High Dietary Cation and Anion Difference and High-Dose Ascorbic Acid Modify Acid–Base and Antioxidant Balance in Dairy Goats Fed under Tropical Conditions
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area, Animals, and Meteorological Data
2.2. Data Collection, Measurement, and Analysis
2.3. Statistical Analyses
3. Results
3.1. Ambient Condition and the Effect of DCAD and AA Supplementation on Tr, RR, DMI, WI, and MY
3.2. Effect of DCAD and AA Supplementation on Blood Gas Parameters, and Urine pH
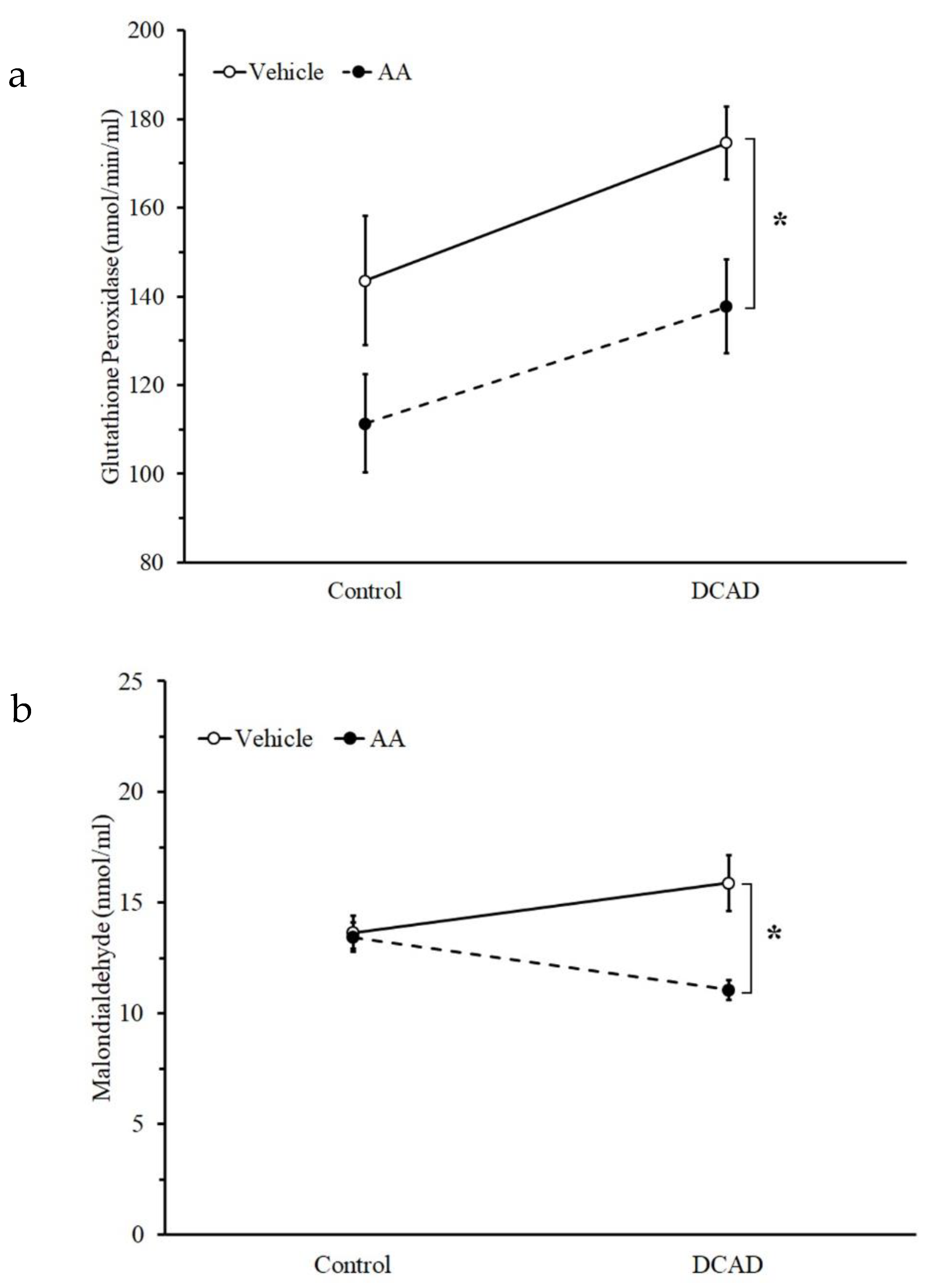
3.3. Effect of DCAD and AA Supplementation on Plasma Antioxidant Capacity and Plasma Cortisol
3.4. Effect of DCAD and AA Supplementation on Milk Composition and Yield of Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thammacharoen, S.; Chanpongsang, S.; Chaiyabutr, N.; Teedee, S.; Pornprapai, A.; Insam-ang, A.; Srisa-ard, C.; Channacoop, N. An analysis of herd-based lactation curve reveals the seasonal effect from dairy cows fed under high ambient temperature. Thai J. Vet. Med. 2020, 50, 169–178. [Google Scholar]
- Saipin, N.; Semsirmboon, S.; Rungsiwiwut, R.; Thammacharoen, S. High ambient temperature directly decreases milk synthesis in the mammary gland in Saanen goats. J. Therm. Biol. 2020, 94, 102783. [Google Scholar] [CrossRef] [PubMed]
- Semsirmboon, S.; Do Nguyen, D.K.; Chaiyabutr, N.; Poonyachoti, S.; Thammacharoen, S. Natural high ambient temperature induced respiratory hypocapnia without activation of the hypothalamic-pituitary-adrenal axis lactating goats. Vet. World 2022, 15, 2611–2616. [Google Scholar] [CrossRef] [PubMed]
- Do Nguyen, D.K.; Semsirmboon, S.; Chaiyabutr, N.; Thammacharoen, S. Effects of low dietary cation and anion difference on blood gas, renal electrolyte, and acid excretion in goat in tropical conditions. Animals 2022, 12, 3444. [Google Scholar] [CrossRef] [PubMed]
- Facanha, D.A.E.; Ferreira, J.; Silveira, R.M.F.; Nunes, T.L.; Carlos de Oliveira, M.G.; Rufino de Sousa, J.E.; Veras de Paula, V. Are locally adapted goats able to recover homeothermy, acid-base and electrolyte equilibrium in a semi-arid region? J. Dairy Sci. 2020, 90, 102593. [Google Scholar] [CrossRef]
- Baumgard, L.H.; Rhoads, R.P., Jr. Effects of heat stress on postabsorptive metabolism and energetics. Annu. Rev. Anim. Biosci. 2013, 1, 311–337. [Google Scholar] [CrossRef] [Green Version]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef] [Green Version]
- Hamzaoui, S.; Salama, A.A.K.; Albenell, E.; Such, X.; Caja, G. Physiological response and lactational performances of late-lactation dairy goats under heat stress conditions. J. Dairy Sci. 2013, 96, 6355–6365. [Google Scholar] [CrossRef] [Green Version]
