Diet and Plastic Ingestion in the Blackmouth Catshark Galeus melastomus, Rafinesque 1810, in Italian Waters
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Data
2.2. Data Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marquard, E.; Wigelt, E.A.; Temperton, V.M.; Roscher, C.; Schumacher, J.; Buchmann, N.; Fischer, M.; Weisser, W.; Schmid, B. Plant species richness and functional composition drive over yielding in a six-year grassland experiment. Ecology 2009, 90, 3290–3302. [Google Scholar] [CrossRef]
- Primarck, R.; Boitani, L. A Primer of Conservation Biology, 5th ed.; Sinauer Associats, Inc., Oxford University Press: Oxford, UK, 2012; pp. 26–34. [Google Scholar]
- Danovaro, R. Biologia Marina, 2nd ed.; UTET Università: Novara, Italy, 2019; pp. 161–164, 240–247. [Google Scholar]
- Estes, J.A.; Terbough, J.; Brashares, J.S.; Power, M.E.; Berger, J.; Bond, W.J.; Carpenter, S.R.; Essington, T.E.; Holt, R.D.; Jackson, J.B.C.; et al. Trophic downgrading of planet earth. Science 2011, 333, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Compagno, L.; Dando, M.; Fowler, S. Sharks of the World; Princeton University Press: Princeton, NJ, USA, 2005; pp. 20–42. [Google Scholar]
- Mancusi, C.; Baino, R.; Fortuna, C.; De Sola, L.G.; Morey, G.; Bradai, M.N.; Kallianotis, A.; Soldo, A.; Hemida, F.; Saad, A.A.; et al. MEDLEM database, a data collection on large Elasmobranchs in the Mediterranean and Black seas. Mediterr. Mar. Sci. 2020, 21, 276–288. [Google Scholar] [CrossRef]
- D’Iglio, C.; Savoca, S.; Rinelli, P.; Spanò, N.; Capillo, G. Diet of the Deep-Sea Sharks Galeus melastomus Rafinesque, 1810, in the Mediterranean Sea: What We Know and What We Should Know. Sustainability 2021, 13, 3962. [Google Scholar] [CrossRef]
- Relini, G.; Lanteri, L. Osteichtyes. Biol. Mar. Mediterr. 2010, 17, 647–649. [Google Scholar]
- Serena, F. Fieled Identification Guide to the Sharks and Rays of the Mediterranean and Black Sea; FAO: Rome, Italy, 2005. [Google Scholar]
- Ungaro, N.; Marano, G.; Marsan, R. Galeus melastomus Rafinesque, 1810 (Selachii, Scyliorhinidae). Distribuzione e biologia sui fondi batiali del basso Adriatico. Att. Rel. Acc. Pugl. Sci. 1994, 49, 195–207. [Google Scholar]
- Ebert, R.; David, A.; Dando, M. Field guide to Sharks, Rays & Chimaeras of Europe and the Mediterranean; Princeton University Press: Princeton, NJ, USA, 2020; p. 304. [Google Scholar]
- Rey, J.; De Sola, L.G.; Massutí, E. Distribution and Biology of the Blackmouth Catshark Galeus melastomus in the Alboran Sea (Southwestern Mediterranean). J. Northwest Atl. Fish. Sci. 2005, 35, 215–233. [Google Scholar] [CrossRef]
- Olaso, I.; Velasco, F.; Sánchez, F.; Serrano, A.; Rodriguez-Cabello, C.; Cendrero, O. Trophic Relations of Lesser-potted Catshark (Scyliorhinus canicula) and Blackmouth Catshark (Galeus melastomus) in the Cantabrian Sea. J. Northwest Atl. Fish. Sci. 2005, 35, 481–494. [Google Scholar] [CrossRef]
- Leonetti, F.L.; Giglio, G.; Leone, A.; Coppola, F.; Romano, C.; Bottaro, M.; Reneiro, R.F.; Milazzo, C.; Micarelli, P.; Tripepi, S.; et al. An updated checklist of chondrichthyans of Calabria (Central Mediterranean, southern Italy), with emphasis on rare species. Mediterr. Mar. Sci. 2020, 21, 794–807. [Google Scholar] [CrossRef]
- Ragonese, S.; Nardone, G.; Ottonello, D.; Gancitano, S.; Giusto, G.B.; Sinacori, G. Distribution and biology of the Blackmouth cathskark Galeus melastomus in the Strait of Sicily (Central Mediterranean Sea). Mediterr. Mar. Sci. 2009, 10, 55–72. [Google Scholar] [CrossRef]
- Pinto, C.; Mannini, A.; Relini, G. Remarks on Galeus melastomus in the northern Ligurian Sea. Biol. Mar. Mediterr. 2010, 17, 224. [Google Scholar]
- Tursi, A.; D’Onghia, G.; Matarrese, A.; Piscitelli, G. Observation on population biology of the Blackmouth catshark Galeus melastomus (Chondrichthyes, Scyliorhinidae) in the Ionian Sea. Cybium 1993, 17, 187–196. [Google Scholar]
