Administration of Beta-Nerve Growth Factor during the Preovulatory Stage Improves Endocrine and Luteal Function in Dairy Heifers
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. NGF Isolation and Purification
2.2. Animals, Synchronization Protocol, Treatments, and Ultrasonography
2.3. Blood Sampling and E2, LH, and NGF Assays
2.4. Uterine Biopsy and Gene Expression
2.5. Effect of NGF on 30-Day Embryo Survival—Field Study
2.6. Statistical Analysis
3. Results
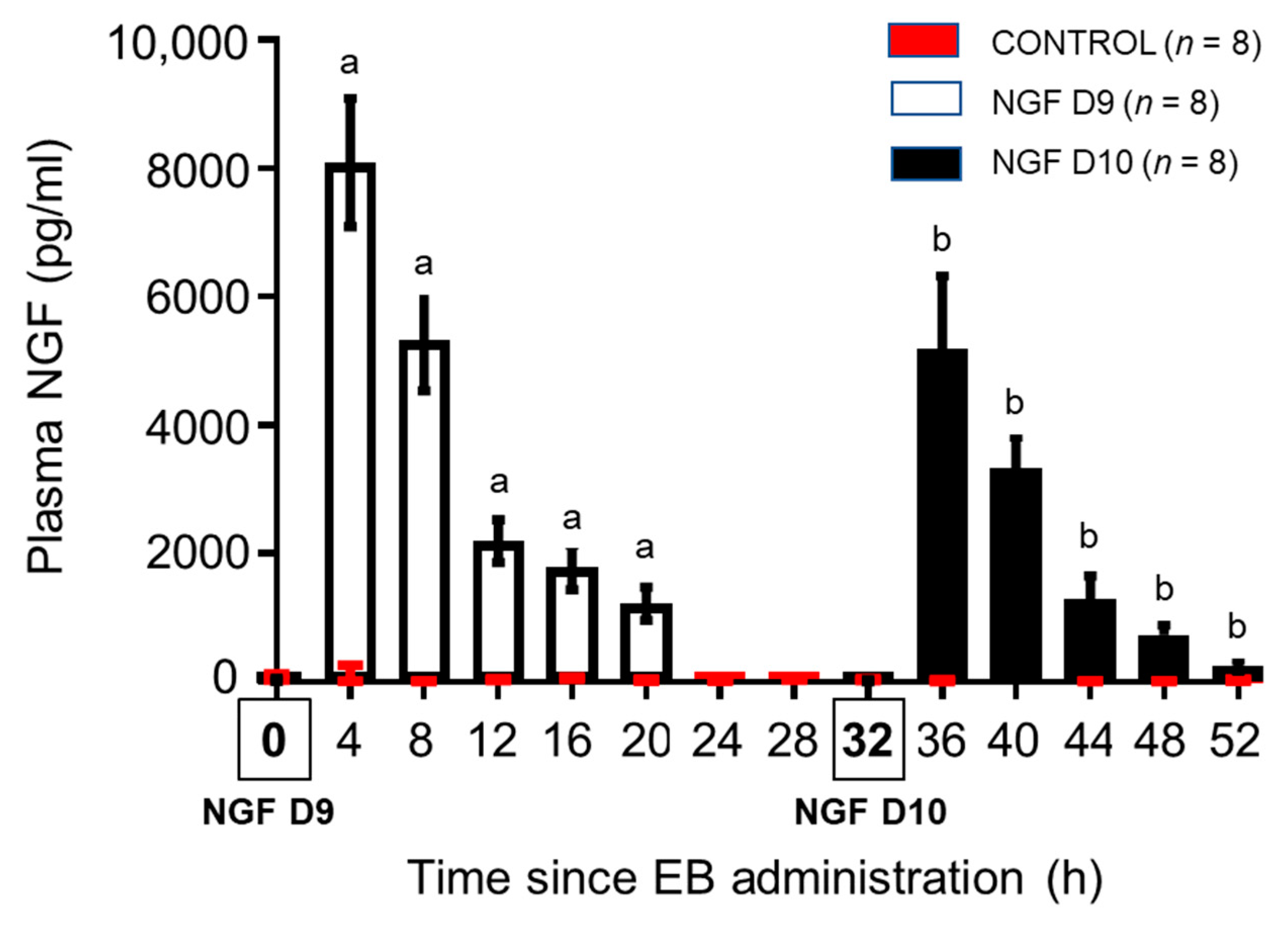
3.1. Bovine Plasma NGF Kinetics
3.2. Effect of NGF on E2 and LH Concentrations
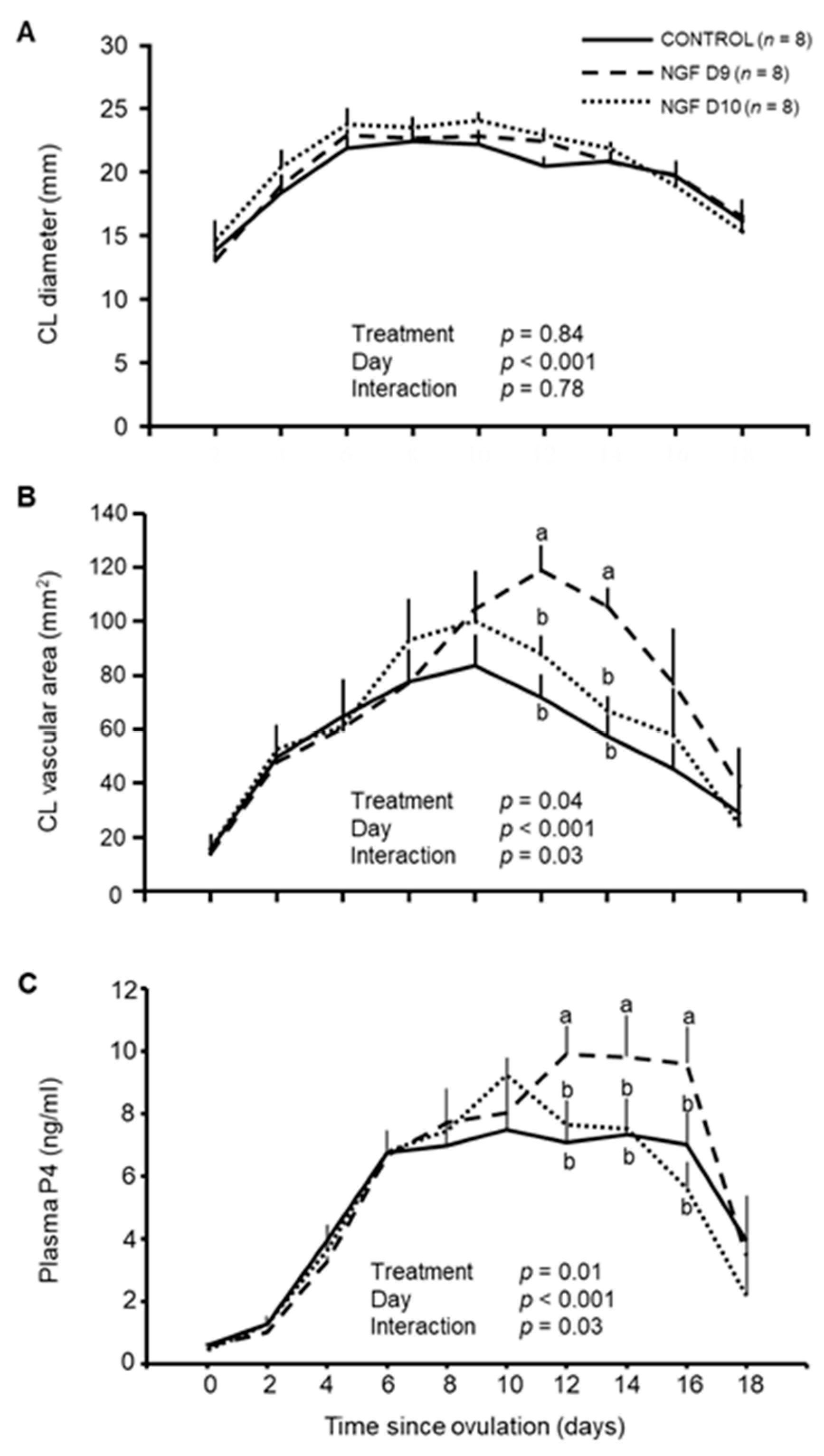
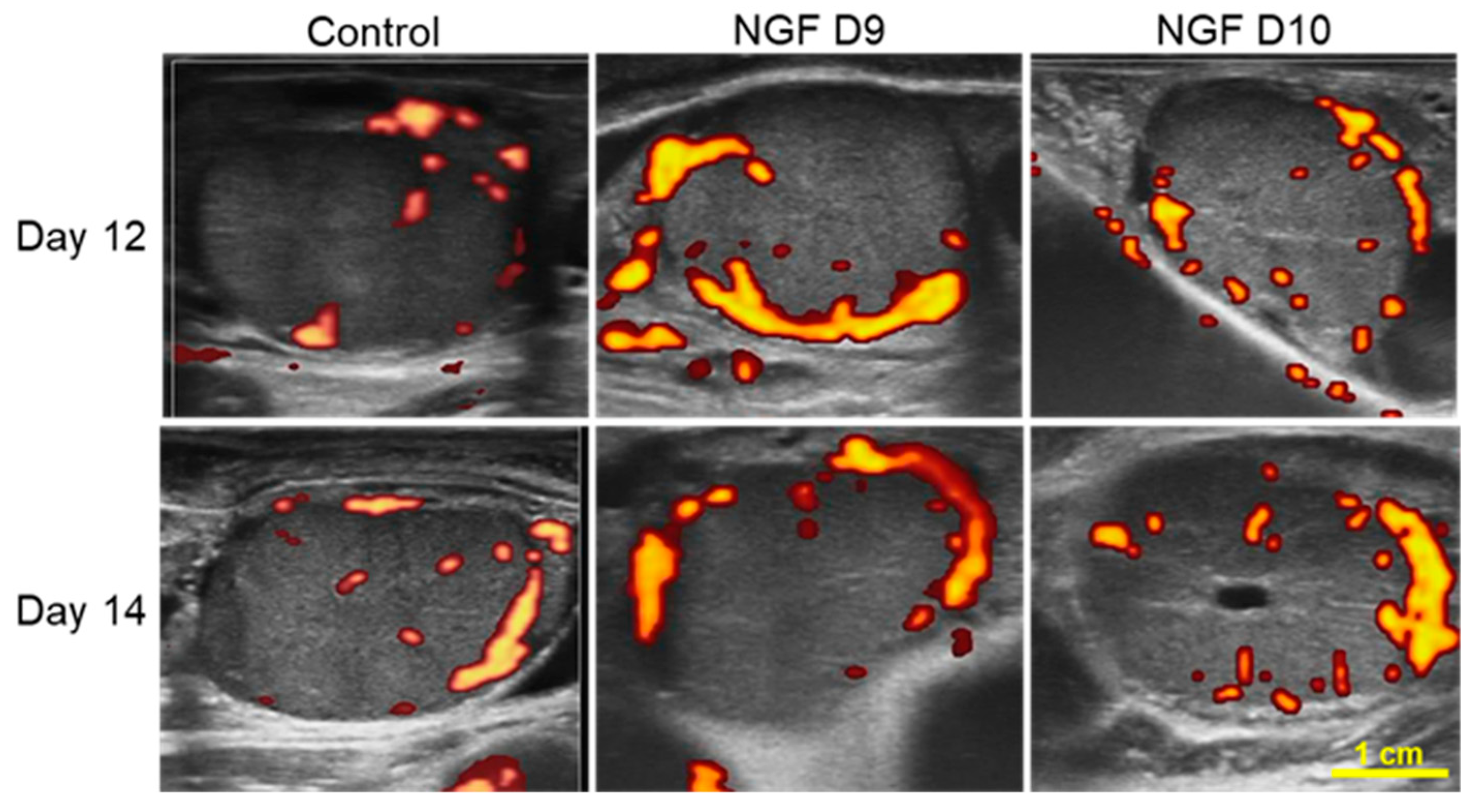
3.3. Effect of NGF on CL Vascular Area and P4 Production
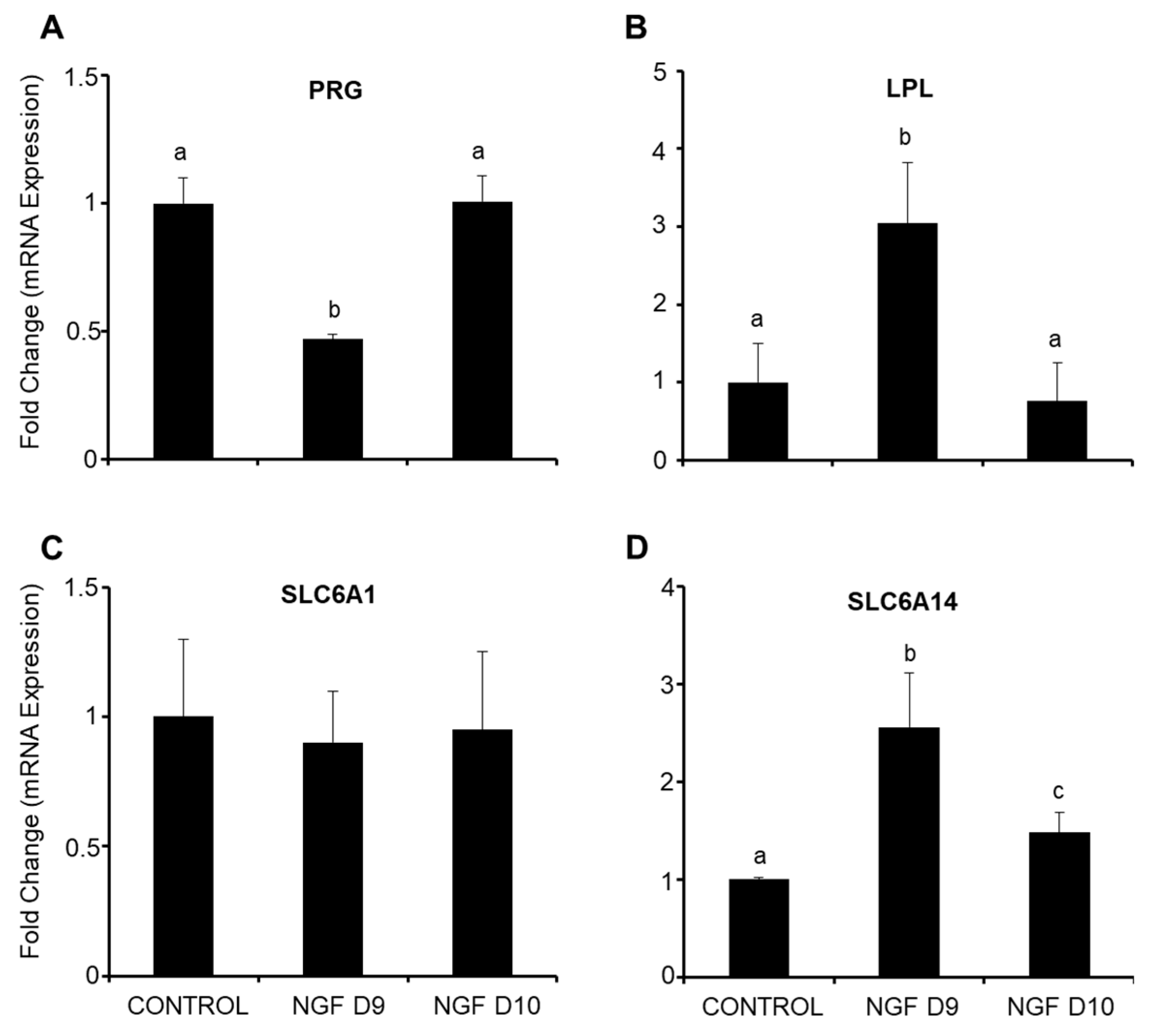
3.4. Endometrial Gene Expression
3.5. Effect of NGF on Conception Day on Day 30
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ratto, M.H.; Leduc, Y.; Valderrama, X.; van Straaten, K.; Delbaere, L.; Pierson, R.; Adams, G. The nerve of ovulation-inducing factor in semen. Proc. Natl. Acad. Sci. USA 2012, 109, 15042–15047. [Google Scholar] [CrossRef] [PubMed]
- Kershaw-Young, C.M.; Druart, X.; Vaughan, J.; Maxwell, W. β-Nerve growth factor is a major component of alpaca seminal plasma and induces ovulation in female alpaca. Reprod. Fertil. Dev. 2012, 24, 1093–1097. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Paiva, L.; Ratto, M. Ovulation mechanism in South American Camelids: The active role of β-NGF as the chemical signal eliciting ovulation in llamas and alpacas. Theriogenology 2020, 150, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Harper, G.; Thoenen, H. The distribution of nerve growth factor in the male sex organs of mammals. J. Neurochem. 