- Sivakumar, A.V.N.; Singh, G.; Varshney, V.P. Antioxidants supplementation on acid base balance during heat stress in goats. Asian-Australas. J. Anim. Sci. 2010, 23, 1462–1468. [Google Scholar] [CrossRef]
- Thammacharoen, S.; Saipin, N.; Nguyen, T.; Chaiyabutr, N. Effects of High Ambient Temperature on Milk Protein Synthesis in Dairy Cows and Goat: Insights from the Molecular Mechanisms Studies, In Milk Protein-New Research Approachs; Chaiyabutr, N., Ed.; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Rodriguez-Villar, S.; Do Vale, B.M.; Fletcher, H.M. The arterial blood gas algorithm: Proposal of a systematic approach to analysis of acid-base disorders. Rev. Esp. Anestesiol. Reanim. 2020, 67, 20–34. [Google Scholar] [CrossRef]
- Ganong, F.S. Chapter 35: Gas transport and pH. In Ganong’s Reviews of Medical Physiology, 26th ed.; Barrett, K.E., Barman, S.M., Brook, H.L., Yuan, J., Eds.; McGraw Hill Education: NewYork, NY, USA, 2019; pp. 1460–1496. [Google Scholar]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Gartner, T.; Zoche-Golob, V.; Redlberger, S.; Reinhold, P.; Donat, K. Acid-base assessment of post-parturient German Holstein dairy cows from jugular venous blood and urine: A comparison of the strong ion approach and traditional blood gas analysis. PLoS ONE 2019, 14, e0210948. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Singh, N.K.; Singh, O.P.; Pandey, V.; Verma, P.K. Oxidative stress and antioxidant status during transition period in dairy cows. Asian-Australas. J. Anim. Sci. 2011, 24, 479–484. [Google Scholar] [CrossRef]
- Putman, A.K.; Brown, J.L.; Gandy, J.C.; Wisnieski, L.; Sordillo, L.M. Change in biomarkers of nutrient metabolism, inflammation, and oxidative stress in dairy cows during the transition into the early dry period. J. Dairy Sci. 2018, 101, 9350–9359. [Google Scholar] [CrossRef] [Green Version]
- Ma, N.; Abaker, J.A.; Wei, G.; Chen, H.; Shen, X.; Chang, G. A high-concentrate diet induces an inflammatory response and oxidative stress and depresses milk fat synthesis in the mammary gland of dairy cows. J. Dairy Sci. 2022, 105, 5493–5505. [Google Scholar] [CrossRef]
- Nedic, S.; Vakanjac, S.; Samardzija, M.; Borozan, S. Paraoxonase 1 in bovine milk and blood as marker of subclinical mastitis caused by Staphylococcus aureus. Res. Vet. Sci. 2019, 125, 323–332. [Google Scholar] [CrossRef]
