The Impact of Beehive Proximity, Human Activity and Agricultural Intensity on Diptera Diversity in a Mediterranean Mosaic of Agroecosystems, with a Focus on Pest Species
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
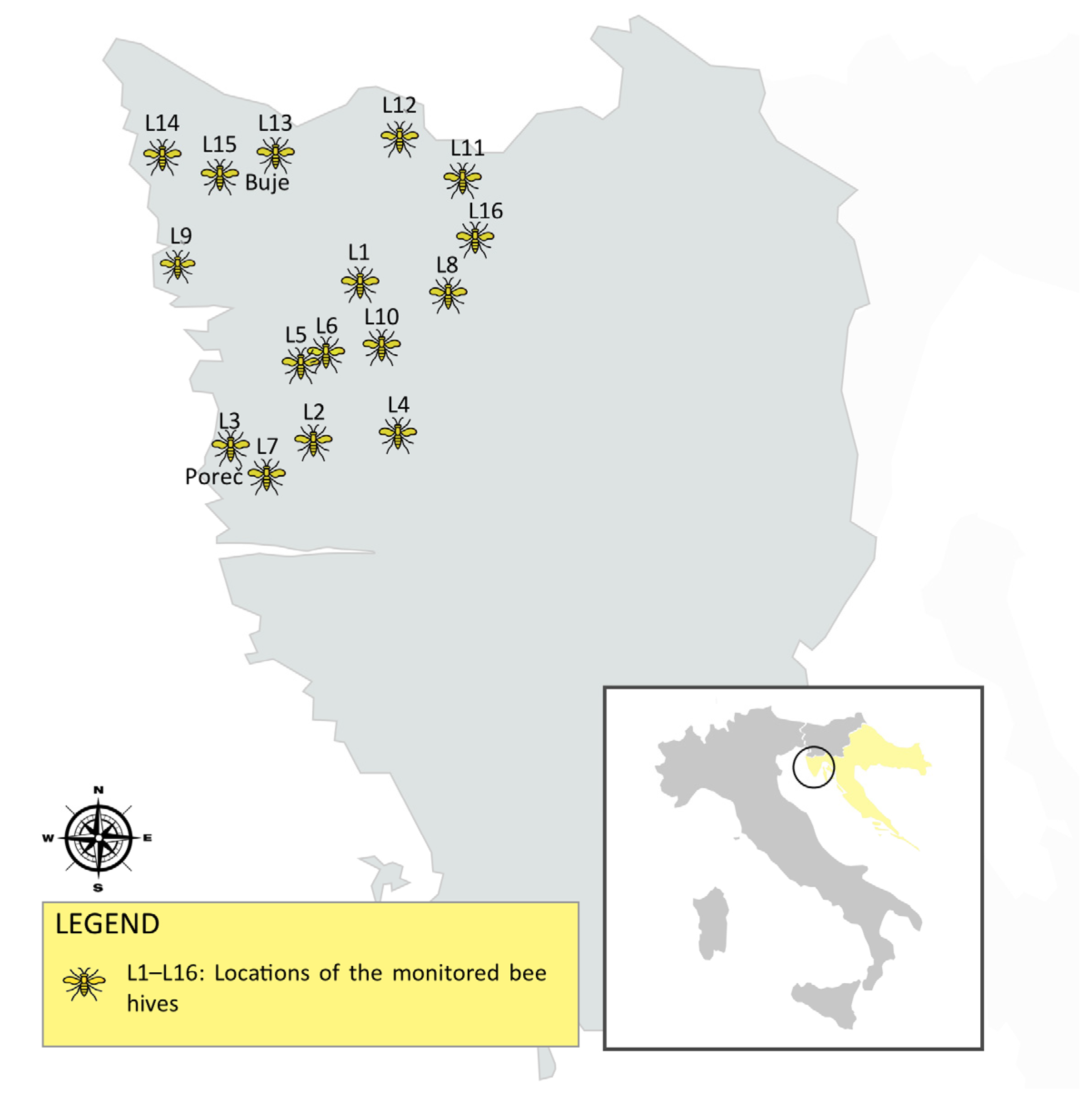
2.1. Study Area
2.2. Descriptive Variables
2.3. Sampling
2.4. Selectivity of the Collecting Method
2.5. Morphological Identification
2.6. Statistical Analyses
3. Results
3.1. Diptera and Vespidae Diversity
3.2. Pests Findings
3.3. First Findings
3.4. Correlation with Environmental Variables
4. Discussion
4.1. Pests Findings
4.2. First Records in Croatia
4.3. Species Diversity in the Mosaic Agricultural Landscape and Correlations with Different Variables
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, E.O. The biological diversity crises. BioScience 1985, 35, 700–706. [Google Scholar] [CrossRef]
- Ostiguy, N. Pests and Pollinators. Nat. Educ. Knowl. 2011, 3, 3. Available online: www.nature.com/scitable/knowledge/library/pests-and-pollinators-23564436/ (accessed on 25 October 2022).