1980, 34, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Harper, G.; Glanville, R.; Thoenen, H. The purification of nerve growth factor from bovine seminal plasma. Biochemical characterization and partial aminoacid sequence. J. Biol. Chem. 1982, 257, 8541–8548. [Google Scholar] [CrossRef]
- Ratto, M.; Huanca, W.; Singh, J.; Adams, G. Comparison of the effect of ovulation-inducing factor (OIF) in the seminal plasma of llamas, alpacas, and bulls. Theriogenology 2006, 66, 1102–1106. [Google Scholar] [CrossRef]
- Tanco, V.; Van Steelandt, M.; Ratto, M.; Adams, G. Effect of purified llama ovulation-inducing factor (OIF) on ovarian function in cattle. Theriogenology 2012, 78, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Tribulo, P.; Bogle, O.; Mapletoft, R.; Adams, G. Bioactivity of ovulation inducing factor (or nerve growth factor) in bovine seminal plasma and its effects on ovarian function in cattle. Theriogenology 2015, 83, 1394–1401. [Google Scholar] [CrossRef]
- Stewart, J.; Mercadante, V.; Dias, N.; Canisso, I.; Yau, P.; Imai, B.; Lima, F. Nerve growth factor-beta, purified from bull seminal plasma, enhances corpus luteum formation and conceptus development in bos taurus cows. Theriogenology 2018, 106, 30–38. [Google Scholar] [CrossRef]
- Gajardo, G.; Ulloa-Leal, C.; Valderrama, X.; Paiva, L.; Ratto, M. Heterologous beta nerve growth factor (β NGF) given at the LH surge enhances luteal function in dairy heifers. Domest. Anim. Endocrinol. 2021, 77, 106645. [Google Scholar] [CrossRef]