- Guo, J.; Gao, S.; Quan, S.; Zhang, Y.; Bu, D.; Wang, J. Blood amino acids profile responding to heat stress in dairy cows. Asian-Australas. J. Anim. Sci. 2018, 31, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Chaiyabutr, N.; Chanchai, W.; Boonsanit, D.; Sitprija, S.; Chanpongsang, S. Different Responses of Oxidative Stress Index in the Plasma of Crossbred Holstein Cattle During Cooling and Supplemental Recombinant Bovine Somatotropin. J. Anim. Vet. Adv. 2011, 10, 1045–1053. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, M.; Kamiya, Y.; Suzuki, T.; Nakai, Y. Effect of high environmental temperatures on ascorbic acid, sulfhydryl residue and oxidized lipid concentrations in plasma of dairy cows. Anim. Sci. J. 2007, 78, 301–306. [Google Scholar] [CrossRef]
- Nguyen, T.; Chaiyabutr, N.; Chanpongsang, S.; Thammacharoen, S. Dietary cation and anion difference: Effects on milk production and body fluid distribution in lactating dairy goats under tropical conditions. Anim. Sci. J. 2018, 89, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Chanpongsang, S.; Chaiyabutr, N.; and Thammacharoen, S. The effect of dietary ions difference on drinking and eating patterns in dairy goats under high ambient temperature. Asian-Australas. J. Anim. Sci. 2019, 32, 599–606. [Google Scholar] [CrossRef]
- Hu, W.; Murphy, M.R. Dietary cation-anion difference effects on prefomance and acid-base status of lactating dairy cows: A meta-analysis. J. Dairy Sci. 2004, 87, 2222–2229. [Google Scholar] [CrossRef]
- Wildman, C.D.; West, J.W.; Bernard, J.K. Effect of dietary cation-anion difference and dietary crude protein on performance of dairy cows during hot weather. J. Dairy Sci. 2007, 90, 1842–1850. [Google Scholar] [CrossRef] [PubMed]
- Chaiyotwittayakun, A.; Erskin, R.J.; Bartlett, T.H.; Herdt, T.H.; Sears, P.M.; Harmon, R.J. The effect of ascorbic acid and l-histidine therapy on acute mammary gland inflammation in dairy cattle. J. Dairy Sci. 2002, 5, 60–67. [Google Scholar] [CrossRef]
- National Research Council (U.S.) Committee on Physioloical Effect of Environment Factors on Animals. A Guide to Environmental Research on Animals; National Academy of Science: Washington, DC, USA, 1971; p. 374. [Google Scholar]
- Mavrogenis, A.P.; Papachristoforou, C. Estimation of the energy value of milk and prediction of fat-corrected milk yield in sheep and goats. Small. Rum. 1988, 1, 229–236. [Google Scholar] [CrossRef]
- Suwannapaporn, P.; Chaiyabutr, N.; Wanasuntronwong, A.; Thammacharoen, S. A low degree of high ambient temperature decreased food intake and activated median preoptic and arcuate nuclei. Physiol. Behav. 2017, 181, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Suwannapaporn, P.; Chaiyabutr, N.; Wanasuntronwong, A.; Thammacharoen, S. Arcuate proopiomelanocortin is part of a novel neural connection for short-term low-degree of high ambient temperature effects on food intake. Physiol. Behav. 2022, 245, 113687. [Google Scholar] [CrossRef] [PubMed]