- Courtney, G.W.; Pape, T.; Skevington, J.H.; Sinclair, B.J. Biodiversity of Diptera. In Insect Biodiversity: Science and Society; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 229–278. [Google Scholar] [CrossRef]
- Ropars, L.; Affre, L.; Thébault, E.; Geslin, B. Seasonal dynamics of competition between honey bees and wild bees in a protected Mediterranean scrubland. OIKOS 2022, 2022, e08915. [Google Scholar] [CrossRef]
- Borges, R.C.; Brito, R.M.; Imperatriz-Fonseca, V.L.; Giannini, T.C. The Value of Crop Production and Pollination Services in the Eastern Amazon. Neotrop. Entomol. 2020, 49, 545–556. [Google Scholar] [CrossRef]
- Mudrić-Stojnić, S.; Andrić, A.; Jòzan, Z.; Vujić, A. Pollinator Diversity (Hymenoptera and Diptera) in Semi-natural Habitats in Serbia during Summer. Arch. Biol. Sci. 2012, 64, 777–786. [Google Scholar] [CrossRef]
- Kremen, C.; Williams, N.M.; Aizen, M.A.; Gemmill-Herren, B.; LeBuhn, G.; Minckley, R.; Packer, L.; Potts, S.G.; Roulston, T.; Steffan-Dewenter, I.; et al. Pollination and other ecosystem services produced by mobile organisms: A conceptual framework for the effects of land-use change. Ecol. Lett. 2007, 10, 299–314. [Google Scholar] [CrossRef]
- Rivers-Moore, J.; Andrieu, E.; Vialatte, A.; Ouin, A. Wooded Semi-Natural Habitats Complement Permanent Grasslands in Supporting Wild Bee Diversity in Agricultural Landscapes. Insects 2020, 11, 812. [Google Scholar] [CrossRef]
- Fahrig, L.; Baudry, J.; Brotons, L.; Burel, F.G.; Crist, T.O.; Fuller, R.J.; Sirami, C.; Siriwardena, G.M.; Martin, J.-L. Functional landscape heterogeneity and animal biodiversity in agricultural landscapes. Ecol. Lett. 2011, 14, 101–112. [Google Scholar] [CrossRef]
- Hass, A.L.; Kormann, U.G.; Tscharntke, T.; Clough, Y.; Baillod, A.B.; Sirami, C.; Fahrig, L.; Martin, J.L.; Baudry, J.; Bertrand, C.; et al. Landscape configurational heterogeneity by small-scale agriculture, not crop diversity, maintains pollinators and plant reproduction in western Europe. Proc. R. Soc. B Biol. Sci. 2018, 285, 20172242. [Google Scholar] [CrossRef]
- Purvis, E.E.N.; Matthew, L.; Meehan, Z.L. Agricultural field margins provide food and nesting resources to bumble bees (Bombus spp., Hymenoptera: Apidae) in Southwestern Ontario, Canada. Insect Conserv. 2020, 13, 219–228. [Google Scholar] [CrossRef]
- Jalut, G.; Dedoubat, J.J.; Fontugne, M.; Otto, T. A Holocene circum-Mediterranean vegetation changes: Climate forcing and human impact. Quat. Int. 2009, 200, 4–18. [Google Scholar] [CrossRef]
- Vimal, R.; Fonderflick, J.; Thompson, J.; Pluvinet, P.; Debussche, M.; Cheylan, M.; Géniez, P.; Mathevet, R.; Acquarone, A.; Lepart, J. Integrating habitat diversity into species conservation in the Mediterranean mosaic landscape. Basic Appl. Ecol. 2017, 22, 36–43. [Google Scholar] [CrossRef]
- Asubonteng, K.O.; Ros-Tonen, M.A.F.; Baud, I.; Pfeffer, K. Envisioning the Future of Mosaic Landscapes: Actor Perceptions in a Mixed Cocoa/Oil-Palm Area in Ghana. Environ. Manag. 2021, 68, 701–719. [Google Scholar] [CrossRef]
- Sole-Senan, X.O.; Juárez-Escario, A.; Conesa, J.A.; Jordi Recasens Guinjuan, J.R. Plant species, functional assemblages and partitioning of diversity in a Mediterranean agricultural mosaic landscape. Agric. Ecosyst. Environ. 2018, 256, 163–172. [Google Scholar] [CrossRef]
- Oplanić, M.; Čehić, A.; Begić, M.; Franić, M. Education for sustainable agricultural development: Case study of Agricultural Secondary school Mate Balota in Poreč. J. Cent. Eur. Agric. 2021, 22, 226–239. [Google Scholar] [CrossRef]
- ARKOD. Overview of the Number and Area of ARKOD by Settlements and Type of Agricultural Land Use. APPRRR. Available online: https://www.arkod.hr (accessed on 31 December 2021).
- Goethe, J.K.; Dorman, S.J.; Huseth, A.S. Local and landscape scale drivers of Euschistus servus and Lygus lineolaris in North Carolina small grain agroecosystems. Agric. Forest Entomol. 2021, 23, 441–451. [Google Scholar] [CrossRef]
- DeFries, R.; Hansen, A.; Turner, B.L.; Reid, R.; Liu, J. Land use change around protected areas: Management to balance human needs and ecological function. Ecol. Appl. 2007, 17, 1031–1038. [Google Scholar] [CrossRef]
- Kennedy, C.M.; Lonsdorf, E.; Neel, M.C.; Williams, N.M.; Ricketts, T.H.; Winfree, R.; Bommarco, R.; Brittain, C.; Burley, A.L.; Cariveau, D.; et al. A global quantitative synthesis of local and landscape effects on wild bee pollinators in agroecosystems. Ecol. Lett. 2013, 16, 584–599. [Google Scholar] [CrossRef]
- Tscharntke, T.; Klein, A.M.; Kruess, A.; Steffan-Dewenter, I.; Thies, C. Landscape perspectives on agricultural intensification and biodiversity—Ecosystem service management. Ecol. Lett. 2005, 8, 857–874. [Google Scholar] [CrossRef]
- Tylianakis, J.M.; Klein, A.M.; Tscharntke, T. Spatiotemporal variation in the diversity of Hymenoptera across a tropical habitat gradient. Ecology 2005, 86, 3296–3302. [Google Scholar] [CrossRef] [Green Version]
- Winfree, R.; Bartomeus, I.; Daniel, P.; Cariveau, D.P. Native pollinators in anthropogenic habitats. Annu. Rev. Ecol. Evol. System. 2011, 42, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Watt, A.; Ekbom, B.; Jones, H. Invasive insect pests: A growing problem? Recent research published in Agricultural and Forest Entomology. Antenna 2019, 43, 27–30. [Google Scholar]
- Triplehorn, C.A.; Johnson, N.F. Borror and DeLong’s Introduction to the Study of Insects, 7th ed.; Brooks/Cole, Thomson Learning: Belmont, CA, USA, 2005. [Google Scholar]
- Pimentel, D.; Lach, L.; Zuniga, R.; Morrison, D. Environmental and Economic Costs of Nonindigenous Species in the United States. BioScience 2000, 50, 53–65. [Google Scholar] [CrossRef] [Green Version]
- Sousa, M.; Araújo, E.; Silva, J.; Barbosa, D.; Fernandes, E. Fruit flies (Diptera: Tephritidae) in commercial mango orchards in a semiarid region of Brazil. Rev. Brasileira Fruticult. 2019, 41. [Google Scholar] [CrossRef] [Green Version]