- Capapé, C.; Zaouali, J. Contribution à la biologie des Scyliorhinidae des côtes tunisiennes. VI: Galeus melastomus Rafinesque, 1810. Répartition géographique et bathymétrique, sexualité, reproduction, fécondité. Cah. Biol. Mar. 1977, 18, 449–463. [Google Scholar]
- Dulvy, N.K.; Reynolds, J.D. Evolutionary transitions among egg-laying, live bearing and maternal inputs in sharks and rays. Proc. R. Soc. B. 1997, 264, 1309–1315. [Google Scholar] [CrossRef]
- Bozzano, A.; Murgia, R.; Vallegra, S.; Hirano, J.; Archer, S. The photoreceptor system in the retinae of two dogfishes, Scyliorhinus canicula and Galeus melastomus: Possibile relationship with depth distribution and predatory lifestyle. J. Fish Biol. 2001, 59, 1258–1270. [Google Scholar] [CrossRef]
- Neves, A.; Figueiredo, I.; Moura, T.; Assis, C.; Gordo, L.S. Diet and feeding strategy of Galeus melastomus in the continental slope off southern Portugal. Vie et Milieu 2007, 57, 165–169. [Google Scholar]
- Carrassón, M.; Stefanescu, C.; Cartes, J.E. Diets and bathymetric distributions of two bathyal sharks of the Catalan deep sea (western Mediterranean). Mar. Ecol. Prog. Ser. 1992, 82, 21–30. [Google Scholar] [CrossRef]
- Fanelli, E.; Rey, K.; Torres, P.; Gil De Sola, L. Feeding habits of blackmouth catshark Galeus melastomus Rafinesque, 1810 and velvet belly lantern shark Etmopterus spinax (Linneus, 1758) in the western Mediterranean. J. Appl. Ichthyol. 2009, 25, 83–93. [Google Scholar] [CrossRef]
- Albo-Puigserver, M.; Navarro, J.; Coll, M.; Aguzzi, J.; Cardona, L.; Sáez-Liante, R. Feeding ecology and trophic position of three sympatric demersal chondrichthyans in the northwestern Mediterranean. Mar. Ecol. Prog. Ser. 2015, 524, 255–268. [Google Scholar] [CrossRef]
- Darna, S.A.; Bendiab, A.A.T.; Mouffok, S.; Belmahi, A.E.; Bouderbala, M. Observation on distribution, biology, growth, diet and feed-ing strategy of blackmouth catshark Galeus melastomus (Rafinesque, 1810) (Chondrichthyes Scyliorhinidae) in Western Algerian coast. Biodivers. J. 2018, 9, 357–368. [Google Scholar] [CrossRef]
- Bendiab, A.A.T.; Mouffok, S.; Boutiba, Z. Feeding Habits of Two Sharks Species Scyliorhinus canicula (Linnaeus, 1758) and Galeus melastomus (Rafinesque, 1810) in the Western Algerian coasts. J. Appl. Environ. Biol. Sci. 2016, 6, 108–116. [Google Scholar]
- Anastasopoulou, A.; Mytilineou, C.H.; Lefkaditou, E.; Dokos, J.; Smith, C.J.; Siapatis, A.; Beaks, P.; Papadopoulou, K.N. Diet and feeding strategy of blackmouth catshark Galeus melastomus. J. Fish Biol. 2013, 83, 1637–1655. [Google Scholar] [CrossRef] [PubMed]
- Bengil, F.; Bengil, E.G.T.; Mavruk, S.; Heral, O.; Karman, O.D.; Ozaydin, O. Feeding Ecology of Four Demersal Shark Species (Etmopterus spinax, Galeus melastomus, Scyliorhinus canicula and Squalus blainville) from the Easter Aegean Sea. Turk. J. Fish. Acquat. Sci. 2018, 19, 457–484. [Google Scholar]
- Relini, G.; Biagi, F.; Serena, F.; Belluscio, A.; Spedicato, M.T.; Rinelli, P.; Follesa, M.C.; Piccinetti, C.; Ungaro, N.; Sion, L.; et al. Selachians fished by otter trawl in the Italian Seas. Biol. Mar. Medit. 2000, 7, 347–384. [Google Scholar]
- Torres, P.; González, M.; Rey, J.; Gil-de-Sola-Simarro, L.; Acosta, J.; Ramos-Segura, A. Rose shrimp fishery’s associated fauna in not exploited grounds on the Alboran Sea slope (Western Mediterranean Sea). Rapp. Comm. Int. Mer Médit. 2001, 36, 330. [Google Scholar]
- Costa, M.E.; Erzini, K.; Borges, T.C. Reproductive biology of Blackmouth catshark, Galeus melastomus (Chondrichthyes: Scyliorhinidae) off the South coast of Portugal. J. Mar. Biol. Assoc. UK 2005, 85, 1173–1183. [Google Scholar] [CrossRef]