- Hubner, A.M.; Canisso, I.; Peixoto, P.W.C., Jr.; Cunha, L.; Ribeiro, L.; Crump, S.; Lima, F. Effect of nerve growth factor-β administered at insemination for lactating Holstein dairy cows bred after timed-artificial insemination protocol. J. Dairy Sci. 2022, 105, 6353–6363. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.L.; Stella, S.; Cunha, L.; Dias, N.; Canisso, I.; Mercadante, V.; Cardoso, R.; Williams, G.; Pohler, K.; Lima, F. Administration of nerve growth factor-β to heifers with a preovulatory follicle enhanced luteal formation and function and promoted LH release. Theriogenology 2020, 148, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Valderrama, X.P.; Goicochea, J.; Silva, M.; Ratto, M. The effect of seminal plasma β-NGF on follicular fluid hormone concentration and gene expression of steroidogenic enzymes in llama granulosa cells. Reprod. Biol. Endocrinol. 2019, 17, 60. [Google Scholar] [CrossRef] [PubMed]
- Valderrama, X.; Ulloa-Leal, C.; Silva, M.; Goicochea, J.; Apichela, S.; Arganaraz, M.; Sari, L.; Paiva, L.; Ratto, V.; Ratto, M. β-NGF stimulates steroidogenic enzyme and VEGFA gene expression, and progesterone secretion via ERK 1/2 pathway in primary culture of llama granulosa cells. Front. Vet. Sci. 2020, 7, 586265. [Google Scholar] [CrossRef]