- Minga, C.; Mazón, M.; Troya, H. Population dynamics, native parasitoids and incidence of Tephritidae (Insecta, Diptera) in cherimoya (Annona cherimola mill.) secondary forests at Southern Ecuador. Int. J. Pest Manag. 2023, 69, 14–21. [Google Scholar] [CrossRef]
- Berrones-Morales, M.; Vanoye-Eligio, V.; Coronado-Blanco, J.M.; Gaona-García, G.; Sánchez-Ramos, G. Species diversity of fruit flies (Diptera: Tephritidae) through different ecosystems in a Neotropical transition zone in Mexico. J. Insect Conserv. 2020, 24, 219–231. [Google Scholar] [CrossRef]
- Pajač Živković, I.; Barić, B.; Šubić, M.; Seljak, G.; Mešić, A. First record of alien species Chymomyza amoena [Diptera, Drosophilidae] in Croatia. Šumarski List 2017, 9–10, 489–492. [Google Scholar]
- Skuhravá, M.; Martinez, M.; Roques, A. Diptera. Alien terrestrial arthropods of Europe. BioRisk 2010, 4, 553–602. [Google Scholar] [CrossRef] [Green Version]
- Allwood, A.J.; Leblanc, L. Losses Caused by Fruit Flies (Diptera: Tephritidae) in Seven Pacific Island Countries. In Management of Fruit Flies in the Pacific, a Regional Symposium, Nadi, Fiji, 28–31 October 1996; Allwood, A.J., Drew, R.A.I., Eds.; Australian Centre for International Agricultural Research: Canberra, ACT, Australia, 1997; ACIAR Proceedings No. 76; p. 267. [Google Scholar]
- Blaser, S.; Heusser, C.; Diem, H.; Felten, A.; Gueuning, M.; Andreou, M.; Boonham, N.; Tomlinson, J.; Müller, P.; Utzinger, J.; et al. Dispersal of harmful fruit fly pests by international trade and a loop-mediated isothermal amplification assay to prevent their introduction. Geospat. Health 2018, 13, 370–373. [Google Scholar] [CrossRef] [Green Version]
- Dias, N.P.; Zotti, M.J.; Montoya, P.; Carvalho, I.R.; Nava, D.E. Fruit fly management research: A systematic review of monitoring and control tactics in the world. Crop Prot. 2018, 112, 187–200. [Google Scholar] [CrossRef]
- Valido, A.; Rodríguez-Rodríguez, M.C.; Jordano, P. Honeybees disrupt the structure and functionality of plant-pollinator networks. Sci. Rep. 2019, 9, 4711. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Honey Market Overview; Office for Food, Farming, Fisheries: Brussels, Belgium, 2020; Directive on honey (2001/110). [Google Scholar]
- Ferro, M.L.; Summerlin, M. Developing a standardized list of entomological collection methods for use in databases. ZooKeys 2019, 861, 145–156. [Google Scholar] [CrossRef] [Green Version]
- Knuff, A.K.; Winiger, N.; Klein, A.M.; Segelbacher, G.; Staab, M. Optimizing sampling of flying insects using a modified window trap. Ecol. Evol. 2019, 10, 1629–1827. [Google Scholar] [CrossRef] [Green Version]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Meth. Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- McCullagh, P.; Nelder, J.A. (Eds.) Generalized Linear Models; Springer: Boston, MA, USA, 1989. [Google Scholar]
- Bacaro, G.; Rocchini, D.; Bonini, I.; Marignani, M.; Maccherini, S.; Chiarucci, A. The role of regional and local scale predictors for plant species richness in Mediterranean forests. Plant Biosyst. 2008, 142, 630–642. [Google Scholar] [CrossRef]
- Calcagno, V. Glmulti: Model Selection and Multimodel Inference Made Easy. R Package Version 1.0.8. 2020. Available online: https://CRAN.R-project.org/package=glmulti (accessed on 4 October 2022).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchinm, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. vegan: Community Ecology Package. R Package Version 2.5-7. 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 5 October 2022).
- De Cáceres, M.; Legendre, P. Associations between species and groups of sites: Indices and statistical inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef]
- Baracchi, D.; Cusseau, G.; Pradella, D.; Turillazzi, S. Defense reaction of Apis mellifera ligustica against the attacks of the European Hornet Vespa crabro. Ethol. Ecol. Evol. 2010, 22, 281–294. [Google Scholar] [CrossRef]
- Tarandek, J. Population of Vinegar Fly Chymomyza amoena (Loew 1862) in Orchard in the Međimurje Area. Master’s Thesis, University of Zagreb Faculty of Agriculture, Zagreb, Croatia, 2019. [Google Scholar]
- Grassi, A.; Palmieri, L.; Giongo, L. Drosophila (Sophophora) suzukii (Matsumura)—New pest of small fruit crops in Trentino. Terra Trent. 2009, 10, 19–23. [Google Scholar]
- Calabria, G.; Máca, J.; Bächli, G.; Serra, L.; Pascual, M. First records of the potential pest species Drosophila suzukii (Diptera: Drosophilidae) in Europe. J. Appl. Entomol. 2010, 136, 139–147. [Google Scholar] [CrossRef]
- Labanowska, B.H.; Piotrowski, W. The spotted wing drosophila Drosophila suzukii (Matsumura, 1931)—Monitoring and first records in Poland. J. Hortic. Res. 2015, 23, 49–57. [Google Scholar] [CrossRef] [Green Version]
- Dreves, A.J.; Walton, V.; Fisher, G. A New Pest Attacking Healthy Ripening Fruit in Oregon: Spotted Wing Drosophila, Drosophila suzukii (Matsumura). Oregon State University. Extension Service. 2009. Available online: http://berrygrape.org/files/Dsuzukii_alert.pdf (accessed on 14 October 2022).
- Lue, C.H.; Mottern, J.L.; Walsh, G.C.; Burrington, M.L. New record for the invasive spotted wing drosophila, Drosophila suzukii (Matsumura, 1931) (Diptera: Drosophilidae) in Anillaco, western Argentina. Proc. Entomol. 2017, 119, 146–150. [Google Scholar] [CrossRef]
- Singh, F.R.; Sarswat, M.; Lhamo, N.; Sati Asha, P.S. Records of Zaprionus indianus and Drosophila suzukii indicus as Invasive Fruit Pests from Mid Valley Region of Garhwal Uttarakhand, India. Drosophila Information Service no. 97. 2014, pp. 119–123. Available online: http://www.ou.edu/journals/dis/DIS97/DIS%2097%20-%202014%20-%20Master%20Copy.pdf (accessed on 12 October 2022).
