Bioclimatic Zoning for Sheep Farming through Geostatistical Modeling in the State of Pernambuco, Brazil
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
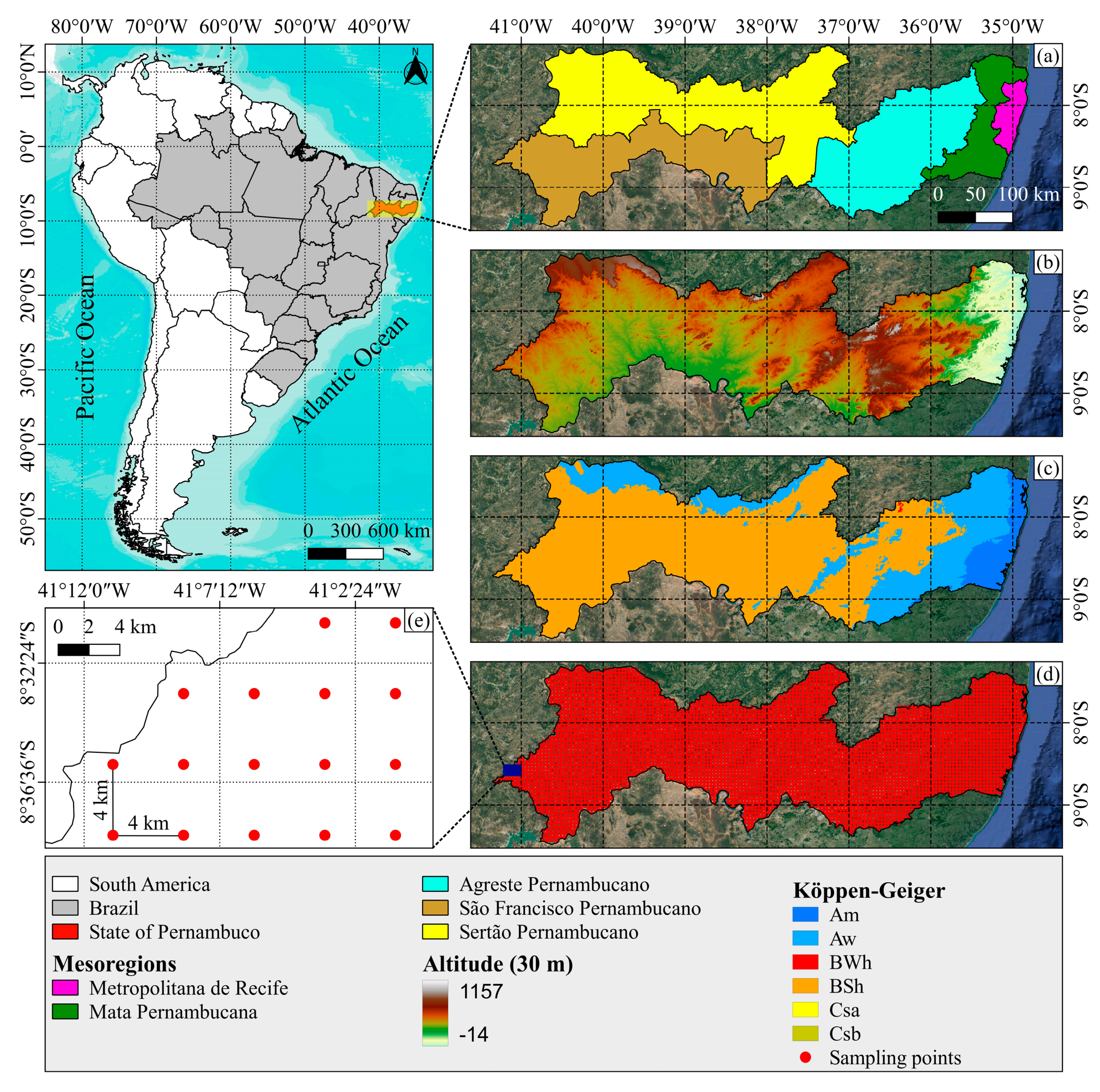
2.1. Characterization of the Study Area
2.2. Climatological and Geospatial Data
2.3. Statistical Analysis
2.4. Geostatistical Analysis
3. Results and Discussion
3.1. Boxplot Analysis
3.2. Descriptive and Geostatistical Analysis of THI
3.3. THI Kriging Maps
3.4. Hair x Wool Breeds
3.5. Main Meat Production Breeds
3.6. Main Milk Production Breeds
3.7. Implications of the Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thornton, P.; Nelson, G.; Mayberry, D.; Herrero, M. Impacts of Heat Stress on Global Cattle Production during the 21st Century: A Modelling Study. Lancet Planet Health 2022, 6, e192–e201. [Google Scholar] [CrossRef] [PubMed]
- Goma, A.A.; Phillips, C.J.C. ‘Can They Take the Heat?’—The Egyptian Climate and Its Effects on Livestock. Animals 2022, 12, 1937. [Google Scholar] [PubMed]
- Rahimi, J.; Mutua, J.Y.; Notenbaert, A.M.O.; Marshall, K.; Butterbach-Bahl, K. Heat Stress Will Detrimentally Impact Future Livestock Production in East Africa. Nat. Food 2021, 2, 88–96. [Google Scholar] [CrossRef]
- Nienaber, J.A.; Hahn, G.L.; Eigenberg, R.A. Quantifying Livestock Responses for Heat Stress Management: A Review. Int. J. Biometeorol. 1999, 42, 183–188. [Google Scholar] [CrossRef]
- Finocchiaro, R.; van Kaam, J.B.C.H.M.; Portolano, B.; Misztal, I. Effect of Heat Stress on Production of Mediterranean Dairy Sheep. J. Dairy Sci. 2005, 88, 1855–1864. [Google Scholar] [CrossRef] [Green Version]
- Singh, K.M.; Singh, S.; Ganguly, I.; Ganguly, A.; Nachiappan, R.K.; Chopra, A.; Narula, H.K. Evaluation of Indian Sheep Breeds of Arid Zone under Heat Stress Condition. Small Rumin. Res. 2016, 141, 113–117. [Google Scholar] [CrossRef]
- Lallo, C.H.O.; Cohen, J.; Rankine, D.; Taylor, M.; Cambell, J.; Stephenson, T. Characterizing Heat Stress on Livestock Using the Temperature Humidity Index (THI)—Prospects for a Warmer Caribbean. Reg. Environ. Change 2018, 18, 2329–2340. [Google Scholar] [CrossRef] [Green Version]
- Ekine-Dzivenu, C.C.; Mrode, R.; Oyieng, E.; Komwihangilo, D.; Lyatuu, E.; Msuta, G.; Ojango, J.M.K.; Okeyo, A.M. Evaluating the Impact of Heat Stress as Measured by Temperature-Humidity Index (THI) on Test-Day Milk Yield of Small Holder Dairy Cattle in a Sub-Sahara African Climate. Livest. Sci. 2020, 242, 104314. [Google Scholar] [CrossRef]
- Da Rosa Ferraz Jardim, A.M.; Araújo Júnior, G.D.N.; da Silva, M.V.; dos Santos, A.; da Silva, J.L.B.; Pandorfi, H.; de Oliveira-Júnior, J.F.; de Castro Teixeira, A.H.; Teodoro, P.E.; de Lima, J.L.M.P.; et al. Using Remote Sensing to Quantify the Joint Effects of Climate and Land Use/Land Cover Changes on the Caatinga Biome of Northeast Brazilian. Remote Sens. 2022, 14, 1911. [Google Scholar] [CrossRef]
- Soares, M.O.; Campos, C.C.; Carneiro, P.B.M.; Barroso, H.S.; Marins, R.V.; Teixeira, C.E.P.; Menezes, M.O.B.; Pinheiro, L.S.; Viana, M.B.; Feitosa, C.V.; et al. Challenges and Perspectives for the Brazilian Semi-Arid Coast under Global Environmental Changes. Perspect. Ecol. Conserv. 2021, 19, 267–278. [Google Scholar] [CrossRef]
- Henriques da Nóbrega, G.; Maria Nunes da Silva, E.; Benício de Souza, B.; Marry Mangueira, J. Animal production under the influence of environmental conditions in the northeastern semiarid. Rev. Verde Agroecol. Desenvolv. Sustentável 2011, 6, 67–73. [Google Scholar]
- Leite, J.H.G.M.; Façanha, D.A.E.; Bermejo, J.V.D.; Guilhermino, M.M.; Bermejo, L.A. Adaptive Assessment of Small Ruminants in Arid and Semi-Arid Regions. Small Rumin. Res. 2021, 203, 106497. [Google Scholar] [CrossRef]
- De, K.; Kumar, D.; Saxena, V.K.; Thirumurugan, P.; Naqvi, S.M.K. Effect of High Ambient Temperature on Behavior of Sheep under Semi-Arid Tropical Environment. Int. J. Biometeorol. 2017, 61, 1269–1277. [Google Scholar] [CrossRef]
- Kumar, D.; De, K.; Sejian, V.; Naqvi, S.M.K. Impact of Climate Change on Sheep Reproduction. In Sheep Production Adapting to Climate Change; Springer: Singapore, 2017; pp. 71–93. [Google Scholar]