- Abella, A.J.; Serena, F. Comparsion of elasmobranch catches from research trawl surveys and commercial landings at port of Viareggio, Italy, in the last decade. J. Northwest Atl. Fish. Sci. 2005, 35, 345–356. [Google Scholar] [CrossRef]
- Ferretti, F.; Worm, B.; Britten, G.L.; Heithaus, M.R.; Lotze, H.K. Patterns and ecosystem consequences of shark declines in the ocean. Ecol. Lett. 2010, 13, 1055–1071. [Google Scholar] [CrossRef]
- Navarro, J.; López, L.; Coll, M.; Barría, C.; Sáez-Liante, R. Short- and long-term importance of small sharks in the diet of the rare deep-sea shark Dalatias licha. Mar. Biol. 2014, 161, 1697–1707. [Google Scholar] [CrossRef]
- Carpenter, E.J.; Smith, K.J., Jr. Plastics on the Sargasso Sea surface. Science 1972, 175, 1240–1241. [Google Scholar] [CrossRef]
- Lusher, A.L.; Hernandez-Milian, G.; O’Brien, J.; Berrow, S.; O’Connor, I.; Officer, R. Microplastics ingestion by a deep diving, oceanic cetacean: The True’s beaked whale Mesoplodon mirus. Environ. Pollut. 2015, 199, 185–191. [Google Scholar] [CrossRef]
- Clark, J.R.; Cole, M.; Lindeque, P.K.; Fileman, E.; Blackford, J.; Lewis, C.; Galloway, T.S. Marine microplastics debris: A targeted plan for understanding and quantifying interactions with marine life. Front. Ecol. Environ. 2016, 14, 317–324. [Google Scholar] [CrossRef]
- Ling, S.D.; Sinclair, M.; Levi, C.J.; Reeves, S.E.; Edgar, G.J. Ubiquity of microplastics in coastal seafloor sediments. Mar. Pollut. Bull. 2017, 121, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Carson, H.S. The incidence of plastic ingestion by fishes: From the prey’s perspective. Mar. Pollut. Bull. 2013, 74, 170–174. [Google Scholar] [CrossRef]
- Li, B.; Liang, W.; Liu, Q.X.; Fu, S.; Ma, C.; Chen, Q.; Su, L.; Craig, N.J.; Shi, H. Fish ingest microplastics unintentionally. Environ. Sci. Technol. 2021, 55, 10471–10479. [Google Scholar] [CrossRef]
- Alomar, C.; Estarellas, F.; Deudero, S. Microplastics in the Mediterranean Sea: Deposition in coastal shallow sediments, spatial variation and preferential grain size. Envrion. Res. 2016, 115, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Parton, K.J.; Godley, B.J.; Santillo, D.; Tausif, M.; Omeyer, L.; Galloway, T.S. Investigating the presence of microplastics in demersal sharks of the North-Eastern Atlantic. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Alomar, C.; Deudero, S. Evidence of microplastic ingestion in the shark Galeus melastomus Rafinesque, 1810 in the continental shelf off the western Mediterranean Sea. Environ. Pollut. 2017, 223, 223–229. [Google Scholar] [CrossRef]
- Anastasopoulou, A.; Mytilieneou, C.; Smith, C.J.; Papadopoulou, K.N. Plastic debris ingested by deep-water fish of the Ionian Sea (Eastern Mediterranean). Deep Sea Res. Part I Oceanogr. Res. Pap. 2013, 74, 11–13. [Google Scholar] [CrossRef]
- Pedà, C.; Battaglia, P.; D’Alessandro, M.; Laface, F.; Malara, D.; Consoli, P.; Vicchio, T.M.; Longo, F.; Andaloro, F.; Baini, M.; et al. Coupling gastro-intestinal tract analysis with an airborne contamination control method to estimate litter ingestion in demersal elasmobranchs. Front. Environ. Sci. 2020, 8, 119. [Google Scholar] [CrossRef]
- Sbrana, A.; Cau, A.; Cicala, D.; Franceschini, S.; Giarrizzo, T.; Gravina, M.F.; Ligas, A.; Maiello, G.; Matiddi, M.; Parisi, A.; et al. Ask the shark: Blackmouth catshark (Galeus melastomus) as a sentinel of plastic waste on the seabed. Mar. Biol. 2022, 169, 98. [Google Scholar] [CrossRef]
- Scacco, U.; Mancini, E.; Marcucci, F.; Tiralongo, F. Microplastics in the Deep: Comparing Dietary and Plastic Ingestion Data between Two Mediterranean Bathyal Opportunistic Feeder Species, Galeus melastomus Rafinesque, 1810, and Coelorinchus caelorhincus (Risso, 1810), throught Stomach Content Analysis. J. Mar. Sci. 2022, 10, 624. [Google Scholar] [CrossRef]