- Noureldin, Y.A.; da Silva, A.; Fahmy, N.; Andonian, S. Is it safe to prescribe ascorbic acid for urinary acidification in stone-forming patients with alkaline urine? Turk. J. Urol. 2017, 43, 183–188. [Google Scholar] [CrossRef]
- Padilla, L.; Matsui, T.; Ikeda, S.; Kitagawa, M.; Yano, H. The effect of vitamin C supplementation on plasma concentration and urinary excretion of vitamin C in cattle. J. Anim. Sci. 2007, 85, 3367–3370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevens, J.B.; Anderson, K.L.; Correa, M.T.; Stewart, W.E.; Braselton, J.R. Hematologic, Blood gas, Blood Chemistry, and serum mineral values for a sample of clinically healthy adult goats. Vet. Clin. Pathol. 1994, 23, 19–24. [Google Scholar] [CrossRef]
- National Research Council (U.S.) Committee on Animal Nutrition. Nutrient Requirements of Goats: Angora, Dairy, and Meat Goats in Temperate and Tropical Countries; National Academy of Science: Washington, DC, USA, 1981; p. 84. [Google Scholar]
- Yun, S.H.; Moon, Y.S.; Sohn, S.H.; Jang, I.S. Effects of cyclic heat stress or vitamin C supplementation during cyclic heat stress on HSP70, inflammatory cytokines, and the antioxidant defense system in Sprague Dawley rats. Exp. Anim. 2012, 61, 543–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colakoglu, H.E.; Yazlik, M.O.; Kaya, U.; Colakoglu, E.C.; Kurt, S.; Oz, B.; Bayramoglu, R.; Vural, M.R.; Kuplulu, S. MDA and GSH-Px activity in transition dairy cows under seasonal variations and their relationship with reproductive performance. J. Vet. Res. 2017, 61, 497–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaiyabutr, C.; Komolvanich, S.; Preuksagorn, S.; Chanpongsang, S. Comparative studies on the utilization of glucose in the mammary gland of crossbred Holstein cattle feeding on different types of roughage during different stages of lactation. Asian-Australas. J. Anim. Sci. 2000, 13, 334–347. [Google Scholar] [CrossRef]
- Garnsworthy, P.C.; Masson, L.L.; Lock, A.L.; Mottram, T.T. Variation of milk citrate with stage of lactation and de novo fatty acid synthesis in dairy cows. J. Dairy Sci. 2006, 89, 1604–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basiricò, L.; Morera, P.; Dipasquale, D.; Tröscher, A.; Bernabucci, U. Comparison between conjugated linoleic acid and essential fatty acids in preventing oxidative stress in bovine mammary epithelial cells. J. Dairy Sci. 2017, 100, 2299–2309. [Google Scholar] [CrossRef] [Green Version]