- IBGE—Instituto Brasileiro de Geografia e Estatística IBGE. Available online: https://www.ibge.gov.br/explica/producao-agropecuaria/pe (accessed on 19 January 2023).
- Hermuche, P.; Guimarães, R.F.; Carvalho, O.A.; Gomes, R.A.T.; Paiva, S.R.; McManus, C.M. Environmental Factors That Affect Sheep Production in Brazil. Appl. Geogr. 2013, 44, 172–181. [Google Scholar] [CrossRef]
- De Azambuja Ribeiro, E.L.; González-García, E. Indigenous Sheep Breeds in Brazil: Potential Role for Contributing to the Sustainability of Production Systems. Trop. Anim. Health Prod. 2016, 48, 1305–1313. [Google Scholar] [CrossRef]
- Silveira, R.M.F.; de Vasconcelos, A.M.; da Silva, V.J.; Vega, W.H.O.; Toro-Mujica, P.; Ferreira, J. Typification, Characterization, and Differentiation of Sheep Production Systems in the Brazilian Semiarid Region. NJAS Impact Agric. Life Sci. 2021, 93, 48–73. [Google Scholar]
- Mcmanus, C.; Hermuche, P.; Paiva, S.R.; Carlos Ferrugem Moraes, J.; Barros De Melo, C.; Mendes, C. Geographical Distribution of Sheep Breeds in Brazil and Their Relationship with Climatic and Environmental Factors as Risk Classification for Conservation. Braz. J. Sci. Technol. 2014, 1, 3. [Google Scholar] [CrossRef] [Green Version]
- McManus, C.; Dallago, B.S.L.; Lehugeur, C.; Ribeiro, L.A.; Hermuche, P.; Guimarães, R.F.; de Carvalho Júnior, O.A.; Paiva, S.R. Patterns of Heat Tolerance in Different Sheep Breeds in Brazil. Small Rumin. Res. 2016, 144, 290–299. [Google Scholar] [CrossRef]
- De Paula Mendes, A.M.; de Azevedo, M.; Lopes, P.M.O.; de Albuquerque Moura, G.B. Zoneamento Bioclimático Para a Raça Ovina Dorper No Estado de Pernambuco. Pesqui. Agropecuária Bras. 2014, 49, 986–993. [Google Scholar] [CrossRef] [Green Version]
- Paiva, S.R.; Silvério, V.C.; Egito, A.A.; McManus, C.; de Faria, D.A.; da Silva Mariante, A.; Castro, S.R.; do Socorro Maués Albuquerque, M.; Dergam, J.A. Genetic Variability of the Brazilian Hair Sheep Breeds. Pesqui. Agropecuária Bras. 2005, 40, 887–893. [Google Scholar] [CrossRef] [Green Version]
- Mcmanus, C.; Louvandini, H.; Do, T.; Paim, P.; Martins, R.S.; Otávio, J.; Barcellos, J.; Cardoso, C.; Guimarães, R.F.; Santana, O.A. The Challenge of Sheep Farming in the Tropics: Aspects Related to Heat Tolerance. Rev. Bras. Zootec. 2011, 40, 107–120. [Google Scholar]
- Giro, A.; Pezzopane, J.R.M.; Barioni Junior, W.; de Faria Pedroso, A.; Lemes, A.P.; Botta, D.; Romanello, N.; do Nascimento Barreto, A.; Garcia, A.R. Behavior and Body Surface Temperature of Beef Cattle in Integrated Crop-Livestock Systems with or without Tree Shading. Sci. Total Environ. 2019, 684, 587–596. [Google Scholar] [CrossRef] [PubMed]
- McManus, C.M.; Faria, D.A.; Lucci, C.M.; Louvandini, H.; Pereira, S.A.; Paiva, S.R. Heat Stress Effects on Sheep: Are Hair Sheep More Heat Resistant? Theriogenology 2020, 155, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Polli, V.A.; Vaz, R.Z.; Carvalho, S.; Costa, P.T.; de Oliveira Mello, R.; Restle, J.; Nigeliskii, A.F.; Silveira, I.D.B.; Pissinin, D. Thermal Comfort and Performance of Feedlot Lambs Finished in Two Climatic Conditions. Small Rumin. Res. 2019, 174, 163–169. [Google Scholar] [CrossRef]
- Li, F.K.; Yang, Y.; Jenna, K.; Xia, C.H.; Lv, S.J.; Wei, W.H. Effect of Heat Stress on the Behavioral and Physiological Patterns of Small-Tail Han Sheep Housed Indoors. Trop. Anim. Health Prod. 2018, 50, 1893–1901. [Google Scholar] [CrossRef]
- Van Wettere, W.H.E.J.; Kind, K.L.; Gatford, K.L.; Swinbourne, A.M.; Leu, S.T.; Hayman, P.T.; Kelly, J.M.; Weaver, A.C.; Kleemann, D.O.; Walker, S.K. Review of the Impact of Heat Stress on Reproductive Performance of Sheep. J. Anim. Sci. Biotechnol. 2021, 12, 26. [Google Scholar] [CrossRef]
- Marcone, G.; Kaart, T.; Piirsalu, P.; Arney, D.R. Panting Scores as a Measure of Heat Stress Evaluation in Sheep with Access and with No Access to Shade. Appl. Anim. Behav. Sci. 2021, 240, 105350. [Google Scholar] [CrossRef]
- Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Jones, G.V.; Alves, F.; Pinto, J.G.; Santos, J.A. Very High Resolution Bioclimatic Zoning of Portuguese Wine Regions: Present and Future Scenarios. Reg. Environ. Change 2014, 14, 295–306. [Google Scholar] [CrossRef]