- Ratto, M.H.; Delbaere, L.; Leduc, Y.; Pierson, R.; Adams, G. Biochemical isolation and purification of ovulation-inducing factor (OIF) in seminal plasma of llamas. Reprod. Biol. Endocrinol. 2011, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Mapletoft, R.J.; Marti, M.; Colazo, M.; Kastelic, J. The use of controlled internal drug release devices for the regulation of bovine reproduction. J. Anim. Sci. 2003, 81 (Suppl. S2), E28–E36. [Google Scholar] [CrossRef]
- Bó, G.; Baruselli, P. Synchronization of ovulation and fixed-time artificial insemination in beef cattle. Animal 2014, 8, 144–150. [Google Scholar] [CrossRef]
- Ulloa-Leal, C.; Bogle, O.; Adams, G.; Ratto, M. Luteotrophic effect of ovulation-inducing factor/nerve growth factor present in the seminal plasma of llamas. Theriogenology 2014, 81, 1101–1107.e1. [Google Scholar] [CrossRef]
- Recabarren, S.E.; Escobar, H.; Lobos, A.; Recabarren, M.; Parilo, J. Luteinizing hormone pulse frequency is increased by arginine infusion in prepubertal sheep. Exp. Clin. Endocrinol. Diabetes 1996, 104, 72–77. [Google Scholar] [CrossRef]
- Berland, M.A.; Ulloa-Leal, C.; Barría, M.; Wright, H.; Dissen, G.; Silva, M.; Ojeda, S.; Ratto, M. Seminal Plasma Induces Ovulation in Llamas in the Absence of a Copulatory Stimulus: Role of Nerve Growth Factor as an Ovulation-Inducing Factor. Endocrinology 2016, 157, 3224–3232. [Google Scholar] [CrossRef]
- Simintiras, C.A.; Sánchez, J.; McDonald, M.; Lonergan, P. Progesterone alters the bovine uterine fluid lipidome during the period of elongation. Reproduction 2019, 157, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Geary, T.W.; Burns, G.; Moraes, J.; Moss, J.; Denicol, A.; Dobbs, K.; Ortega, M.; Hansen, P.; Wehrman, M.; Neibergs, H.; et al. Identification of Beef Heifers with superior Uterine Capacity for Pregnancy. Biol. Reprod. 2016, 95, 47. [Google Scholar] [CrossRef] [PubMed]