- Stark, A. Nachweis der „Pfauenfliege“ Callopistromyia annulipes (Macquart, 1855) in Sachsen-Anhalt (Diptera, Ulidiidae). Entomol. Nachr. Ber. 2017, 61, 108. [Google Scholar]
- Dvořák, L.; Čejka, T.; Semebauer, M. New records of Callopistromyia annulipes (Diptera: Ulidiidae) from Slovakia. Folia Oecologica 2017, 9, 18–21. [Google Scholar]
- Korneyev, V.; Dvořák, L.; Kameneva, E. New Records of Callopistromyia annulipes Macquart (Diptera: Ulidiidae: Otitinae: Myennidini) in Europe. Ukr. Entomofaunistyka 2014, 5, 10. [Google Scholar]
- Kasalo, N.; Topić, M.; Tarandek, A. The first record of the peacock fly Callopistromyia annulipes (Macquart, 1855) (Diptera: Ulidiidae) in Croatia revealed by social media. Nat. Croat. 2021, 30, 523–528. [Google Scholar] [CrossRef]
- Akmeșe, V.; Sertkaya, E.; Yucel, C. Türkiye’de sorgumda yeni bir zararlı, Atherigona varia (Meigen, 1826) (Diptera: Muscidae). Türkiye Entomoloji Bülteni 2016, 6, 261. [Google Scholar] [CrossRef] [Green Version]
- Riyazaddin, M.; Kavi Kishor, P.B.; Ashok Kumar, A.; Reddy, B.V.S.; Munghate, R.S.; Sharma, H.C. Mechanisms and diversity of resistance to sorghum shoot fly, Atherigona soccata. Plant Breed 2015, 134, 423–436. [Google Scholar] [CrossRef] [Green Version]
- Jong, H.; Zuijlen, J. Chymomyza amoena (Diptera: Drosophilidae) new for The Netherlands. Entomol. Ber. 2003, 63, 103–104. [Google Scholar]
- Band, H.T.; Bächli, G.; Band, R.N. Behavioral constancy for interspecies dependency enables Nearctic Chymomyza amoena (Loew) (Diptera: Drosophilidae) to spread in orchards and forests in Central and Southern Europe. Biol. Invasions 2005, 7, 509–530. [Google Scholar] [CrossRef]
- Pontikakos, V.M.; Tsiligiridis, T.A.; Yialouris, C.P.; Kontodimas, D.C. Pest management control of olive fruit fly (Bactrocera oleae) based on a location-aware agro-environmental system. Comput. Electron. Agric. 2012, 87, 39–50. [Google Scholar] [CrossRef]
- Bon, M.C.; Hoelmer, K.A.; Pickett, C.H.; Kirk, A.A.; He, Y.; Mahmood, R.; Daane, K.M. Populations of Bactrocera oleae (Diptera: Tephritidae) and Its Parasitoids in Himalayan Asia. Ann. Entomol. 2016, 109, 81–91. [Google Scholar] [CrossRef] [Green Version]
- Khamis, F.M.; Karam, S.N.; Ekesi, M.D.E.; Meyer, A.; Bonomi, L.M.; Gomulski, F.; Scolari, P.; Gabrieli, P.; Siciliano, D.; Masiga; et al. Uncovering the tracks of a recent and rapid invasion: The case of the fruit ßy pest Bactrocera invadens (Diptera: Tephritidae) in Africa. Mol. Ecol. 2009, 18, 4798. [Google Scholar] [CrossRef]
- Popović, L.; Bjeliš, M. Black fig fly—Silba adipata McAlpin (Diptera, Lonchaeidae), pest of growing importance in Croatian fig cultivation. In Proceedings of the 12th Slovenian Conference on Plant Protection with International Participation, Ptuj, Slovenia, 2–3 March 2015; p. 95. [Google Scholar]
- Abbes, K.; Abir, H.; Harbi, A.; Mars, M.; Brahim, C. The black fig fly Silba adipata (Diptera: Lonchaeidae) as an emerging pest in Tunisia: Preliminary data on geographic distribution, bioecology and damage. Phytoparasitica 2021, 49, 1. [Google Scholar] [CrossRef]
- Giliomee, J.H. Recent Establishment of Many Alien Insects in South Africa—A Cause for Concern. Afr. Entomol. 2011, 19, 151–155. [Google Scholar] [CrossRef]
- Elaini, R.; Mazih, A. Current Status and Future Prospects of Ceratitis capitata Wiedemann (Diptera: Tephritidae) Control in Morocco. J. Entomol. 2018, 15, 47–55. [Google Scholar]
- Öztemiz, S.; Doğanlar, M. Invasive plant pests (Insecta and Acarina) of Turkey. Munis Entomol. Zool. 2015, 10, 144–159. [Google Scholar]
- De Villiers, M.; Manrakhan, A.; Addison, P.; Hattingh, V. The distribution, relative abundance, and seasonal phenology of Ceratitis capitata, Ceratitis rosa, and Ceratitis cosyra (Diptera: Tephritidae) in South Africa. Environ. Entomol. 2013, 42, 831–840. [Google Scholar] [CrossRef]
- GBIF Home Page. Available online: https://www.gbif.org (accessed on 20 January 2023).
- Merz, B. A revision of the Herina lugubris species group (Diptera, Ulididae, Otitinae) with the description of two new species. Rev. Suisse Zool. 2002, 109, 407–431. [Google Scholar] [CrossRef]
- Roháček, J.; Barták, M. Trixoscelididae. In Diptera in an Industrially Affected Region (North-Western Bohemia, Bílina and Duchcov environs), II.—Folia Facultatis Scientiarium Naturalium Universitatis Masarykianae Brunensis, Biologia; Barták, M., Vaňhara, J., Eds.; Masaryk University Brno: Brno, Czech Republic, 2021; Volume 105, pp. 407–409. [Google Scholar]
- Carles-Tojrá, M.; Verdugo Páez, A. Periscelis piricercus sp. n.: A new periscelidid species from Spain (Diptera: Periscelididae). Heteropterus. Rev. Entomol. 2009, 9, 101–104. [Google Scholar]
- Papp, L.; Withers, P. A revision of the Palaearctic periscelidinae with notes on some New World species (Diptera: Periscelididae). Ann. Hist. Nat. Mus. Nat. Hung. 2011, 103, 345–371. [Google Scholar]
- Wallace, P.F.; O’Connor, J.P. Palloptera muliebris (Harris) (Dipt., Pallopteridae) discovered in Dublin City. Entomol. Mon. Mag. 1997, 133, 114. [Google Scholar]
- Ozerov, A.L. Review of the family Pallopteridae (Diptera) of the fauna of Russia. Russ. Entomol. J. 2009, 18, 129–146. [Google Scholar]
- Roháček, J.; Andrade, R. Periscelis fugax sp. nov., an overlooked European species of Periscelididae (Diptera), with notes on the morphology and terminology of terminalia. Acta Entomol. Musei Nat. Pragae 2017, 57, 229–251. [Google Scholar] [CrossRef] [Green Version]
- Carles-Tolrá, M.; Kameneva, E.P. Nuevos datos faunísticos sobre Ulidiidae de España y Portugal (Diptera, Ulidiidae). Heteropterus Rev. Entomol. 2008, 8, 47–51. [Google Scholar]
- Almeida, J. New records of picture-winged flies (Diptera, Ulidiidae) for Portugal. Arq. Entomoloxicos 2013, 8, 149–154. [Google Scholar]
- Brake, I. Milichiidae of the Lake Kerkini region in Greece. Milichiidae Online. 2010. Available online: http://milichiidae.info/ (accessed on 22 October 2022).