- Tavares, G.F.; Carnevskis, E.L.; Schiassi, L.; Filho, R.C.; da Silva Miranda, K.O.; de Miranda, J.H. Bioclimatic Zoning for Beef Cattle in Brazil with the Aid of Intelligent Systems. J. Anim. Behav. Biometeorol. 2020, 4, 116–123. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, H.; Ahmadi, F. Mapping Thermal Comfort in Iran Based on Geostatistical Methods and Bioclimatic Indices. Arab. J. Geosci. 2017, 10, 342. [Google Scholar] [CrossRef]
- De Oliveira Aparecido, L.E.; Lorençone, J.A.; Lorençone, P.A.; Torsoni, G.B.; da Silva Cabral de Moraes, J.R.; de Meneses, K.C. Bioclimatic Zoning for Dairy Cows in Brazil by Statistical Modeling. J. Sci. Food Agric. 2022, 102, 3847–3857. [Google Scholar] [CrossRef]
- Da Silva, V.C.; de Sousa Nascimento, R.; Neto, J.P.L.; Miranda, J.R.; de Melo Lopes, F.F.; Furtado, D.A. Bioclimatic Spatial Zoning for Small Ruminants in the State of Paraíba, Brazil. Acta Sci. 2022, 44. [Google Scholar] [CrossRef]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and Future Köppen-Geiger Climate Classification Maps at 1-Km Resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef] [Green Version]
- Martins, F.R.; Abreu, S.L.; Pereira, E.B. Scenarios for Solar Thermal Energy Applications in Brazil. Energy Policy 2012, 48, 640–649. [Google Scholar] [CrossRef]
- Abatzoglou, J.T.; Dobrowski, S.Z.; Parks, S.A.; Hegewisch, K.C. TerraClimate, a High-Resolution Global Dataset of Monthly Climate and Climatic Water Balance from 1958–2015. Sci. Data 2018, 5, 170191. [Google Scholar] [CrossRef] [Green Version]
- Segnalini, M.; Bernabucci, U.; Vitali, A.; Nardone, A.; Lacetera, N. Temperature Humidity Index Scenarios in the Mediterranean Basin. Int. J. Biometeorol. 2013, 57, 451–458. [Google Scholar] [CrossRef]
- Ciobotaru, A.M.; Andronache, I.; Dey, N.; Petralli, M.; Daneshvar, M.R.M.; Wang, Q.; Radulovic, M.; Pintilii, R.D. Temperature-Humidity Index Described by Fractal Higuchi Dimension Affects Tourism Activity in the Urban Environment of Focşani City (Romania). Theor. Appl. Climatol. 2019, 136, 1009–1019. [Google Scholar] [CrossRef]
- Da Silva, M.V.; Pandorfi, H.; de Almeida, G.L.P.; da Rosa Ferraz Jardim, A.M.; Batista, P.H.D.; da Silva, R.A.B.; Lopes, I.; de Oliveira, M.E.G.; da Silva, J.L.B.; Moraes, A.S. Spatial Variability and Exploratory Inference of Abiotic Factors in Barn Compost Confinement for Cattle in the Semiarid. J. Therm. Biol. 2020, 94, 102782. [Google Scholar] [CrossRef]
- Da Rosa Ferraz Jardim, A.M.; da Silva, M.V.; Silva, A.R.; dos Santos, A.; Pandorfi, H.; de Oliveira-Júnior, J.F.; de Lima, J.L.M.P.; de Souza, L.S.B.; do Nascimento Araújo Júnior, G.; Lopes, P.M.O.; et al. Spatiotemporal Climatic Analysis in Pernambuco State, Northeast Brazil. J. Atmos. Sol. Terr. Phys. 2021, 223, 105733. [Google Scholar] [CrossRef]
- De Oliveira-Júnior, J.F.; de Gois, G.; de Lima Silva, I.J.; de Oliveira Souza, E.; da Rosa Ferraz Jardim, A.M.; da Silva, M.V.; Shah, M.; Jamjareegulgarn, P. Wet and Dry Periods in the State of Alagoas (Northeast Brazil) via Standardized Precipitation Index. J. Atmos. Sol. Terr. Phys. 2021, 224, 105746. [Google Scholar] [CrossRef]
- De Oliveira-Júnior, J.F.; Shah, M.; Abbas, A.; Iqbal, M.S.; Shahzad, R.; de Gois, G.; da Silva, M.V.; da Rosa Ferraz Jardim, A.M.; Souza, A. de Spatiotemporal Analysis of Drought and Rainfall in Pakistan via Standardized Precipitation Index: Homogeneous Regions, Trend, Wavelet, and Influence of El Niño-Southern Oscillation. Theor. Appl. Climatol. 2022, 149, 843–862. [Google Scholar] [CrossRef]
- Warrick, A.W.; Nielsen, D.R. Spatial Variability of Soil Physical Properties in the Field. Appl. Soil Phys. 1980, 13, 319–344. [Google Scholar]
- Zhang, J.; Li, X.; Yang, R.; Liu, Q.; Zhao, L.; Dou, B. An Extended Kriging Method to Interpolate Near-Surface Soil Moisture Data Measured by Wireless Sensor Networks. Sensors 2017, 17, 1390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, K.; Qin, C.; Lee, B.; Lee, I. Modified Screening-Based Kriging Method with Cross Validation and Application to Engineering Design. Appl. Math. Model 2019, 70, 626–642. [Google Scholar] [CrossRef]