- Valente, T.; Sbrana, A.; Sacco, U.; Jacomini, C.; Banchi, J.; Palazzo, L.; De Lucia, G.A.; Silvestri, C.; Mattidi, M. Expoloring microplastic ingestion by three deep-water elasmbranch species: A case of stud from the Tyrrhenian Sea. Environ. Pollut. 2019, 253, 342–350. [Google Scholar] [CrossRef]
- Pierini, S.; Simioli, A. A wind-driven circulation model of the Tyrrhenian Sea area. J. Mar. Syst. 1998, 18, 161–178. [Google Scholar] [CrossRef]
- Zavatarielli, M.; Mellor, G.L. A numerical study of the Mediterranean Sea circulation. J. Phys. Oceanogr. 1995, 25, 1384–1414. [Google Scholar] [CrossRef]
- Robinson, A.R.; Leslie, W.G.; Theocharis, A.; Lascaratos, A. Mediterranean Sea circulation. In Ocean Currents, 2nd ed.; Elsevier Ltd.: Oxford, UK, 2001; pp. 283–297. [Google Scholar]
- Pinet, P.R. The General Circulation Pattern in Mediterranean Sea. In Invitation to Oceanography, 3rd ed.; St Jones & Bartlett Publisher: Mississauga, ON, Canada, 2003; p. 350. [Google Scholar]
- Bertrand, J.; De Sola, L.G.; Papaconstantinou, C.; Relini, G.; Souplet, A. An international bottom trawl survey in the Mediterranean: The MEDITS programme. In Proceedings of the ICES Annual Science Conference, Theme Session: Synthesis and Critical Evolution of Research Surveys, Trani, Italy, 25 March 1997. [Google Scholar]
- Bertrand, J.; Relini, G. Demersal resources in the Mediterranean. In Proceedings of the Symposium, Pisa, Italy, 18–21 March 1998; Actes de Colloques. IFREMER: Plouzané, France, 1998; Volume 26, pp. 76–93. [Google Scholar]
- Bertrand, J.A.; De Sola, L.G.; Papaconstantinou, C.; Relini, G.; Souplet, A. An International Bottom Trawl Survey in the Mediterranean: The MEDITS Programme; Actes de Colloques; IFREMER: Plouzané, France, 2000; pp. 76–96. [Google Scholar]
- International Bottom Trawl Survey in the Mediterranean (MEDITS). Medits-Handbook, Version N°9; Medits Working Group: Split, Croatia, 2017; 106p. [Google Scholar]
- Comas, M.; Ribas, A.; Milazzo, C.; Sperone, E.; Tripepi, S. High levels of prevalence related to age and body condition: Host-parasite interactions in a water frog Pelophylax kl hispanicus. Acta Herpetol. 2014, 9, 25–31. [Google Scholar]
- Falciai, L.; Minervini, R. Guida dei Crostacei Decapodi d’Europa; Franco Muzzio Editore: Padova, Italy, 1992; p. 316. [Google Scholar]
- Lu, C.C.; Ickernigill, R. Cephalopod beak identification and biomass estimation techniques: Tools for dietary studies of southern Australian finfishes. Museum Victoria, Sci. Rep. 2002, 6, 1–65. [Google Scholar] [CrossRef]
- Campana, S.E. Photographic Atlas of Fish Otoliths of the Northwest Atlantic Ocean; NRC Research Press: Ottawa, ON, Canada, 2004; No. 133; p. 284. [Google Scholar]
- Tuset, V.M.; Lombarte, A.; Assis, C.A. Otolith atlas for the western Mediterranean, north and central eastern Atlantic. Sci. Mar. 2008, 72, 7–198. [Google Scholar] [CrossRef]
- Xavier, J.C.; Cherel, Y. Cephalopod Beak Guide for the Southern Ocean; BAS: Cambridge, UK, 2009; p. 129. [Google Scholar]
- Açik, S.; Salman, A. Estimation of body size from the beaks of eight sepiolid species (Cephalopoda: Sepiolidae) from the Eastern Mediterranean Sea. Molluscan Res. 2010, 3, 154–164. [Google Scholar]
- Eriksen, M.; Lebreton, L.C.M.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic pollution in the world’s oceans: More than 5 trillion plastic pieces weighing over 250.000 tons afloat at sea. PLoS ONE 2014, 9, 111913. [Google Scholar] [CrossRef] [PubMed]
- Galgani, F.; Leaute, J.P.; Moguedet, P.; Souplet, A.; Verin, Y.; Carpentier, A.; Goraguer, H.; Latrouite, D.; Andral, B.; Cadiou, Y.; et al. Litter on the sea floor along European coasts. Mar. Pollut. Bull. 2000, 40, 516–527. [Google Scholar] [CrossRef]
- Galgani, F.; Hanke, G.; Werner, S.; Oosterbaan, L.; Nilsson, P.; Fleet, D.; Kinsey, S.; Thompson, R.; Van-Franeker, K.; Vlachogianni, T.; et al. Monitoring Guidance for Marine Litter in European Seas; JRC Scientific and Policy Reports; Centro Oceanográfico de Vigo: Vigo, Spain, 2013. [Google Scholar]