- Brake, I. The type material of Milichiidae and Carnidae (Insecta: Diptera: Schizophora) in the Naturhistorisches Museum Wien. Ser. B Bot. Zool. 2008, 110, 67–76. [Google Scholar]
- Roháček, J. Acalyptrate flies (Diptera) on glacial sand deposits in the Hlučínsko region (NE Czech Republic): Most interesting records. Acta Musei Sil. Sci. Nat. 2016, 65, 33–46. [Google Scholar] [CrossRef] [Green Version]
- Nita, A.; Hartel, T.; Manolache, S.; Ciocanea, C.M.; Miu, I.V.; Rozylowicz, L. Who is researching biodiversity hotspots in Eastern Europe? A case study on the grasslands in Romania. PLoS ONE 2019, 14, e0217638. [Google Scholar] [CrossRef] [Green Version]
- Papp, L.; Ševčík, J. Grzegorzekia hungarica sp. n. and new records of European Mycetophilidae and Bolithophilidae (Diptera). Acta Zool. Univ. Comenianae Entomol. Gaz. 2015, 66, 53–60. [Google Scholar]
- Hauser, M.; Hogue, J.N.; Fiesler, E. Lamprolonchaea smaragdi (Walker, 1849) (Diptera: Lonchaeidae) newly established in Los Angeles County, California: First record for North America. Pan-Pac. Entomol. 2017, 93, 61–64. [Google Scholar] [CrossRef]
- De Bree, E.; Ketelaar, R. Neoalticomerus formosus new for the fauna of The Netherlands (Diptera: Odiniidae). Entomol. Ber. 2018, 78, 226–228. [Google Scholar]
- Withers, P.; Papp, L. The Palaearctic species of Neoalticomerus Hendel (Diptera, Odiniidae). Dipter. Digest 2012, 19, 53–63. [Google Scholar]
- Wang, Y.L.; Cao, H.L.; Chen, H.W. Molecular phylogeny and species delimitation of Amiota alboguttata and Amiota basdeni species groups (Diptera: Drosophilidae) from East Asia. Zool. J. Linnean Soc. 2020, 189, 1370–1397. [Google Scholar] [CrossRef]
- Smith, A.H.; Gill, W.M.; Pinkard, E.A.; Mohammed, C.L. Anatomical and histochemical defence responses induced in juvenile leaves of Eucalyptus globulus and Eucalyptus nitens by Mycosphaerella infection. For. Pathol. 2007, 37, 361–373. [Google Scholar] [CrossRef]
- Brockerhoff, E.G.; Bain, J.; Kimberley, M.; Knížek, M. Interception frequency of exotic bark and ambrosia beetles (Coleoptera: Scolytinae) and relationship with establishment in New Zealand and worldwide. Canad. J. Forest Res. 2006, 36, 289–298. [Google Scholar] [CrossRef]
- U.S. Congress, Office of Technology Assessment (OTA). Harmful Non-Indigenous Species in the United States; OTA-F-565; U.S. Government Printing Office: Washington, DC, USA, September 1993. [Google Scholar]
- Kiritani, K.; Yamamura, K. Exotic insects and their pathways for invasion. In Invasive Species-Vectors and Management Strategies; Ruiz, G.M., Carlton, J.T., Eds.; Island Press: Washington, DC, USA, 2003; pp. 44–67. [Google Scholar]
- Maynard, G.V.; Hamilton, J.G.; Grimshaw, J.F. Quarantine-Phytosanitary, sanitary and incursion management: An Australian entomological perspective. Aust. Entomol. 2004, 43, 318–328. [Google Scholar] [CrossRef]
- Kenis, M.; Rabitsch, W.; Auger-Rozenberg, M.A.; Roques, A. How can alien species inventories and interception data help us prevent insect invasions? Bull. Entomol. Res. 2007, 97, 489–502. [Google Scholar] [CrossRef] [Green Version]
- Pajač Živković, I.; Skendžić, S.; Lemić, D. Rapid spread and first massive occurrence of Halyomorpha halys (Stål, 1855) in agricultural production in Croatia. J. Cent. Eur. Agric. 2021, 22, 531–538. [Google Scholar] [CrossRef]
- Pajač Živković, I.; Barić, B.; Šubić, M.; Mešić, A. The spread of Drosophila suzukii (Matsumura, 1931) in northwestern Croatia. In Zbornik Sažetaka 2. Hrvatskog Simpozija o Invazivnim Vrstama; Jelaska, S.D., Ed.; Hrvatsko Ekološko Društvo: Zagreb, Croatia, 2016; p. 68. [Google Scholar]
- Krčmar, S.; Whitmore, D.; Pape, T.; Buenaventura, E. Checklist of the Sarcophagidae (Diptera) of Croatia, with new records from Croatia and other Mediterranean countries. ZooKeys 2019, 831, 95–155. [Google Scholar] [CrossRef] [Green Version]
- Zielke, E.; Banar, P. First records of the genus Mydaea and of some other Muscidae species from Croatia (Diptera). Acta Musei Morav. Sci. Biol. 2017, 102, 43–48. [Google Scholar]
- Zielke, E.; Banar, P. More records of Muscidae (Diptera) from Croatia with a short comment on findings of Helina interfusa (PandellÈ) reported to date in Europe. Acta Musei Morav. Sci. Biol. 2018, 103, 281–285. [Google Scholar]
- Merdic, E.; Boca, I.; Bogojević, M.S.; Landeka, N. Mosquitoes of Istria, a contribution to the knowledge of Croatian mosquito fauna (Diptera, Culicidae). Period 2008, 110, 351–360. [Google Scholar]
- Verves, Y.G.; Barták, M. New faunistic data on Sarcophagidae (Diptera) from Croatia. Kharkov Entomol. Soc. Gaz. 2021, 29, 71–76. [Google Scholar] [CrossRef]
- Masten Milek, T.; Seljak, G.; Šimala, M.; Bjeliš, M. Prvi nalaz Drosophila suzukii (Matsumara, 1931) (Diptera Drosophilidae) u Hrvatskoj. Glasilo Biljne Zaštite 2015, 5, 323–327. [Google Scholar]
- Bjeliš, M.; Buljubašić, I.; Popović, L.; Masten Milek, T. Spread of the spotted wing drosophila—Drosophila suzukii (Diptera: Drosophilidae) and new distribution records in Dalmatia region of Croatia. OEPP/EPPO Bull. 2015, 45, 214–217. [Google Scholar] [CrossRef]
- Pajač Živković, I.; Barić, B. Drosophila suzukii (Matsumura, 1931)—Potential pest of stone fruits in Croatia. Pomol. Croat. 2010, 16, 43–50. [Google Scholar]
