplementation enhances antiviral immunity in chickens challenged with low pathogenic avian influenza virus subtype H9N2. Vet. Immunol. Immunopathol. 2019, 207, 62–68. [Google Scholar]
- Ala, M.; Kheyri, Z. The rationale for selenium supplementation in inflammatory bowel disease: A mechanism-based point of view. Nutrition 2021, 85, 111153. [Google Scholar] [CrossRef]
- Ferreira, R.L.U.; Sena, E.K.C.M.; Azevedo, E.P.; Pinheiro, F.I.; Cobucci, R.N.; Pedrosa, L.F.C. Selenium in Human Health and Gut Microflora: Bioavailability of Selenocompounds and Relationship With Diseases. Front. Nutr. 2021, 8, 685317. [Google Scholar] [CrossRef]
- Ahmadi, M.; Poorghasemi, M.; Seidavi, A.; Hatzigiannakis, E.; Milis, C. An optimum level of nano-selenium supplementation of a broiler diet according to the performance, economical parameters, plasma constituents and immunity. J. Elem. 2020, 25, 1187–1198. [Google Scholar]
- Ghaderzadeh, S.; Aghjegheshlagh, F.M.; Nikbin, S.; Navidshad, B. Correlation effects of nano selenium and conjugated linoleic acid on the performance, lipid metabolism and immune system of male Moghani lambs. Iran. J. Appl. Anim. Sci. 2020, 9, 443–451. [Google Scholar]
- Verma, A.K.; Kumar, A.; Rahal, A.; Kumar, V.; Roy, D. Inorganic versus organic selenium supplementation: A review. Pak. J. Biol. Sci. 2012, 15, 418–425. [Google Scholar]
- Arin, B.; Abhishek, B.; Sudin, B. Selenium nanoparticles are less toxic than inorganic and organic selenium to mice in vivo. Nucleus 2019, 62, 259–268. [Google Scholar]
- Alidad, B.; Ali, A.S.; Seyed, N.M.; Mohamad, C.; Nasser, K. The Effects of Organic, Inorganic, and Nano-Selenium on Blood Attributes in Broiler Chickens Exposed to Oxidative Stress. Acta Sci. Vet. 2015, 43, 1264. [Google Scholar]
- Hu, S.Q.; Hu, W.C.; Li, Y.R.; Li, S.J.; Tian, H.F.; Lu, A.; Wang, J.G. Construction and structure activity mechanism of polysaccharide nanoselenium carrier. Carbohydr. Polym. 2020, 236, 116052. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.R.; Wu, Z.C.; Ma, Y.; Jian, W.J.; Xiong, H.J.; Zhou, L.N. Preparation and growth-promoting effect of selenium nanoparticles capped by polysaccharide-protein complexes on tilapia. J. Sci. Food Agric. 2021, 101, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shen, B.X.; Nie, S.L.; Duan, Z.H.; Chen, K.S. A combination of selenium and polysaccharides: Promising therapeutic potential. Carbohydr. Polym. 2019, 206, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.H.; Zhang, S.M.; Song, C.W.; Zhang, Y.B.; Ling, Q.J.; Hoffmann, P.R.; Li, J.; Chen, T.F.; Zheng, W.J.; Huang, Z. Selenium nanoparticles decorated with Ulva lactuca polysaccharide potentially attenuate colitis by inhibiting NF-kB mediated hyper inflammation. J. Nanobiotechnol. 2017, 15, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Tang, J.B.; Wang, X.K.; Sun, F.X.; Liang, S.J. An immunostimulatory polysaccharide (SCP-IIa) from the fruit of Schisandra chinensis (Turcz.) Baill. Int. J. Biol. Macromol. 2012, 50, 844–848. [Google Scholar] [CrossRef]
- Zhu, B.Q.; Qian, C.D.; Zhou, F.M.; Guo, J.J.; Chen, N.P.; Gao, C.X.; Jin, B.; Ding, Z.S. Antipyretic and antitumor effects of a purified polysaccharide from aerial parts of Tetrastigma hemsleyanum. J. Ethnopharmacol. 2020, 253, 112663. [Google Scholar] [CrossRef]
- Wang, C.C.; Feng, L.; Su, J.Y.; Cui, L.; Liu, D.; Yan, J.; Ding, C.L.; Tan, X.B.; Jia, X.B. Polysaccharides from Epimedium koreanum Nakai with immunomodulatory activity and inhibitory effect on tumor growth in LLC-bearing mice. J. Ethnopharmacol. 2017, 207, 8–18. [Google Scholar] [CrossRef]
- Xiao, Y.D.; Huang, Q.L.; Zheng, Z.M.; Guan, H.; Liu, S.Y. Construction of a Cordyceps sinensis exopolysaccharide-conjugated selenium nanoparticles and enhancement of their antioxidant activities. Int. J. Biol. Macromol. 2017, 99, 483–491. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, H.H.; Ma, L.; Zhang, H.R.; Ren, D.F. Preparation and characterization of selenium nanoparticles decorated by Spirulina platensis polysaccharide. J. Food Biochem. 2020, 44, 13363. [Google Scholar] [CrossRef]