- França, M.R.; da Silva, M.; Pugliesi, G.; Van Hoeck, V.; Binelli, M. Evidence of endometrial amino acid metabolism and transport modulation by peri-ovulatory endocrine profiles driving uterine receptivity. J. Anim. Sci. Biotechnol. 2017, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Forde, N.; Carter, F.; Spencer, T.; Bazer, F.; Sandra, O.; Mansouri-Attia, N.; Okumu, L.; McGettigan, P.; Mehta, J.; McBride, R.; et al. Conceptus-induced changes in the endometrial transcriptome: How soon does the cow know she is pregnant? Biol. Reprod. 2011, 85, 144–156. [Google Scholar] [CrossRef]
- Bogle, A.; Ratto, M.; Adams, G. Ovulation-inducing factor (OIF) induces LH secretion from pituitary cells. Anim. Reprod. Sci. 2012, 133, 117–122. [Google Scholar] [CrossRef]
- Paiva, L.; Silva, M.; Carrasco, R.; Ratto, M. The ovulatory and luteotropic actions of the male-derived beta-nerve growth factor in South American camelids. Anim. Front. 2022, 12, 87–94. [Google Scholar] [CrossRef]
- Dissen, G.A.; Hill, D.; Costa, M.; Dees, C.L.; Lara, H.; Ojeda, S. A role for trkA nerve growth factor receptors in mammalian ovulation. Endocrinology 1996, 137, 198–209. [Google Scholar] [CrossRef]
- Gibbs, R.B. Estrogen and Nerve Growth Factor-related Systems in Brain. Ann. N. Y. Acad. Sci. 1994, 743, 165–196. [Google Scholar] [CrossRef]