- Walsh, D.B.; Bolda, M.P.; Goodhue, R.E.; Dreves, A.J.; Lee, J.; Bruck, D.J. Drosophila suzukii (Diptera: Drosophilidae): Invasive pest of ripening soft fruit expanding its geographic range and damage potencial. J. Integr. Pest Manag. 2011, 2, 1–8. [Google Scholar] [CrossRef]
- Dos Santos, L.A.; Mendes, M.F.; Krüger, A.P.; Blauth, M.L.; Gottschalk, M.S.; Garcia, F.R. Global potential distribution of Drosophila suzukii (Diptera, Drosophilidae). PLoS ONE 2017, 12, e0174318. [Google Scholar] [CrossRef] [Green Version]
- Mazzetto, F.; Lessio, F.; Giacosa, S.; Rolle, L.; Alma, A. Relationships between Drosophila suzukii and grapevine in North-western Italy: Seasonal presence and cultivar susceptibility. Bull. Insectol. 2020, 73, 29–38. [Google Scholar]
- Ravoet, J.; Farinelle, C. The peacock fly Callopistromyia annulipes (Macquart, 1855): A long-expected new addition to the Belgian fauna (Diptera: Ulidiidae). Bull. Ann. Soc. R. Belge Entomolog. 2017, 153, 121–122. [Google Scholar]
- Smit, J.T.; Hamers, B. De invasieve Noord-Amerikaanse pauwvlieg Callopistromyia annulipes nieuw voor Nederland (Diptera: Ulidiidae). Ned. Faun. Meded. 2011, 36, 23–27. [Google Scholar]
- Singh, B.U.; Sharma, H.C. Natural Enemies of Sorghum Shoot Fly, Atherigona soccata Rondani (Diptera: Muscidae). Biocontrol Sci. Technol. 2002, 12, 307–323. [Google Scholar] [CrossRef]
- Follak, S.; Essl, F. Spread dynamics and agricultural impact of Sorghum halepense, an emerging invasive species in Central Europe. Weed Res. 2013, 53, 53–60. [Google Scholar] [CrossRef]
- Mâca, J.; Bächli, G. On the distribution of Chymomyza amoena (Loew), a species recently introduced into Europe. Mitt. Der Schweiz. Entomol. Gesellschaft. 1994, 67, 183–188. [Google Scholar]
- Escher, S.A.; Ekenstedt, J.; Karpa, A.; Saura, A. The Drosophilidae (Diptera) of Latvia. Latvijas Entomol. 2002, 39, 62–69. [Google Scholar]
- Band, H.T. Behavior and Taxonomy of a Chymomyzid Fly (Chymomyzia amoena). Int. J. Comp. Psychol. 1988, 2, 1–26. [Google Scholar] [CrossRef]
- Dminić, I.; Bažok, R.; Igrc Barčić, J. Reduction of olive fruit fly damage by early harvesting and impact on oil quality parameters. Eur. J. Lipid Sci. Technol. 2015, 117, 103–111. [Google Scholar] [CrossRef]
- Caleca, V.; Antista, G.; Campisi, G.; Caruso, T.; Verde, G.; Maltese, M.; Rizzo, R.; Planeta, D. High Quality Extra Virgin Olive Oil from Olives Attacked by the Olive Fruit Fly, Bactrocera oleae (Rossi) (Diptera Tephritidae): Which Is the Tolerable Limit? Data from Experimental ‘Nocellara Del Belice’ and ‘Cerasuola’ Olive Groves in Sicily. Chem. Eng. Trans. 2017, 58, 451–456. [Google Scholar] [CrossRef]
- Bažok, R.; Lađarević, I.; Dminić, I. Praćenje dinamike pojave i prognoza maslinine muhe (Bactrocera oleae Gmelin) na trima sortama masline u zapadnoj Istri pomoću žutih ploča. Fragm. Phytomed. Herbol. 2011, 31, 13–30. [Google Scholar]
- Radonjić, S.; Hrnčić, S. The black fig fly Silba adipata McAlpine (Diptera, Lonchaeidae), a little known fig pest in Montenegro. Agro-Knowl. J. 2009, 10, 31–40. [Google Scholar]
- Kovačević, Ž. Voćna mušica Ceratitis capitata Wied. kao ekološki problem. Agron. Glas. 1960, 4, 161–170. [Google Scholar]
- Bjeliš, M.; Dugalić, K.; Đugum, J.; Budinšćak, Ž.; Popović, L.; Benc, D.; Perleta, M.; Cardoso-Pereira, R. Provedba akcijskog plana suzbijanja mediteranske voćne muhe na području doline Neretve. In Zbornik Sažetaka 62. Seminara Biljne Zaštite; Renata, B., Ed.; Hrvatsko Društvo Biljne Zaštite: Zagreb, Croatia, 2018; p. 25. [Google Scholar]
- Kameneva, E.P. New and little-known Ulidiidae (Diptera, Tephritoidea) from Europe. Vestn. Zool. 2008, 42, 45–72. [Google Scholar] [CrossRef] [Green Version]
- Roháček, J.; Mác, J. New and interesting records of Diptera (Asteiidae, Aulacigastridae, Milichiidae, Sphaeroceridae) from the Czech Republic. Čas. Slez. Muz. Opava (A) 2010, 59, 165–170. [Google Scholar]
- Pollini Paltrinieri, L.; Roháček, J. Periscelis (Myodris) haennii sp. nov., a new species of Periscelididae (Diptera) from Ticino, Switzerland with a new key to European species of the subgenus. Alp. Entomol. 2022, 6, 39–49. [Google Scholar] [CrossRef]
- Roháček, J. The true identity of Periscelis winnertzii and description of P. laszloi sp. nov. from Europe (Diptera: Periscelididae). Acta Entomol. Musei Natl. Pragae 2022, 62, 359–381. [Google Scholar] [CrossRef]
- Rotheray, G.E. Development sites, feeding modes and early stages of seven European Palloptera species (Diptera, Pallopteridae). Zootaxa 2014, 3900, 50–76. [Google Scholar] [CrossRef] [Green Version]
- Cannings, R.A.; Gibson, J.F. Toxonevra muliebris (Harris) (Diptera: Pallopteridae): A European fly new to North America. J. Entomol. Soc. Brit. Columbia 2020, 116, 64–68. [Google Scholar]
- Vikhrev, N.E.; Erofeeva, E.A. Review of the Phaonia pallida group (Diptera: Muscidae). Russ. Entomol. J. 2018, 27, 315–322. [Google Scholar] [CrossRef] [Green Version]
- Mantič, M.; Sikora, T.; Roháček, J.; Sevcik, J. New and interesting records of Bibionomorpha (Diptera) from the Czech and Slovak Republics. Acta Musei Sil. Sci. Natl. 2015, 64, 141–149. [Google Scholar] [CrossRef] [Green Version]
- Williams, P.H. Some properties of rarity scores used in site quality assessment. Br. J. Entomol. Nat. Hist. 2000, 13, 73–86. [Google Scholar]