- Song, J.Y.; Zhou, J.J.; Li, X.; Li, P.L.; Tian, G.Z.; Zhang, C.; Zhou, D.Z. Nano-selenium stablilized by Konjac Glucommannan and its biological activity in vitro. LWT-Food Sci. Technol. 2022, 161, 113289. [Google Scholar] [CrossRef]
- Keyhani, A.; Mahmoudvand, H.; Shakibaie, M. Histopathological and toxicological study of selenium nanoparticles in BALB/C Mice. Entomol. Appl. Sci. Lett. 2018, 5, 31. [Google Scholar]
- Liang, Y.C.; Liu, H.J.; Chen, S.H.; Chen, C.C.; Chou, L.S.; Tsai, L.H. Effect of lipopolysaccharide on diarrhea and gastrointestinal transit in mice: Roles of nitric oxide and prostaglandin E2. World J. Gastroenterol. 2005, 11, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Peng, Z.M.; Zhou, L.; Peng, R.Q.; Li, X.H.; Zuo, W.; Gou, J.H.; Zhou, F.X.; Yu, S.J.; Huang, M.; et al. Short-Chain Fatty Acid Decreases the Expression of CEBPB to Inhibit miR-145-Mediated DUSP6 and Thus Further Suppresses Intestinal Inflammation. Inflammation 2022, 45, 372–386. [Google Scholar] [CrossRef]
- Shi, X.D.; Tian, Y.Q.; Wu, J.L.; Wang, S.Y. Synthesis, characterization, and biological activity of selenium nanoparticles conjugated with polysaccharides. Crit. Rev. Food Sci. Nutr. 2021, 61, 2225–2236. [Google Scholar] [CrossRef]
- Cui, D.X.; Ma, J.; Liang, T.T.; Sun, L.Q.; Meng, L.Q.; Liang, T.G.; Li, Q.S. Selenium nanoparticles fabricated in laminarin polysaccharides solutions exert their cytotoxicities in HepG2 cells by inhibiting autophagy and promoting apoptosis. Int. J. Biol. Macromol. 2019, 137, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.F.; Hu, T.; Amr, M.B.; Zheng, Z.M.; Xiao, Y.D.; Huang, Q.L. Effect of ultrasound on size, morphology, stability and antioxidant activity of selenium nanoparticles dispersed by a hyperbranched polysaccharide from Lignosus rhinocerotis. Ultrason. Sonochem. 2018, 42, 823–831. [Google Scholar] [CrossRef]
- Wang, L.; Li, C.; Huang, Q.; Fu, X. Biofunctionalization of selenium nanoparticles with a polysaccharide from Rosa roxburghii fruit and their protective effect against H2O2-induced apoptosis in INS-1 cells. Food Funct. 2019, 10, 539. [Google Scholar] [CrossRef]
- Zhang, S.J.; Song, Z.T.; Shi, L.J.; Zhou, L.N.; Zhang, J.; Cui, J.L.; Li, Y.H.; Jin, D.Q.; Ohizumi, Y.; Xu, J.; et al. A dandelion polysaccharide and its selenium nanoparticles: Structure features and evaluation of anti-tumor activity in zebrafish models. Carbohydr. Polym. 2021, 270, 118365. [Google Scholar] [CrossRef]
- Li, H.Y.; Liu, D.D.; Li, S.H.; Xue, C.H. Synthesis and cytotoxicity of selenium nanoparticles stabilized by α-D-glucan from Castanea mollissima Blume. Int. J. Biol. Macromol. 2019, 129, 818–826. [Google Scholar] [CrossRef]
- Zhang, L.L.; Wei, X.B.; Zhang, R.J.; Si, D.Y.; James, N.P.; Baseer, A.; Zhang, M.Y. A Novel Peptide Ameliorates LPS-Induced Intestinal Inflammation and Mucosal Barrier Damage via Its Antioxidant and Antiendotoxin Effects. Int. J. Mol. Sci. 2019, 20, 3974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.Y.; Wang, W.J.; Wang, L.M.; Yu, C.; Zhang, G.L.; Zhu, H.L.; Wang, C.W.; Zhao, S.J.; Hu, C.A.A.; Liu, Y.L. Lentinan modulates intestinal microbiota and enhances barrier integrity in a piglet model challenged with lipopolysaccharide. Food Funct. 2019, 10, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.L.; Gao, R.R.; Gu, X.J.; Yu, B.; Wu, Y.; Li, Q.S.; Xiang, P.; Xu, H. A New Insight into Toxicity of Colchicine Analogues by Molecular Docking Analysis Based on Intestinal Tight Junction Protein ZO-1. Molecules 2022, 27, 1797. [Google Scholar] [CrossRef] [PubMed]