- Maranesi, M.; Petrucci, L.; Leonardi, L.; Piro, F.; Rebollar, P.; Millán, P.; Cocci, P.; Vullo, C.; Parillo, F.; Moura, A.; et al. New insights on a NGF-mediated pathway to induce ovulation in rabbits (Oryctolagus cuniculus). Biol. Reprod. 2018, 98, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, R.; Singh, J.; Adams, G. The dynamics of trkA expression in the bovine ovary are associated with a luteotrophic effect of ovulation-inducing factor/nerve growth factor (OIF/NGF). Reprod. Biol. Endocrinol. 2016, 14, 47. [Google Scholar] [CrossRef]
- Minten, M.A.; Bilby, T.; Bruno, R.; Allen, C.; Madsen, C.; Wang, Z.; Sawyer, J.; Tibary, A.; Neibergs, H.; Geary, T.; et al. Effects of fertility on gene expression and function of the bovine endometrium. PLoS ONE 2013, 8, e69444. [Google Scholar] [CrossRef]
- Forde, N.; Lonergan, P. Transcriptomic analysis of the bovine endometrium: What is required to establish uterine receptivity to implantation in cattle? J. Reprod. Dev. 2012, 58, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Forde, N.; Carter, F.; Fair, T.; Crowe, M.; Evans, A.; Spencer, T.; Bazer, F.; McBride, R.; Boland, M.; O’Gaora, P.; et al. Progesterone-regulated changes in endometrial gene expression contribute to advanced conceptus development in cattle. Biol. Reprod. 2009, 81, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Brogliatti, G.M.; Dominguez, G.; Lüssenhoff, M.; Perkins, J.; Bó, G. Deep intrauterine fixed-time artificial insemination using sexed semen in Holstein heifers. Reprod. Fertil. Dev. 2009, 22, 165. [Google Scholar] [CrossRef]