- Belkhiri, L.; Tiri, A.; Mouni, L. Spatial Distribution of the Groundwater Quality Using Kriging and Co-Kriging Interpolations. Groundw. Sustain. Dev. 2020, 11, 100473. [Google Scholar] [CrossRef]
- Chen, G.; Yang, Y.; Liu, X.; Wang, M. Spatial Distribution Characteristics of Heavy Metals in Surface Soil of Xilinguole Coal Mining Area Based on Semivariogram. ISPRS Int. J. Geo-Inf. 2021, 10, 290. [Google Scholar] [CrossRef]
- Jo, H.; Pyrcz, M.J. Automatic Semivariogram Modeling by Convolutional Neural Network. Math. Geosci. 2022, 54, 177–205. [Google Scholar] [CrossRef]
- Eze, P.N.; Kumahor, S.K. Gaussian Process Simulation of Soil Zn Micronutrient Spatial Heterogeneity and Uncertainty—A Performance Appraisal of Three Semivariogram Models. Sci. Afr. 2019, 5, e00110. [Google Scholar] [CrossRef]
- Houlong, J.; Daibin, W.; Chen, X.; Shuduan, L.; Hongfeng, W.; Chao, Y.; Najia, L.; Yiyin, C.; Lina, G. Comparison of Kriging Interpolation Precision between Grid Sampling Scheme and Simple Random Sampling Scheme for Precision Agriculture. Eurasian J. Soil Sci. 2016, 5, 62. [Google Scholar] [CrossRef] [Green Version]
- Johnston, K.; Hoef, J.M.V.; Krivoruchko, K.; Lucas., N. Using ArcGIS Geostatistical Analysis; GIS User Manual by ESRI; ESRI: Redlands, CA, USA, 2011. [Google Scholar]
- Cambardella, C.A.; Moorman, T.B.; Novak, J.M.; Parkin, T.B.; Karlen, D.L.; Turco, R.F.; Konopka, A.E. Field-Scale Variability of Soil Properties in Central Iowa Soils. Soil Sci. Soc. Am. J. 1994, 58, 1501–1511. [Google Scholar] [CrossRef]
- Silva, L.; Hermsdorf, J.; Guedes, V.; Teixeira, F.; Fernandes, J.; Bispo, B.; Teixeira, J.P. Outliers Treatment to Improve the Recognition of Voice Pathologies. Procedia Comput. Sci. 2019, 164, 678–685. [Google Scholar] [CrossRef]
- Komorowski, M.; Marshall, D.C.; Salciccioli, J.D.; Crutain, Y. Exploratory Data Analysis. In Secondary Analysis of Electronic Health Records; Springer: Cham, Switzerland, 2016; pp. 185–203. [Google Scholar]
- De Morais Inocêncio, T.; Ribeiro Neto, A.; Oertel, M.; Meza, F.J.; Scott, C.A. Linking Drought Propagation with Episodes of Climate-Induced Water Insecurity in Pernambuco State—Northeast Brazil. J. Arid Environ. 2021, 193, 104593. [Google Scholar] [CrossRef]
- Silva, T.R.B.F.; dos Santos, C.A.C.; Silva, D.J.F.; Santos, C.A.G.; da Silva, R.M.; de Brito, J.I.B. Climate Indices-Based Analysis of Rainfall Spatiotemporal Variability in Pernambuco State, Brazil. Water 2022, 14, 2190. [Google Scholar] [CrossRef]
- Da Silva, M.V.; Pandorfi, H.; da Rosa Ferraz Jardim, A.M.; de Oliveira-Júnior, J.F.; da Divincula, J.S.; Giongo, P.R.; da Silva, T.G.F.; de Almeida, G.L.P.; de Albuquerque Moura, G.B.; Lopes, P.M.O. Spatial Modeling of Rainfall Patterns and Groundwater on the Coast of Northeastern Brazil. Urban Clim. 2021, 38, 100911. [Google Scholar] [CrossRef]
- Robinson, T.P.; Metternicht, G. Comparing the Performance of Techniques to Improve the Quality of Yield Maps. Agric. Syst. 2005, 85, 19–41. [Google Scholar] [CrossRef]
- Lacetera, N. Impact of Climate Change on Animal Health and Welfare. Anim. Front. 2019, 9, 26–31. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; Carpentier, L.; Teng, G.; Liu, M.; Wang, C.; Norton, T. Assessment of Laying Hens’ Thermal Comfort Using Sound Technology. Sensors 2020, 20, 473. [Google Scholar] [CrossRef] [Green Version]
- Dias Batista, P.H.; de Almeida, G.L.P.; Pandorfi, H.; da Silva, M.V.; da Silva, R.A.B.; da Silva, J.L.B.; Santana, T.C.; de Moraes Rodrigues, J.A. Thermal Images to Predict the Thermal Comfort Index for Girolando Heifers in the Brazilian Semiarid Region. Livest. Sci. 2021, 251, 104667. [Google Scholar] [CrossRef]
- De Medeiros, R.M.; de Holanda, R.M.; de França, M.V.; Saboya, L.M.F.; Filho, M.C.; de Araújo, W.R. Urban Variability in Recife—PE, through Contributions: Precipitation, Temperature and Relative Air Humidity. Res. Soc. Dev. 2022, 11. [Google Scholar]
- IBGE—Instituto Brasileiro de Geografia e Estatística Divisões Regionais do Brasil. Available online: https://www.ibge.gov.br/geociencias/organizacao-do-territorio/divisao-regional/15778-divisoes-regionais-do-brasil.html?=&t=acesso-ao-produto (accessed on 18 January 2023).