- MSFD Technical Subgroup on Marine Litter. Guidance on Monitoring of Marine Litter in European Seas; Publication Office: Luxembourg, 2013. [Google Scholar]
- Bernardini, I.; Garibaldi, F.; Canesi, L.; Fossi, M.C.; Baini, M. First data on plastic ingestion by blue sharks (Prionace glauca) from the Ligurian Sea (North-Wstern Mediterranean Sea). Mar. Pollut. Bull. 2018, 135, 303–310. [Google Scholar] [CrossRef]
- Schirinzi, G.F.; Pedà, C.; Battaglia, P.; Laface, F.; Galli, M.; Baini, M.; Consoli, P.; Scotti, G.; Esposito, V.; Faggio, C.; et al. A new digestion approach for the extraction of microplastics from gastrointestinal tracts (GITs) of the common dolphinfish (Coryphaena hippurus) from the western Mediterranean Sea. J. Hazard. Mater. 2020, 397, 122794. [Google Scholar] [CrossRef] [PubMed]
- Preti, A.; Soykan, C.U.; Dewar, H.; Wells, R.J.; Spear, N.; Kohin, S. Comparative feeding ecology of shortfin mako, blue and thresher sharks in the California Current. Environ. Biol. Fishes 2012, 95, 127–146. [Google Scholar] [CrossRef]
- Ganesh, K.; Geetha, B. Biodiversity status and conservation strategy of elasmobranchs (sharks) at gulf of mannar, Thoothukudi district. Int. J. Multidiscip. Res. Dev. 2017, 4, 116–122. [Google Scholar]
- Janžekovič, F.; Klenovšek, T. The biogeography of diet diversity of barn owls on Mediterranean islands. J. Biogeogr. 2020, 47, 2353–2361. [Google Scholar] [CrossRef]
- D’Iglio, C.; Albano, M.; Tiralongo, F.; Famulari, S.; Rinelli, P.; Savoca, S.; Spanò, N.; Capillo, G. Biological and ecological aspects of the blackmouth catshark (Galeus melastomus Rafinesque, 1810) in the southern Tyrrhenian Sea. J. Mar. Sci. Eng. 2021, 9, 967. [Google Scholar] [CrossRef]
- Madurell, T. Feeding Strategies and Trophodynamic Requirements of Deep-Sea Demersal Fish in the Eastern Mediterranean. Ph.D. Thesis, University of the Balearic Island, Palma, Spain, 2003. [Google Scholar]
- Preciado, I.; Cartes, J.E.; Serrano, A.; Velasco, F.; Olaso, I.; Sánchez, F.; Frutos, I. Resource utilization by deep-sea sharks at the Le Danois Bank, Cantabrian Sea, north-east Atlantic Ocean. J. Fish Biol. 2009, 75, 1331–1355. [Google Scholar] [CrossRef]
- Barría, C.; Navarro, J.; Coll, M. Feeding habits of four sympatric sharks in two deep-water fishery areas of the western Mediterranean Sea. Deep Sea Res. Part I Oceanogr. Res. Pap. 2018, 142, 34–43. [Google Scholar] [CrossRef]
- Yemişken, E.; Forero, M.G.; Megalofonou, P.; Eryilmaz, L.; Navarro, J. Feeding habits of three Batoids in the Levantine Sea (north-eastern Mediterranean Sea) based on stomach content and isotopic data. J. Mar. Biolog. Assoc. 2018, 98, 89–96. [Google Scholar] [CrossRef]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of microplastic on shorelines worldwide: Sources and sinks. ES&T 2011, 45, 9175–9179. [Google Scholar]
- Napper, I.E.; Thompson, R.C. Release of synthetic microplastic plastic fibres from domestic washing machines: Effects of fabric type and washing conditions. Mar. Pollut. Bull. 2016, 112, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Romeo, T.; Peda, C.; Fossi, M.C.; Andaloro, F.; Battaglia, P. First record of plastic debris in the stomach of Mediterranean lanternfishes. Acta Adriat. Int. J. Mar. Sci. 2016, 57, 115–122. [Google Scholar]
- Miccoli, A.; Mancini, E.; Saraceni, P.R.; Dalla Ventura, G.; Scapigliati, G.; Picchietti, S. First evidence of in vitro cytotoxic effects of marine microlitter on Merluccius merluccius and Mullus barbatus, two Mediterranean commercial fish species. Sci. Total Environ. 2022, 813, 152618. [Google Scholar] [CrossRef] [PubMed]
- WWF Italia. Available online: https://www.wwf.it/uploads/Report-Squali_Dal-mare-al-banco-del-pesce.pdf (accessed on 13 July 2021).