- Lauber, M.R.; McMullen, B.; Parrish, J.; Fricke, P. Short communication: Effect of timing of induction of ovulation relative to timed artificial insemination using sexed semen on pregnancy outcomes in primiparous Holstein cows. J. Dairy Sci. 2020, 103, 10856–10861. [Google Scholar] [CrossRef]
- Li, C.; Sun, Y.; Yi, K.; Ma, Y.; Sun, Y.; Zhang, W.; Zhou, X. Detection of nerve growth factor (NGF) and its specific receptor (TrkA) in ejaculated bovine sperm, and the effects of NGF on sperm function. Theriogenology 2010, 74, 1615–1622. [Google Scholar] [CrossRef]


| Accession No. | Entrez Gene Symbol | Primer Sequence (5′-3′) | Amplicon Size (bp) |
|---|---|---|---|
| NM_001205356.1 | PGR | F: GAGAGCTCATCAAGGCAATTGG R: CACCATCCCTGCCAATATCTTG | 199 |
| NM_001077836.1 | SLC6A1 | F: GTGTCTCCATTTCCTGGTTT R: GCACAGCACTGAAGATGAA | 150 |
| NM_001098461.1 | SLC6A14 | F: GATGCTGCCACACAGATATT R: TAGCAAATCCAGCGAACAC | 154 |
| NM_001075120.1 | LPL | F: CAGGTCGAAGTATCGGAATCCA R: GAAAGTGCCTCCGTTAGGGTAAA | 67 |
| XM_027528015 | ACTB | F: GGATGAGGCTCAGAGCAAGAGA R: TCGTCCCAGTTGGTGACGAT | 77 |
| Control | NGF D9 | NGF D10 | p-Value | |
|---|---|---|---|---|
| Follicle size at DIB removal (mm) | 13.2 ± 1.1 | 11.5 ± 0.5 | 12.8 ± 1.6 | p = 0.8 |
| Follicle size at Day 9 of synchronization protocol (mm) | 15.4 ± 1.6 | 13.9 ± 0.6 | 14.4 ± 1.0 | p = 0.6 |
| Follicle size at Day 10 of synchronization protocol (mm) | 16.4 ± 1.4 | 15.6 ± 0.5 | 15.4 ± 0.7 | p = 0.7 |
| Interval from second EB administration to ovulation (h) | 45.0 ± 3.0 | 46.5 ± 2.7 | 43.5 ± 3.9 | p = 0.9 |
| Ovulation rate (%) | 8/8 (100%) | 8/8 (100%) | 8/8 (100%) | p = 1.0 |
| CL diameter (mm) | 24.0 ± 1.3 | 23.0 ± 0.5 | 24.4 ± 1.2 | p = 0.7 |