- Leite, J.H.G.M.; Façanha, D.A.E.; Costa, W.P.; Chaves, D.F.; Silva, W.S.T.; Bermejo, L.A. Thermoregulatory Responses Related to Coat Traits of Brazilian Native Ewes: An Adaptive Approach. J. Appl. Anim. Res. 2017, 46, 353–359. [Google Scholar] [CrossRef]
- Wojtas, K.; Cwynar, P.; Kołacz, R. Effect of Thermal Stress on Physiological and Blood Parameters in Merino Sheep. Bull. Vet. Inst. Pulawy 2014, 58, 283–288. [Google Scholar] [CrossRef] [Green Version]
- Titto, C.G.; Veríssimo, C.J.; Pereira, A.M.F.; de Mira Geraldo, A.; Katiki, L.M.; Titto, E.A.L. Thermoregulatory Response in Hair Sheep and Shorn Wool Sheep. Small Rumin. Res. 2016, 144, 341–345. [Google Scholar] [CrossRef]
- Belhadj Slimen, I.; Najar, T.; Ghram, A.; Abdrrabba, M. Heat Stress Effects on Livestock: Molecular, Cellular and Metabolic Aspects, a review. J. Anim. Physiol. Anim. Nutr. 2016, 100, 401–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashan, N.E.J.; Manafi Azar, G.H.; Afzalzadeh, A.; Salehi, A. Growth Performance and Carcass Quality of Fattening Lambs from Fat-Tailed and Tailed Sheep Breeds. Small Rumin. Res. 2005, 60, 267–271. [Google Scholar] [CrossRef]
- Pourlis, A.F. A Review of Morphological Characteristics Relating to the Production and Reproduction of Fat-Tailed Sheep Breeds. Trop. Anim. Health Prod. 2011, 43, 1267–1287. [Google Scholar] [CrossRef]
- Mohapatra, A.; Shinde, A.K. Fat-Tailed Sheep-An Important Sheep Genetic Resource for Meat Production in Tropical Countries: An Overview. Indian J. Small Rumin. 2018, 24, 1. [Google Scholar] [CrossRef]
- Mariante, A.S.; do Socorro Maués Albuquerque, M.; Ramos, A.F. Criopreservação de Recursos Genéticos Animais Brasileiros. Rev. Bras. Reprod. Anim. 2011, 35, 64–68. [Google Scholar]
- Paiva, S.R.; Facó, O.; Faria, D.A.; Lacerda, T.; Barretto, G.B.; Carneiro, P.L.S.; Lobo, R.N.B.; McManus, C. Molecular and Pedigree Analysis Applied to Conservation of Animal Genetic Resources: The Case of Brazilian Somali Hair Sheep. Trop. Anim. Health Prod. 2011, 43, 1449–1457. [Google Scholar] [CrossRef]
- McManus, C.; Paiva, S.R.; de Araújo, R.O. Genetics and Breeding of Sheep in Brazil. Rev. Bras. Zootec. 2010, 39, 236–246. [Google Scholar] [CrossRef]
- ARCO. ARCO—Associação Brasileira de Criadores de Ovinos. Available online: http://www.arcoovinos.com.br/PadraoRacial/Details/13 (accessed on 19 January 2023).
- Souza, A.G.S.S.; Ribeiro Neto, A.; de Souza, L.L. Soil Moisture-Based Index for Agricultural Drought Assessment: SMADI Application in Pernambuco State-Brazil. Remote Sens. Environ. 2021, 252, 112124. [Google Scholar] [CrossRef]
- Van der Merwe, D.A.; Brand, T.S.; Hoffman, L.C. Slaughter Characteristics of Feedlot-Finished Premium South African Lamb: Effects of Sex and Breed Type. Foods 2020, 9, 648. [Google Scholar] [CrossRef]
- Van der Merwe, D.A.; Brand, T.S.; Hoffman, L.C. Application of Growth Models to Different Sheep Breed Types in South Africa. Small Rumin. Res. 2019, 178, 70–78. [Google Scholar] [CrossRef]
- Brand, T.S.; van der Westhuizen, E.J.; van der Merwe, D.A.; Hoffman, L.C. Effect of Days in Feedlot on Growth Performance and Carcass Characteristics of Merino, South African Mutton Merino and Dorper Lambs. S. Afr. J. Anim. Sci. 2017, 47, 26–33. [Google Scholar] [CrossRef] [Green Version]
- De Jesus Junior, C.; Rodrigues, L.S.; de Moraes, V.E.G. Ovinocaprinocultura de Corte: A Convivência dos Extremos; Banco Nacional de Desenvolvimento Econômico e Social: Rio de Janeiro, Brazil, 2010. [Google Scholar]
- Amarante, A.F.T.; Bricarello, P.A.; Rocha, R.A.; Gennari, S.M. Resistance of Santa Ines, Suffolk and Ile de France Sheep to Naturally Acquired Gastrointestinal Nematode Infections. Vet. Parasitol. 2004, 120, 91–106. [Google Scholar] [CrossRef]