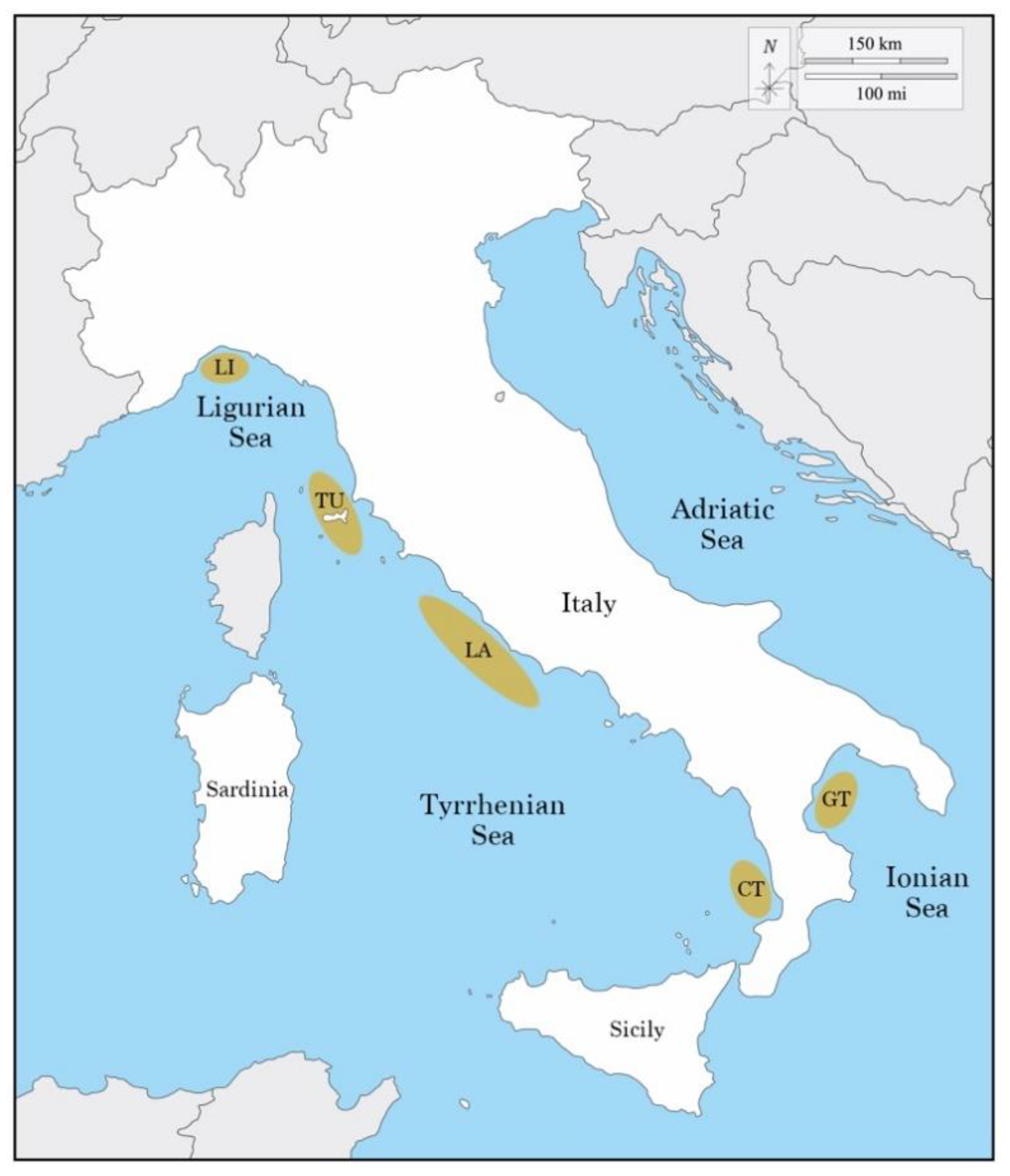






| Region | Sampling Sites Coordinates | Date | Number of Individuals | Depth | |
|---|---|---|---|---|---|
| Longitude | Latitude | (m) | |||
| Liguria | 43°48′450″ | 9°38′270″ | 20 October 2020 | 16 | 412.5 |
| 44°06′680″ | 9°32′050″ | 19 October 2020 | 6 | 392 | |
| 44°15′740″ | 8°40′840″ | 18 October 2020 | 4 | 582 | |
| 43°58′520″ | 8°17′270″ | 17 October 2020 | 19 | 471.5 | |
| Tuscany | 43°35′310″ | 9°35′520″ | 20 October 2020 | 26 | 634.5 |
| 42°15′190″ | 10°11′220″ | 14 October 2020 | 2 | 251 | |
| Latium | 41°22′780″ | 12°17′580″ | 3 November 2020 | 6 | 282 |
| 41°29′740″ | 12°08′500″ | 1 November 2020 | 29 | 497.5 | |
| 41°27′580″ | 12°10′980″ | 1 November 2020 | 34 | 432.5 | |
| 41°48′110″ | 11°48′990″ | 31 October 2020 | 12 | 476.5 | |
| 42°04′770″ | 11°09′790″ | 30 October 2020 | 103 | 347 | |
| Calabria | 38°94′1037″ | 16°12′5332″ | 7 November 2020 | 6 | 40 |
| 38°94′1037″ | 16°12′5332″ | 11 February 2021 | 12 | 40 | |
| 38°94′1037″ | 16°12′5332″ | 12 April 2021 | 17 | 40 | |
| Gulf of Taranto | 40°04′1865″ | 16°88′4223″ | 2 February 2021 | 2 | 300 |
| 40°04′1865″ | 16°88′4223″ | 12 August 2021 | 8 | 300 | |
| Taxa | Calabria | Tuscany | Latium | Liguria | Gulf of Taranto | N% | FO% |
|---|---|---|---|---|---|---|---|
| Crustacea | |||||||
| DECAPODA | |||||||
| Carcinus aestuarii | 1 | 0.06 | 0.40 | ||||
| Natantia | |||||||
| Superfamily: Penaeidae | 8 | 1 | 0.54 | 2.37 | |||
| Aristaeopsis edwardsiana | 1 | 0.06 | 0.40 | ||||
| Aristeus antennatus | 6 | 0.36 | 1.19 | ||||
| Parapenaeus longirostris | 1 | 7 | 4 | 1 | 0.78 | 4.35 | |
| Family: Pasiphaeidae | |||||||
| Pasiphaea sp. | 1 | 0.06 | 0.40 | ||||
| Pasiphaea sivado | 6 | 1 | 0.42 | 2.77 | |||
| Pasiphaea multidentata | 1 | 1 | 0.12 | 0.79 | |||
| Family: Sergestidae | |||||||
| Eusergestes arcticus | 1 | 0.06 | 0.40 | ||||
| ISOPODA | 1 | 1 | 4 | 1 | 0.42 | 2.77 | |
| Unidentified crustacean | 10 | 17 | 53 | 40 | 2 | 7.28 | 39.53 |
| Mollusca | |||||||
| Family: Brachioteuthidae | |||||||