- Vrdoljak, S.M.; Samways, M.J. Agricultural mosaics maintain significant flower and visiting insect biodiversity in a global hotspot. Biodivers. Conserv. 2016, 23, 133–148. [Google Scholar] [CrossRef]
- Kevan, P.G. Pollinators as bioindicators of the state of the environment: Species, activity and diversity. Agric. Ecosyst. Environ. 1999, 74, 373–393. [Google Scholar] [CrossRef]
- Shackelford, G.; Steward, P.R.; Benton, T.G.; Kunin, W.E.; Potts, S.G.; Biesmeijer, J.C.; Sait, S.M. Comparison of pollinators and natural enemies: A meta-analysis of landscape and local effects on abundance and richness in crops. Biol. Rev. Camb. Philos. Soc. 2013, 88, 1002–1021. [Google Scholar] [CrossRef]
- Altieri, M.A.; Nicholls, C.I. Agroecology Scaling Up for Food Sovereignty and Resiliency. In Sustainable Agriculture Reviews; Lichtfouse, E., Ed.; Springer: Dordrecht, The Netherlands, 2012; Volume 11. [Google Scholar] [CrossRef]
- Dhamorikar, A.H. Flies matter: A study of the diversity of Diptera families (Insecta: Diptera) of Mumbai Metropolitan Region, Maharashtra, India, and notes on their ecological roles. J. Threat. Taxa 2017, 9, 10865–10879. [Google Scholar] [CrossRef] [Green Version]
- Fischer, J.; Lindenmayer, D.B.; Adrian, D.; Manning, A.D. Biodiversity, ecosystem function, and resilience: Ten guiding principles for commodity production landscapes. Front. Ecol. Environ. 2006, 4, 80–86. [Google Scholar] [CrossRef] [Green Version]
- Biesmeijer, J.C.; Roberts, S.P.; Reemer, M.; Ohlemüller, R.; Edwards, M.; Peeters, T.; Schaffers, A.P.; Potts, S.G.; Kleukers, R.; Thomas, C.D.; et al. Parallel declines in pollinators and insect-pollinated plants in Britain and The Netherlands. Science 2006, 313, 351–354. [Google Scholar] [CrossRef]
- Rollin, O.; Pérez-Méndez, N.; Bretagnolle, V.; Henry, M. Preserving habitat quality at local and landscape scales increase wild bee diversity in intensive farming systems. Agric. Ecosyst. Environ. 2019, 275, 73–80. [Google Scholar] [CrossRef]
- Kremen, C.; Williams, N.M.; Thorp, R.W. Crop pollination from native bees at risk from agricultural intensification. Proc. Natl. Acad. Sci. USA 2002, 99, 16812–16816. [Google Scholar] [CrossRef] [Green Version]
- Diekötter, T.; Crist, T.O. Quantifying habitat-specific contributions to insect diversity in agricultural mosaic landscapes. Insect Conser. Divers. 2013, 6, 607–618. [Google Scholar] [CrossRef]
- Kaluza, B.F.; Wallace, H.M.; Heard, T.A.; Minden, V.; Klein, A.; Leonhardt, S.D. Social Bees Are Fitter in More Biodiverse Environments. Sci. Rep. 2018, 8, 12353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casanelles-Abella, J.; Moretti, M. Challenging the sustainability of urban beekeeping using evidence from Swiss cities. Npj Urban Sustain. 2022, 2, 3–7. [Google Scholar] [CrossRef]
- Mallinger, R.E.; Gaines-Day, H.R.; Gratton, C. Do managed bees have negative effects on wild bees? A systematic review of the literature. PLoS ONE 2017, 12, e0189268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torné-Noguera, A.; Rodrigo, A.; Cañadas, S.O.; Bosch, J. Collateral effects of beekeeping: Impacts on pollen-nectar resources and wild bee communities. Basic App. Ecol. 2015, 17, 199–209. [Google Scholar] [CrossRef] [Green Version]
- Martín-Vega, D.; Cifrián, B.; Díaz-Aranda, L.M.; Baz, A. Environmental correlates of species diversity for sarcosaprophagous Diptera across a pronounced elevational gradient in central Spain. Ital. J. Zool. 2014, 81, 415–424. [Google Scholar] [CrossRef] [Green Version]
- Loos, J.; Turtureanu, D.P.; Von Wehrden, H.; Hanspach, J.; Dorresteijn, I.; Frink, J.P.; Fischer, J. Plant diversity in a changing agricultural landscape mosaic in Southern Transylvania (Romania). Agric. Ecosyst. 2015, 199, 350–357. [Google Scholar] [CrossRef]
- Tlak Gajger, I.; Laklija, I.; Jurković, M.; Košćević, A.; Dar, S.A.; Ševar, M. The Impact of Different Biotopes and Management Practices on the Burden of Parasites in Artificial Nests of Osmia spp. (Megachilidae) Bees. Diversity 2022, 14, 226. [Google Scholar] [CrossRef]





| Species/ Family | Number of Specimens | Location | Invasive * and Pest Status in Croatia | Pest Status Worldwide/References | Host Plants |
|---|---|---|---|---|---|
| Drosophila suzukii Drosophilidae | 17,352 | L1-L16 | Important pest/invasive | Worldwide pest; Italy [48], Spain [49], Poland [50], USA [51], Argentina [52], and India [53]. | fruits vegetables |
| Callopistromyia annulipes Ulidiidae | 265 | L1-L11, L13, L15, L16 | n.d., the second record, invasive | Pest in Europe: Germany [54], Slovakia [55], France [56], and Croatia [57]. | deciduous dead trees |
| Atherigona varia Muscidae | 89 | L1, L3, L5-L9, L13, L16 | Important pest/invasive | Pest in Europe and Asia: Turkey [58] and India [59] | corn, sorghum |
| Chymomyza amoena Drosophilidae | 34 | L1, L2, L6, L8, L9, L15 | Important pest, invasive, third appearance in Croatia | Pest in Europe; Croatia [30], The Netherlands [60], and Switzerland [61]. | fruits, nuts |
| Bactrocera oleae Tephritidae | 27 | L1, L3, L6, L7, L9, L14, L15 | Important pest/domestic | Worldwide pest: Greece [62], Pakistan, India, Nepal [63], Kenya, Tanzania, Zanzibar, Uganda, and DR Congo [64]. | olive trees, fruit |