- Lôbo, R.N.B.; Pereira, I.D.C.; Facó, O.; McManus, C.M. Economic Values for Production Traits of Morada Nova Meat Sheep in a Pasture Based Production System in Semi-Arid Brazil. Small Rumin. Res. 2011, 96, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Vargas, F.M.; Martins, C.F.; Pinto, G.S.; Ferreira, M.B.; Ricardo, H.A.; Leonardo, A.P.; Fernandes, A.R.M.; Teixeira, A. Carcass Measurements, Non-Carcass Components and Cut Production of Local Brazilian Pantaneiro Sheep and Crossbreeds of Texel and Santa Inês with Pantaneiro. Small Rumin. Res. 2015, 124, 55–62. [Google Scholar] [CrossRef] [Green Version]
- De Farias Jucá, A.; Faveri, J.C.; Melo Filho, G.M.; de Lisboa Ribeiro Filho, A.; Azevedo, H.C.; Muniz, E.N.; Pedrosa, V.B.; Pinto, L.F.B. Effects of Birth Type and Family on the Variation of Carcass and Meat Traits in Santa Ines Sheep. Trop. Anim. Health Prod. 2016, 48, 435–443. [Google Scholar] [CrossRef]
- Paim, T.P.; Bianchini, E.; Esteves, G.; Daltro, D.S.; Cardoso, C.C.; Braccini Neto, J.; McManus, C. Meat Production Performance from Crossbreeding between Locally-Adapted Hair Sheep and Specialized Breeds. Arch. Zootec. 2019, 68, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Davenport, K.M.; Hiemke, C.; McKay, S.D.; Thorne, J.W.; Lewis, R.M.; Taylor, T.; Murdoch, B.M. Genetic Structure and Admixture in Sheep from Terminal Breeds in the United States. Anim. Genet. 2020, 51, 284–291. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Yang, Y.L.; Li, G.Q.; Yu, Q.; Yang, J. Identifications of Immune-Responsive Genes for Adaptative Traits by Comparative Transcriptome Analysis of Spleen Tissue from Kazakh and Suffolk Sheep. Sci. Rep. 2021, 11. [Google Scholar] [CrossRef]
- Zhang, M.; Warner, R.D.; Dunshea, F.R.; DiGiacomo, K.; Joy, A.; Abhijith, A.; Osei-Amponsah, R.; Hopkins, D.L.; Ha, M.; Chauhan, S.S. Impact of Heat Stress on the Growth Performance and Retail Meat Quality of 2nd Cross (Poll Dorset × (Border Leicester × Merino)) and Dorper Lambs. Meat Sci. 2021, 181, 108581. [Google Scholar] [CrossRef] [PubMed]
- Malhado, C.H.M.; Carneiro, P.L.S.; Affonso, P.R.A.M.; Souza, A.A.O.; Sarmento, J.L.R. Growth Curves in Dorper Sheep Crossed with the Local Brazilian Breeds, Morada Nova, Rabo Largo, and Santa Inês. Small Rumin. Res. 2009, 84, 16–21. [Google Scholar] [CrossRef]
- Da Silva, W.E.; Leite, J.H.G.M.; de Sousa, J.E.R.; Costa, W.P.; da Silva, W.S.T.; Guilhermino, M.M.; Asensio, L.A.B.; Façanha, D.A.E. Daily Rhythmicity of the Thermoregulatory Responses of Locally Adapted Brazilian Sheep in a Semiarid Environment. Int. J. Biometeorol. 2017, 61, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- McManus, C.M.; Hermuche, P.; Guimarães, R.F.; de Carvalho Júnior, O.A.; Dallago, B.S.L.; Vieira, R.A.; de Faria, D.A.; Blackburn, H.; Moraes, J.C.F.; Souza, C.H.; et al. Integration of Georeferenced and Genetic Data for the Management of Biodiversity in Sheep Genetic Resources in Brazil. Trop. Anim. Health Prod. 2021, 53, 126. [Google Scholar] [CrossRef] [PubMed]
- Sousa, D.L.; Marcondes, M.I.; Silva, L.P.; Lima, F.W.R.; Herbster, C.J.L.; Souza, J.G.; Rodrigues, J.P.P.; Bezerra, L.R.; Oliveira, R.L.; Pereira, E.S. Macromineral and Trace Element Requirements for Santa Ines Sheep. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- De Andrade Pantoja, M.H.; Esteves, S.N.; Jacinto, M.A.C.; Pezzopane, J.R.M.; de Paz, C.C.P.; da Silva, J.A.R.; de Brito Lourenço Junior, J.; Brandão, F.Z.; Moura, A.B.B.; Romanello, N.; et al. Thermoregulation of Male Sheep of Indigenous or Exotic Breeds in a Tropical Environment. J. Therm. Biol. 2017, 69, 302–310. [Google Scholar] [CrossRef]
- Matos, J.C.; Menezes, V.G.; Gois, G.C.; de Araújo, G.G.L.; de Carvalho Barcellos, B.S.; Soares, M.G.; de Matos, M.H.T.; Moraes, E.A.; Menezes, D.R.; Queiroz, M.A.Á. Histological and Physical–Mechanical Characteristics of the Skin of Dorper Sheep Related to Residual Feed Intake and the Confinement Environment. Trop. Anim. Health Prod. 2022, 54, 314. [Google Scholar] [CrossRef]