- Oshima, T.; Miwa, H. Gastrointestinal mucosal barrier function and diseases. J. Gastroenterol. 2016, 51, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Zachary, M.S.; Anthony, T.B. The Integral Role of Tight Junction Proteins in the Repair of Injured Intestinal Epithelium. Int. J. Mol. Sci. 2020, 21, 972. [Google Scholar]
- Ikenouchi, J.; Umeda, K.; Tsukita, S.; Furuse, M.; Tsukita, S. Requirement of ZO-1 for the formation of belt-like adherens junctions during epithelial cell polarization. J. Cell Biol. 2007, 176, 779–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poritz, L.S.; Garver, K.I.; Green, C.; Fitzpatrick, L.; Ruggiero, F.; Koltun, W.A. Loss of the Tight Junction Protein ZO-1 in Dextran Sulfate Sodium Induced Colitis. J. Surg. Res. 2007, 140, 12–19. [Google Scholar] [CrossRef]
- Wei, J.; Feng, J.X. Signaling pathways associated with inflammatory bowel disease. Recent Pat. Inflamm. Allergy Drug Discov. 2010, 4, 105–117. [Google Scholar] [CrossRef]
- Gabay, C.; Lamacchia, C.; Palmer, G. IL-1 pathways in inflammation and human diseases. Nat. Rev. Rheumatol. 2010, 6, 232–241. [Google Scholar] [CrossRef]
- Neurath, F.M. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef]
- Konstantinos, H.K.; Papadakis, K.A. Inflammatory Bowel Disease: Updates on Molecular Targets for Biologics. Gut Liver 2017, 11, 455–463. [Google Scholar]
- Kim, K.Y.; Oh, T.W.; Do, H.J.; Yang, J.H.; Yang, I.J.; Jeon, Y.H.; Go, Y.H.; Ahn, S.C.; Ma, J.Y.; Park, K.I. Acer palmatum thumb. ethanol extract alleviates interleukin-6-induced barrier dysfunction and dextran sodium sulfate-induced colitis by improving intestinal barrier function and reducing inflammation. J. Immunol. Res. 2018, 2018, 5718396. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.Y.; Zhu, H.; Yao, X.M.; Qian, J.P.; Yang, J.; Pan, X.D.; Chen, X.D. Metformin regulates tight junction of intestinal epithelial cells via MLCK-MLC signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 5239–5246. [Google Scholar] [PubMed]
- Wu, Y.P.; Zhu, C.; Chen, Z.; Chen, Z.J.; Zhang, W.N.; Ma, X.Y.; Wang, L.; Yang, X.F.; Jiang, Z.Y. Protective effects of Lactobacillus plantarum on epithelial barrier disruption caused by enterotoxigenic Escherichia coli in intestinal porcine epithelial cells. Vet. Immunol. Immunopathol. 2016, 172, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Loris, R.L.; Chowdhry, S.; Pizarro, T.T. Opposing functions of classic and novel IL-1 family members in gut health and disease. Front. Immunol. 2013, 4, 181. [Google Scholar]
- Matthias, F.; Mathilde, P.; Fiona, P. Cytokine Networks in the Pathophysiology of Inflammatory Bowel Disease. Immunity 2019, 50, 992–1006. [Google Scholar]
| Gene | Primer Sequence (5′-3′) | Product Size (bp) |
|---|---|---|
| IL-1β | Forward CACTACAGGCTCCGAGATGAACAAC Reverse TGTCGTTGCTTGGTTCTCCTTGTAC | 145 145 |
| IL-6 | Forward CTTCTTGGGACTGATGCTGGTGAC Reverse TCTGTTGGGAGTGGTATCCTCTGTG | 91 91 |
| TNF-α | Forward CGCTCTTCTGTCTACTGAACTTCGG Reverse GTGGTTTGTGAGTGTGAGGGTCTG | 113 113 |
| β-actin | Forward TTCCTTCCTGGGTATGGAAT Reverse GAGGAGCAATGATCTTGATC | 206 206 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, H.; Tan, X.; Li, Z.; Dong, H.; Su, L.; He, Z.; Ma, Q.; Dong, S.; Ramachandran, M.; Liu, J.; et al. Effects of Schisandra chinensis Polysaccharide-Conjugated Selenium Nanoparticles on Intestinal Injury in Mice. Animals 2023, 13, 930. https://doi.org/10.3390/ani13050930
Du H, Tan X, Li Z, Dong H, Su L, He Z, Ma Q, Dong S, Ramachandran M, Liu J, et al. Effects of Schisandra chinensis Polysaccharide-Conjugated Selenium Nanoparticles on Intestinal Injury in Mice. Animals. 2023; 13(5):930. https://doi.org/10.3390/ani13050930
Chicago/Turabian StyleDu, Hongxu, Xiaoyan Tan, Zhangxun Li, Hong Dong, Lijuan Su, Zhengke He, Qi Ma, Shiqi Dong, Mythili Ramachandran, Juan Liu, and et al. 2023. "Effects of Schisandra chinensis Polysaccharide-Conjugated Selenium Nanoparticles on Intestinal Injury in Mice" Animals 13, no. 5: 930. https://doi.org/10.3390/ani13050930