| Silba adipata Lonchaeidae | 15 | L7 | Important pest | Pest in the Mediterranean, South Africa: Croatia [65], Tunisia [66], and South Africa [67]. | fruit |
| Ceratitis capitata Tephritidae | 1 | L7 | Important pest | Worldwide pest: Morocco [68], Turkey [69], and South African Republic [70]. | fruit, vegetable, nuts |
| Species/ Family | Number of Specimens | Location | Other Records Inside Europe/References | Host Plant |
|---|---|---|---|---|
| Ulidia apicalis (Wiedemann, 1824) Ulidiidae | 464 | L1, L2, L3, L4, L5, L6, L7, L8, L9, L10, L11, L12, L13. L14, L15, L16 | Italy Spain, and Portugal [71] | possibly flowers |
| Herina lacustris (Meigen, 1826) Ulidiidae | 58 | L1 | Spain [72] and France [71] | n.d. |
| Desmometopa microps (Lamb 1914) Milichiidae | 4 | L7, L10, L12 | Czech Republic and The Netherlands [71,73] | n.d. |
| Periscelis (Myodris) piricercus (Carles-Tolrá & Verdugo Páez, 2009) Periscelididae | 3 | L9, L10 | Spain [74] and Portugal [75] | trees |
| Toxoneura muliebris (Harris, 1780 Pallopteridae | 3 | L4, L15 | Ireland [76]; Russia [77], Italy France, Spain, The Netherlands, Greece, and Portugal [71] | possibly saprophagous species, flowers |
| Periscelis (P.) winnertzii (Egger, 1862) Periscelididae | 2 | L10, L11 | Portugal and Czech Republic [78], Finland, France, and UK [71] | n.d. |
| Cephalia rufipes (Meigen, 1826) Ulidiidae | 1 | L4 | Spain [79], Portugal [80], France, Germany, The Netherlands, and Austria [71] | n.d. |
| Desmometopa discipalpis (Papp, 1993) Milichiidae | 1 | L2 | Greece [81], Germany [82], Czech Republic [83], Sweden, and Denmark [71] | n.d. (possibly saprophagous species) |
| Phaonia regalis (Stein, 1900) Phaonia | / | / | Austria and Bulgaria [71] | n.d. |
| Grzegorzekia hungarica (Papp & Ševčík, 2007) Mycetophilidae | / | / | Hungary and Romania [84,85] | n.d. |
| Lonchaea peregrina (Becker, 1895) Lonchaeidae | / | / | ||
| Lamprolonchaea smaragdi (Walker, 1849) Lonchaeidae | / | / | Spain, Portugal, and Greece [71,86] | vegetables, crops |
| Neoalticomerus formosus (Loew, 1844) Odiniidae | / | / | The Netherlands [87], Sweden, Finland [71], Poland, France, and Italy [87] | n.d. |
| Odinia ornata (Zetterstedt, 1838) Odiniidae | / | / | Sweden, Finland, and UK [71] | n.d. |
| Amiota alboguttata (Wahlberg, 1839) Drosophilidae | / | / | Sweden, Finland, UK, and Norway [71] | possibly fermenting tree sap |
| Scaptodrosophila deflexa (Duda, 1924) Drosophilidae | / | / | UK, Sweden, Finland, Switzerland, and The Netherlands [71,88] | n.d. |
| Selected Variables | Degree of Freedom | Sum Sq | Coefficient Sign | p-Value (F Tests) |
|---|---|---|---|---|
| Agriculture Intensity | 2 | 59.43 | Factor | 0.071 |
| Anthropic Disturbance | 2 | 98.08 | Factor | 0.025 * |
| Distance from the sea | 1 | 4.62 | −0.932 | 0.458 |
| N° of hornets | 1 | 36.16 | 0.253 | 0.064 |
| N° of bee colonies | 1 | 112.66 | −0.348 | 0.006 ** |
| R-squared | 0.855 ** |
| Selected Variables | Degree of Freedom | Sum Sq | Coefficient Sign | p-Value (F Tests) |
|---|---|---|---|---|
| Agriculture Intensity | 2 | 4.52 | Factor | 0.168 |
| Anthropic Disturbance | 2 | 10.20 | Factor | 0.034 * |
| Elevation | 1 | 2.63 | −0.004 | 0.145 |
| R-squared | 0.621 ** |
| Agriculture Intensity—level 3 | |||||
| Species | A | B | stat | p-value | significance |
| Bactrocera oleae | 0.7835 | 1 | 0.885 | 0.0186 | * |
| Scathophaga stercoraria | 0.8082 | 0.75 | 0.779 | 0.0224 | * |
| Agriculture Intensity—combined levels 2 and 3 | |||||
| A | B | stat | p-value | significance | |
| Atherigona varia | 0.8992 | 1 | 0.948 | 0.0038 | ** |
| Anthropic disturbance—level 3 | |||||
| A | B | stat | p-value | significance | |
| Bactrocera oleae | 0.807 | 1 | 0.898 | 0.0038 | ** |
| Habitat type—habitat H (herbaceous) | |||||
| A | B | stat | p-value | significance | |
| Bercaea africa | 0.7903 | 1 | 0.889 | 0.0098 | ** |
| Heteronychia filia | 1 | 0.6667 | 0.816 | 0.021 | * |
| Habitat type—habitat S (shrubs) | |||||
| A | B | stat | p-value | significance | |
| Chymomyza amoena | 1 | 0.6667 | 0.816 | 0.4446 | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sladonja, B.; Tlak Gajger, I.; Uzelac, M.; Poljuha, D.; Garau, C.; Landeka, N.; Barták, M.; Bacaro, G. The Impact of Beehive Proximity, Human Activity and Agricultural Intensity on Diptera Diversity in a Mediterranean Mosaic of Agroecosystems, with a Focus on Pest Species. Animals 2023, 13, 1024. https://doi.org/10.3390/ani13061024
Sladonja B, Tlak Gajger I, Uzelac M, Poljuha D, Garau C, Landeka N, Barták M, Bacaro G. The Impact of Beehive Proximity, Human Activity and Agricultural Intensity on Diptera Diversity in a Mediterranean Mosaic of Agroecosystems, with a Focus on Pest Species. Animals. 2023; 13(6):1024. https://doi.org/10.3390/ani13061024
Chicago/Turabian StyleSladonja, Barbara, Ivana Tlak Gajger, Mirela Uzelac, Danijela Poljuha, Clara Garau, Nediljko Landeka, Miroslav Barták, and Giovanni Bacaro. 2023. "The Impact of Beehive Proximity, Human Activity and Agricultural Intensity on Diptera Diversity in a Mediterranean Mosaic of Agroecosystems, with a Focus on Pest Species" Animals 13, no. 6: 1024. https://doi.org/10.3390/ani13061024