- Costa, J.H.S.; de Araújo Furtado, D.; Lopes Neto, J.P.; Ribeiro, N.L.R.; dos Santos Damaceno, L.d.F.; da Silva Neves, R.; de Medeiros, G.R. Thermal Comfort and Integumentary Structure of Sheep Kept in a Covered and Uncovered Environment. Braz. J. Dev. 2020, 6, 20449–20461. [Google Scholar] [CrossRef]
- Correa, M.P.C.; Cardoso, M.T.; Castanheira, M.; Landim, A.V.; Dallago, B.S.L.; Louvandini, H.; McManus, C. Heat Tolerance in Three Genetic Groups of Lambs in Central Brazil. Small Rumin. Res. 2012, 104, 70–77. [Google Scholar] [CrossRef]
- Ángeles Hernández, J.C.; Schilling, S.R.; Arias, M.A.V.; Echeverría Pérez, R.A.; Castelán-Ortega, O.A.; Ramírez Pérez, A.H.; Ronquillo, M.G.; Angeles Hern Andez, J.C.; Echeverría Pérez, R.A.; Castel An-Ortega, O.A.; et al. Effect of Live Weight Pre- and Post-Lambing on Milk Production of East Friesian Sheep. Ital. J. Anim. Sci. 2017, 17, 184–194. [Google Scholar] [CrossRef] [Green Version]
- Robles Jimenez, L.E.; Angeles Hernandez, J.C.; Palacios, C.; Abecia, J.A.; Naranjo, A.; Avalos, J.O.; Gonzalez-Ronquillo, M. Milk Production of Lacaune Sheep with Different Degrees of Crossing with Manchega Sheep in a Commercial Flock in Spain. Animals 2020, 10, 520. [Google Scholar] [CrossRef] [Green Version]
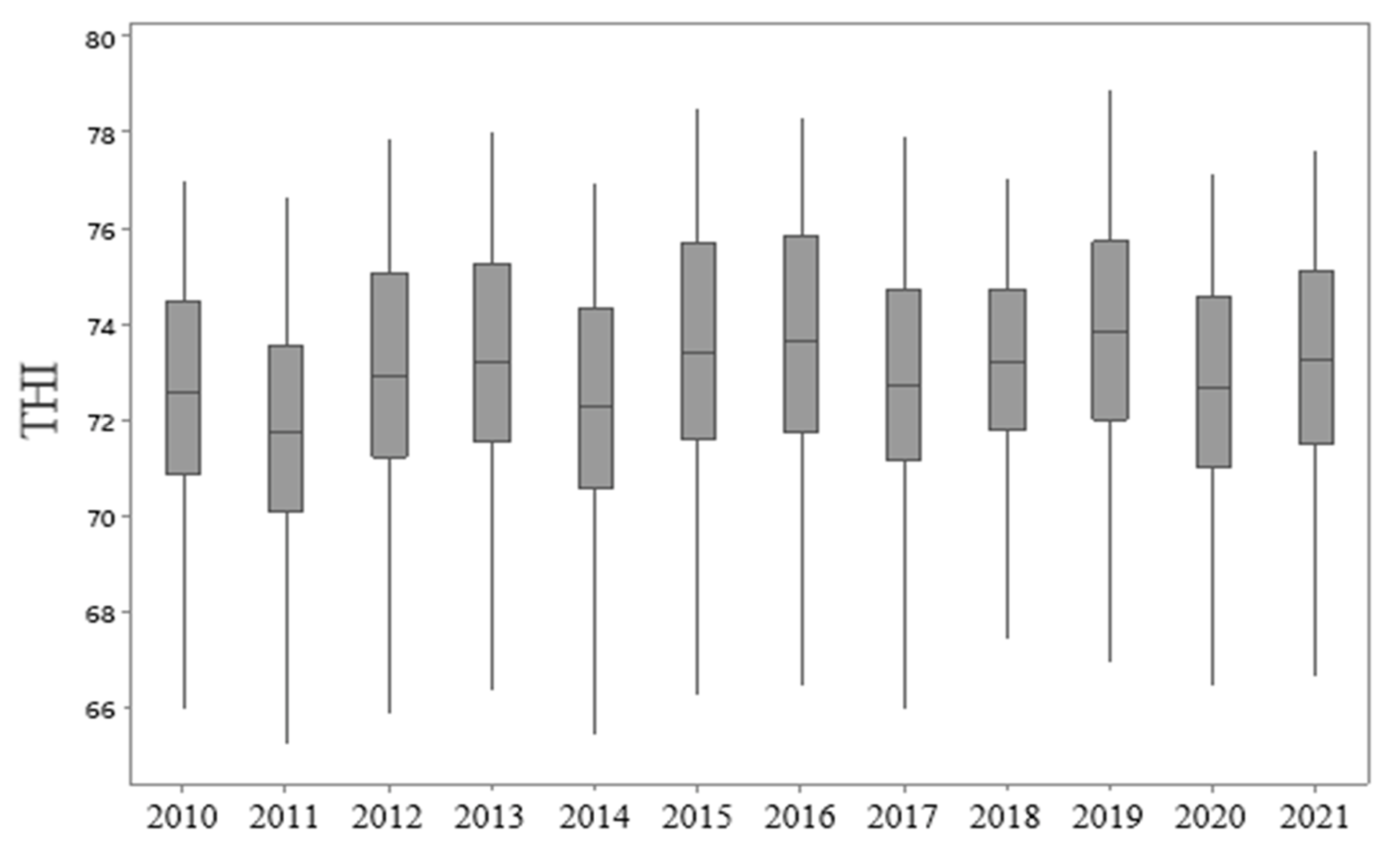


| Year | Mean | 1 Med | 2 Min | 3 Max | 4 SD | 5 CV | 6 A | 7 K |
|---|---|---|---|---|---|---|---|---|
| 2010 | 72.45 | 72.56 | 65.94 | 76.97 | 2.25 | 3.10 | −0.32 | −0.79 |
| 2011 | 71.65 | 71.71 | 65.21 | 76.63 | 2.23 | 3.11 | −0.21 | −0.74 |
| 2012 | 72.84 | 72.89 | 65.85 | 77.86 | 2.50 | 3.43 | −0.27 | −0.73 |
| 2013 | 73.12 | 73.18 | 66.32 | 77.99 | 2.39 | 3.27 | −0.27 | −0.75 |
| 2014 | 72.18 | 72.26 | 65.39 | 76.90 | 2.39 | 3.31 | −0.28 | −0.79 |
| 2015 | 73.34 | 73.40 | 66.24 | 78.47 | 2.56 | 3.48 | −0.23 | −0.82 |
| 2016 | 73.50 | 73.61 | 66.42 | 78.30 | 2.53 | 3.45 | −0.31 | −0.84 |
| 2017 | 72.67 | 72.70 | 65.95 | 77.89 | 2.39 | 3.29 | −0.23 | −0.68 |
| 2018 | 73.05 | 73.20 | 67.02 | 77.05 | 1.93 | 2.64 | −0.44 | −0.60 |
| 2019 | 73.69 | 73.81 | 66.89 | 78.87 | 2.37 | 3.21 | −0.24 | −0.75 |
| 2020 | 72.64 | 72.65 | 66.43 | 77.10 | 2.13 | 2.93 | −0.20 | −0.91 |
| 2021 | 73.09 | 73.24 | 66.63 | 77.59 | 2.20 | 3.01 | −0.33 | −0.82 |
| Spherical | |||||
|---|---|---|---|---|---|
| Year | ME | RMSE | MSE | RMSSE | ASE |
| 2010 | −6.39 × 10−5 | 0.259214 | −0.00048 | 0.7138 | 0.362777 |
| 2011 | −5.08 × 10−5 | 0.257907 | −0.00045 | 0.690961 | 0.372801 |
| 2012 | −5.07 × 10−5 | 0.259716 | −0.00046 | 0.694066 | 0.373771 |
| 2013 | −5.26 × 10−5 | 0.260369 | −0.00047 | 0.710843 | 0.36587 |
| 2014 | −7.27 × 10−5 | 0.258842 | −0.00049 | 0.711379 | 0.363453 |
| 2015 | −5.34 × 10−5 | 0.261177 | −0.00052 | 0.749804 | 0.34791 |
| 2016 | −5.26 × 10−5 | 0.260877 | −0.00047 | 0.69588 | 0.374477 |
| 2017 | −4.15 × 10−5 | 2.59 × 10−1 | −0.00045 | 0.704609 | 0.367699 |
| 2018 | −2.75 × 10−5 | 0.260611 | −0.00044 | 0.716682 | 0.363221 |
| 2019 | −6.23 × 10−5 | 0.261536 | −0.00045 | 0.703081 | 0.371541 |
| 2020 | −7.11 × 10−5 | 0.260421 | −0.00047 | 0.71442 | 0.364073 |
| 2021 | −6.07 × 10−5 | 0.260687 | −0.00046 | 0.701996 | 0.370905 |
| Gaussian | |||||
| Year | ME | RMSE | MSE | RMSSE | ASE |
| 2010 | −0.00234 | 0.361035 | −0.00638 | 0.944639 | 0.382196 |
| 2011 | −0.00235 | 0.359701 | −0.00639 | 0.934722 | 0.384795 |
| 2012 | −0.00252 | 0.359492 | −0.00695 | 0.950751 | 0.378098 |
| 2013 | −0.00256 | 0.361166 | −0.007 | 0.948248 | 0.380862 |
| 2014 | −0.00234 | 0.360502 | −0.0064 | 0.944673 | 0.381605 |
| 2015 | −0.00255 | 0.36118 | −0.00698 | 0.947964 | 0.380983 |
| 2016 | −0.00254 | 0.362973 | −0.0069 | 0.945865 | 0.383743 |
| 2017 | −0.0024 | 0.361205 | −0.00657 | 0.945479 | 0.382008 |
| 2018 | −0.0025 | 0.364844 | −0.0067 | 0.934608 | 0.39035 |
| 2019 | −0.00229 | 0.363888 | −0.00619 | 0.944569 | 0.385213 |
| 2020 | −0.00212 | 0.366506 | −0.00555 | 0.917904 | 0.399259 |
| 2021 | −0.00229 | 0.365006 | −0.00612 | 0.934848 | 0.390413 |