| Day of maximum CL diameter (mm) | 6.7 ± 0.4 | 6.2 ± 0.4 | 7.0 ± 0.4 | p = 0.8 |
| CL diameter at Day 8 after ovulation | 22.7 ± 1.6 | 22.6 ± 0.5 | 24.1 ± 1.0 | p = 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gajardo, G.; Paiva, L.; Ulloa-Leal, C.; Valderrama, X.; López, G.; Carrasco, A.; Hidalgo, A.I.; Silva, M.E.; Palma, P.I.; Ratto, M.H. Administration of Beta-Nerve Growth Factor during the Preovulatory Stage Improves Endocrine and Luteal Function in Dairy Heifers. Animals 2023, 13, 1004. https://doi.org/10.3390/ani13061004
Gajardo G, Paiva L, Ulloa-Leal C, Valderrama X, López G, Carrasco A, Hidalgo AI, Silva ME, Palma PI, Ratto MH. Administration of Beta-Nerve Growth Factor during the Preovulatory Stage Improves Endocrine and Luteal Function in Dairy Heifers. Animals. 2023; 13(6):1004. https://doi.org/10.3390/ani13061004
Chicago/Turabian StyleGajardo, Gonzalo, Luis Paiva, Cesar Ulloa-Leal, Ximena Valderrama, Gerardo López, Albert Carrasco, Alejandra Isabel Hidalgo, Mauricio E. Silva, Patricio I. Palma, and Marcelo H. Ratto. 2023. "Administration of Beta-Nerve Growth Factor during the Preovulatory Stage Improves Endocrine and Luteal Function in Dairy Heifers" Animals 13, no. 6: 1004. https://doi.org/10.3390/ani13061004
APA StyleGajardo, G., Paiva, L., Ulloa-Leal, C., Valderrama, X., López, G., Carrasco, A., Hidalgo, A. I., Silva, M. E., Palma, P. I., & Ratto, M. H. (2023). Administration of Beta-Nerve Growth Factor during the Preovulatory Stage Improves Endocrine and Luteal Function in Dairy Heifers. Animals, 13(6), 1004. https://doi.org/10.3390/ani13061004