| Brachioteuthis riisei | 3 | 0.18 | 0.79 | ||||
| Family: Chiroteuthidae | |||||||
| Chiroteuthis veranii | 2 | 2 | 0.24 | 1.58 | |||
| Family: Enoploteuthidae | |||||||
| Abralia veranyi | 14 | 1 | 9 | 9 | 1 | 2.04 | 11.58 |
| Family: Loliginidae | |||||||
| Loligo forbesii | 1 | 2 | 0.18 | 0.79 | |||
| Family: Histioteuthidae | |||||||
| Histioteuthis spp. | 1 | 1 | 2 | 0.24 | 1.19 | ||
| Histioteuthis bonnellii | 1 | 3 | 1 | 1 | 0.36 | 1.58 | |
| Histioteuthis reversa | 5 | 11 | 2 | 5 | 1.38 | 5.93 | |
| Family: Ommastrephidae | |||||||
| Illex coindetii | 1 | 1 | 2 | 2 | 0.36 | 2.37 | |
| Todarodes sagittatus | 1 | 16 | 1 | 1 | 9 | 1.68 | 6.32 |
| Family: Onychoteuthidae | |||||||
| Ancistroteuthis spp. | 1 | 4 | 0.30 | 0.79 | |||
| Ancistroteuthis lichtensteinii | 2 | 4 | 1 | 0.42 | 2.77 | ||
| Onychoteuthis sp. | 1 | 0.06 | 0.40 | ||||
| Onychoteuthis banksii | 2 | 17 | 3 | 3 | 5 | 1.80 | 8.70 |
| Family: Sepiolidae | 4 | 0.24 | 0.79 | ||||
| Heteroteuthis dispar | 77 | 22 | 81 | 23 | 18 | 13.29 | 39.92 |
| Rossia macrosoma | 1 | 1 | 0.12 | 0.79 | |||
| Sepietta sp. | 1 | 0.06 | 0.40 | ||||
| Octopoda | |||||||
| Argonauta argo | 1 | 0.06 | 0.40 | ||||
| Eledone cirrhosa | 1 | 0.06 | 0.40 | ||||
| Unidentified cephalopods | 29 | 97 | 35 | 9 | 11.13 | 45.06 | |
| Osteichthyes | |||||||
| Family: Caristiidae | 1 | 0.06 | 0.40 | ||||
| Family: Gadidae | 1 | 0.06 | 0.40 | ||||
| Family: Nettastomatidae | |||||||
| Nettastoma melanorum | 1 | 0.06 | 0.40 | ||||
| Ordine: Myctophiformes | 5 | 0.30 | 0.79 | ||||
| Family: Myctophidae | 3 | 4 | 0.42 | 1.58 | |||
| Benthosema glaciale | 1 | 2 | 0.18 | 1.19 | |||
| Ceratoscopelus sp. | 1 | 0.06 | 0.40 | ||||
| Ceratoscopelus maderensis | 2 | 3 | 1 | 4 | 0.60 | 2.77 | |
| Diaphus sp. | 5 | 1 | 0.36 | 2.37 | |||
| Diaphus rafinesquii | 1 | 1 | 1 | 0.18 | 1.19 | ||
| Electrona risso | 19 | 6 | 1.50 | 6.72 | |||
| Lampanyctus sp. | 1 | 0.06 | 0.40 | ||||
| Lampanyctus crocodilus | 3 | 0.18 | 1.19 | ||||
| Myctophum punctatum | 2 | 1 | 0.18 | 1.19 | |||