| Exponential | |||||
| Year | ME | RMSE | MSE | RMSSE | ASE |
| 2010 | 1.48 × 10−4 | 0.261364 | 0.00 | 0.585199 | 0.445757 |
| 2011 | 0.000167 | 0.260073 | 1.37 × 10−5 | 0.563429 | 0.460613 |
| 2012 | 0.000183 | 0.261855 | 2.46 × 10−5 | 0.568621 | 0.45959 |
| 2013 | 0.00019 | 0.26251 | 3.63 × 10−5 | 0.526857 | 0.497263 |
| 2014 | 0.000141 | 0.260972 | −2.58 × 10−5 | 0.559033 | 0.4659 |
| 2015 | 0.000184 | 0.263107 | 2.39 × 10−5 | 0.555818 | 0.472411 |
| 2016 | 0.000184 | 0.263034 | 1.97 × 10−5 | 0.558827 | 0.469761 |
| 2017 | 0.000181 | 0.26155 | 2.33 × 10−5 | 0.604687 | 0.431644 |
| 2018 | 0.000208 | 0.262778 | 6.41 × 10−5 | 0.60554 | 0.43308 |
| 2019 | 0.000154 | 0.263722 | 3.33 × 10−6 | 0.572355 | 0.459804 |
| 2020 | 0.00012 | 0.262605 | −4.41 × 10−5 | 0.52963 | 0.494776 |
| 2021 | 0.000158 | 0.262874 | 2.72 × 10−6 | 0.604837 | 0.433709 |
| Year | Model | Nugget Effect | Sill | Range | 1 DSD |
|---|---|---|---|---|---|
| 2010 | Gaussian | 0.1585 | 1.6217 | 59,902 | 9.77 |
| 2011 | Gaussian | 0.1316 | 1.7403 | 54,541 | 7.52 |
| 2012 | Gaussian | 0.1267 | 1.7982 | 57,355 | 7.00 |
| 2013 | Gaussian | 0.1287 | 1.7805 | 63,124 | 7.22 |
| 2014 | Gaussian | 0.1294 | 1.7170 | 55,485 | 7.51 |
| 2015 | Gaussian | 0.1287 | 1.8061 | 62,548 | 7.12 |
| 2016 | Gaussian | 0.1308 | 1.7594 | 61,548 | 7.39 |
| 2017 | Gaussian | 0.1296 | 1.7412 | 58,563 | 7.44 |
| 2018 | Gaussian | 0.1356 | 1.7220 | 62,551 | 7.87 |
| 2019 | Gaussian | 0.1318 | 1.7792 | 58,961 | 7.36 |
| 2020 | Gaussian | 0.1422 | 1.7163 | 60,512 | 8.27 |
| 2021 | Gaussian | 0.1356 | 1.7313 | 56,548 | 7.83 |
| Breed | Cover Type | THI |
|---|---|---|
| Rabo Largo | Hair | 81.93 |
| Morada Nova | Hair | 80.81 |
| Somali | Hair | 80.64 |
| Bergamácia | Wool | 79.40 |
| Cariri | Hair | 79.34 |
| Santa Inês | Hair | 78.27 |
| Dorper | Hair | 78.26 |
| White Dorper | Hair | 76.96 |
| 1 SAMM | Wool | 75.92 |
| Suffolk | Wool | 74.61 |
| Poll Dorset | Wool | 74.55 |
| East Friesian | Wool | 74.55 |
| Texel | Wool | 73.67 |
| Border Leicester | Wool | 72.77 |
| Lacaune | Wool | 72.72 |
| Merino | Wool | 72.66 |
| Corriedale | Wool | 72.63 |
| Ilê de France | Wool | 72.63 |
| Karakul | Wool | 72.40 |
| Crioula | Wool | 72.10 |
| Hampshire | Wool | 71.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marinho, G.T.B.; Pandorfi, H.; da Silva, M.V.; Montenegro, A.A.d.A.; de Sousa, L.d.B.; Desenzi, R.; da Silva, J.L.B.; de Oliveira-Júnior, J.F.; Mesquita, M.; de Almeida, G.L.P.; et al. Bioclimatic Zoning for Sheep Farming through Geostatistical Modeling in the State of Pernambuco, Brazil. Animals 2023, 13, 1124. https://doi.org/10.3390/ani13061124
Marinho GTB, Pandorfi H, da Silva MV, Montenegro AAdA, de Sousa LdB, Desenzi R, da Silva JLB, de Oliveira-Júnior JF, Mesquita M, de Almeida GLP, et al. Bioclimatic Zoning for Sheep Farming through Geostatistical Modeling in the State of Pernambuco, Brazil. Animals. 2023; 13(6):1124. https://doi.org/10.3390/ani13061124
Chicago/Turabian StyleMarinho, Gabriel Thales Barboza, Héliton Pandorfi, Marcos Vinícius da Silva, Abelardo Antônio de Assunção Montenegro, Lizandra de Barros de Sousa, Raquel Desenzi, Jhon Lennon Bezerra da Silva, José Francisco de Oliveira-Júnior, Márcio Mesquita, Gledson Luiz Pontes de Almeida, and et al. 2023. "Bioclimatic Zoning for Sheep Farming through Geostatistical Modeling in the State of Pernambuco, Brazil" Animals 13, no. 6: 1124. https://doi.org/10.3390/ani13061124
APA StyleMarinho, G. T. B., Pandorfi, H., da Silva, M. V., Montenegro, A. A. d. A., de Sousa, L. d. B., Desenzi, R., da Silva, J. L. B., de Oliveira-Júnior, J. F., Mesquita, M., de Almeida, G. L. P., Guiselini, C., da Rosa Ferraz Jardim, A. M., & Silva, T. G. F. d. (2023). Bioclimatic Zoning for Sheep Farming through Geostatistical Modeling in the State of Pernambuco, Brazil. Animals, 13(6), 1124. https://doi.org/10.3390/ani13061124