- Zachut, M.; Kra, G.; Portnik, Y.; Shapiro, F.; Silanikove, N. Milk glucose-6-phosphate dehydrogenase activity and glucose-6-phosphate are associated with oxidative stress and serve as indicators of energy balance in dairy cows. RSV Adv. 2016, 6, 65412–65417. [Google Scholar] [CrossRef]


| Feed Composition (%) | Hay | Control | DCAD |
|---|---|---|---|
| Dry matter | 92.68 | 91.26 | 87.76 |
| Protein | 4.2 | 16.1 | 15.5 |
| Crude Fat | 1.0 | 3.9 | 2.6 |
| NDF | 78.9 | - | - |
| ADF | 48.4 | - | - |
| Ash | 7.7 | 7.1 | 7.1 |
| Ca | 0.8 | 1.2 | 1.4 |
| P | 0.1 | 0.5 | 0.6 |
| Na | 14 | 1 | 13 |
| K | 36 | 32 | 57 |
| Cl | 9 | 15 | 17 |
| S | 12 | 12 | 8 |
| DCAD (mEq/100 g DM) | 29 | 6 | 45 |
| Control | DCAD | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| Vehicle | AA | Vehicle | AA | SEM | DCAD | AA | DCAD × AA | |
| 4th week of supplement | ||||||||
| RR (TPM) | 67 | 90 | 93 | 102 | 21.84 | 0.28 | 0.11 | 0.47 |
| Tr (°C) | 38.9 | 39.0 | 38.7 | 38.9 | 0.30 | 0.60 | 0.23 | 0.95 |
| DMI (%kgBw) | 3.04 | 3.05 | 3.40 | 3.58 | 0.21 | 0.31 | 0.35 | 0.26 |
| WI (%kgBw) | 14.2 | 14.0 | 13.5 | 15.2 | 1.36 | 0.92 | 0.19 | 0.13 |
| MY (kg/8 h) | 0.53 | 0.56 | 0.53 | 0.54 | 0.03 | 0.91 | 0.19 | 0.48 |
| 4%FCM (kg/8 h) | 1.21 | 1.28 | 1.30 | 1.32 | 0.08 | 0.78 | 0.23 | 0.51 |
| 8th week of supplement | ||||||||
| RR (TPM) | 51 | 60 | 81 | 70 | 18.76 | 0.22 | 0.95 | 0.22 |
| Tr (°C) | 38.9 | 38.9 | 39.0 | 38.7 | 0.20 | 1.00 | 0.05 | 0.18 |
| DMI (%kgBw) | 3.22 | 3.47 | 3.70 | 3.49 | 0.28 | 0.56 | 0.83 | 0.08 |
| WI (%kgBw) | 12.6 | 12.7 | 12.4 | 12.0 | 2.13 | 0.88 | 0.85 | 0.75 |
| MY (kg/8 h) | 0.46 | 0.48 | 0.48 | 0.48 | 0.04 | 0.90 | 0.51 | 0.35 |
| 4%FCM (kg/8 h) | 1.03 | 1.08 | 1.15 | 1.13 | 0.08 | 0.69 | 0.61 | 0.32 |
| Control | DCAD | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| Vehicle | AA | Vehicle | AA | SEM | DCAD | AA | DCAD × AA | |
| 4th week of supplement | ||||||||
| Blood pH | 7.43 | 7.44 | 7.50 | 7.49 | 0.03 | 0.02 | 0.69 | 0.24 |
| PCO2 (mmHg) | 35.37 | 33.82 | 34.42 | 34.15 | 2.54 | 0.86 | 0.40 | 0.55 |
| HCO3 (mmol/L) | 23.35 | 22.68 | 27.28 | 25.50 | 2.65 | 0.09 | 0.28 | 0.62 |
| Urine pH | 8.17 | 8.06 | 8.13 | 8.08 | 0.1 | 0.9 | 0.07 | 0.47 |
| 8th week of supplement | ||||||||
| Blood pH | 7.47 | 7.48 | 7.48 | 7.50 | 0.03 | 0.46 | 0.18 | 0.74 |
| PCO2 (mmHg) | 35.02 | 35.00 | 36.45 | 35.87 | 1.57 | 0.36 | 0.65 | 0.67 |
| HCO3 (mmol/L) | 25.48 | 26.23 | 27.32 | 28.02 | 1.70 | 0.31 | 0.32 | 0.97 |
| Urine pH | 8.22 | 8.04 | 8.31 | 8.24 | 0.05 | 0.0547 | <0.01 | 0.025 |