| Family: Scomberesocidae | |||||||
| Scomberesox saurus | 9 | 1 | 0.60 | 3.16 | |||
| Family: Stomiidae | 4 | 1 | 0.30 | 1.98 | |||
| Stomias boa | 1 | 1 | 1 | 0.18 | 1.19 | ||
| Unidentified fishes | 142 | 30 | 517 | 121 | 24 | 50.15 | 87.75 |
| Others | |||||||
| Annelida | 1 | 1 | 1 | 0.18 | 1.19 | ||
| Turdus merula | 1 | 0.06 | 0.40 | ||||
| Echinodermata | 1 | 0.06 | 0.40 | ||||
| Sylvia curruca | 1 | 0.06 | 0.40 |
| Plastics Percentage | |||
|---|---|---|---|
| Microplastics | Mesoplastics | Macroplastics | |
| x < 1 mm | 1 < x < 5 mm | 5 < x < 25 mm | 25 mm > x |
| 2.6% | 32.2% | 64.8% | 0.3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zicarelli, G.; Romano, C.; Gallo, S.; Valentino, C.; Pepe Bellomo, V.; Leonetti, F.L.; Giglio, G.; Neri, A.; Marsili, L.; Milazzo, C.; et al. Diet and Plastic Ingestion in the Blackmouth Catshark Galeus melastomus, Rafinesque 1810, in Italian Waters. Animals 2023, 13, 1039. https://doi.org/10.3390/ani13061039
Zicarelli G, Romano C, Gallo S, Valentino C, Pepe Bellomo V, Leonetti FL, Giglio G, Neri A, Marsili L, Milazzo C, et al. Diet and Plastic Ingestion in the Blackmouth Catshark Galeus melastomus, Rafinesque 1810, in Italian Waters. Animals. 2023; 13(6):1039. https://doi.org/10.3390/ani13061039
Chicago/Turabian StyleZicarelli, Giorgia, Chiara Romano, Samira Gallo, Carmen Valentino, Victor Pepe Bellomo, Francesco Luigi Leonetti, Gianni Giglio, Alessandra Neri, Letizia Marsili, Concetta Milazzo, and et al. 2023. "Diet and Plastic Ingestion in the Blackmouth Catshark Galeus melastomus, Rafinesque 1810, in Italian Waters" Animals 13, no. 6: 1039. https://doi.org/10.3390/ani13061039
APA StyleZicarelli, G., Romano, C., Gallo, S., Valentino, C., Pepe Bellomo, V., Leonetti, F. L., Giglio, G., Neri, A., Marsili, L., Milazzo, C., Faggio, C., Mancusi, C., & Sperone, E. (2023). Diet and Plastic Ingestion in the Blackmouth Catshark Galeus melastomus, Rafinesque 1810, in Italian Waters. Animals, 13(6), 1039. https://doi.org/10.3390/ani13061039