| Control | DCAD | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| Vehicle | AA | Vehicle | AA | SEM | DCAD | AA | DCAD × AA | |
| Milk composition | ||||||||
| Fat (%) | 4.68 | 4.73 | 5.05 | 4.81 | 0.27 | 0.63 | 0.42 | 0.21 |
| Protein (%) | 2.68 | 2.74 | 2.61 | 2.67 | 0.05 | 0.46 | 0.01 | 0.89 |
| Lactose (%) | 4.25 | 4.23 | 4.24 | 4.26 | 0.06 | 0.99 | 0.99 | 0.12 |
| Citrate (%) | 0.125 | 0.133 | 0.098 | 0.110 | 0.007 | 0.28 | 0.01 | 0.57 |
| FFA (%) | 0.57 | 0.51 | 0.88 | 0.77 | 0.07 | 0.15 | 0.01 | 0.29 |
| Glucose (µM) | 70 | 72 | 79 | 95 | 14.13 | 0.61 | 0.15 | 0.27 |
| G6P (µM) | 166 | 182 | 170 | 186 | 29.44 | 0.83 | 0.21 | 0.97 |
| G6P: Glu ratio | 2.92 | 3.10 | 3.73 | 3.11 | 0.77 | 0.72 | 0.50 | 0.23 |
| Yield of composition (8-h) | ||||||||
| Fat (g) | 20.98 | 22.42 | 23.76 | 22.02 | 1.96 | 0.72 | 0.85 | 0.07 |
| Protein (g) | 12.16 | 13.11 | 12.54 | 12.65 | 0.92 | 0.98 | 0.19 | 0.29 |
| Lactose (g) | 19.34 | 20.33 | 20.40 | 20.26 | 1.40 | 0.89 | 0.47 | 0.36 |
| Citrate (g) | 0.54 | 0.61 | 0.50 | 0.56 | 0.06 | 0.65 | 0.02 | 0.99 |
| FFA (g) | 0.026 | 0.025 | 0.039 | 0.033 | 0.003 | 0.18 | 0.03 | 0.10 |
| Glucose(µmol) | 30 | 32 | 39 | 49 | 9.20 | 0.48 | 0.13 | 0.32 |
| G6P (µmol) | 76 | 86 | 84 | 91 | 14.30 | 0.83 | 0.18 | 0.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semsirmboon, S.; Do Nguyen, D.K.; Chaiyabutr, N.; Poonyachoti, S.; Lutz, T.A.; Thammacharoen, S. High Dietary Cation and Anion Difference and High-Dose Ascorbic Acid Modify Acid–Base and Antioxidant Balance in Dairy Goats Fed under Tropical Conditions. Animals 2023, 13, 970. https://doi.org/10.3390/ani13060970
Semsirmboon S, Do Nguyen DK, Chaiyabutr N, Poonyachoti S, Lutz TA, Thammacharoen S. High Dietary Cation and Anion Difference and High-Dose Ascorbic Acid Modify Acid–Base and Antioxidant Balance in Dairy Goats Fed under Tropical Conditions. Animals. 2023; 13(6):970. https://doi.org/10.3390/ani13060970
Chicago/Turabian StyleSemsirmboon, Sapon, Dang Khoa Do Nguyen, Narongsak Chaiyabutr, Sutthasinee Poonyachoti, Thomas A. Lutz, and Sumpun Thammacharoen. 2023. "High Dietary Cation and Anion Difference and High-Dose Ascorbic Acid Modify Acid–Base and Antioxidant Balance in Dairy Goats Fed under Tropical Conditions" Animals 13, no. 6: 970. https://doi.org/10.3390/ani13060970
APA StyleSemsirmboon, S., Do Nguyen, D. K., Chaiyabutr, N., Poonyachoti, S., Lutz, T. A., & Thammacharoen, S. (2023). High Dietary Cation and Anion Difference and High-Dose Ascorbic Acid Modify Acid–Base and Antioxidant Balance in Dairy Goats Fed under Tropical Conditions. Animals, 13(6), 970. https://doi.org/10.3390/ani13060970






