Red Blood Cell Distribution Width as a Novel Parameter in Canine Disorders: Literature Review and Future Prospective
Abstract
:Simple Summary
Abstract
1. Introduction
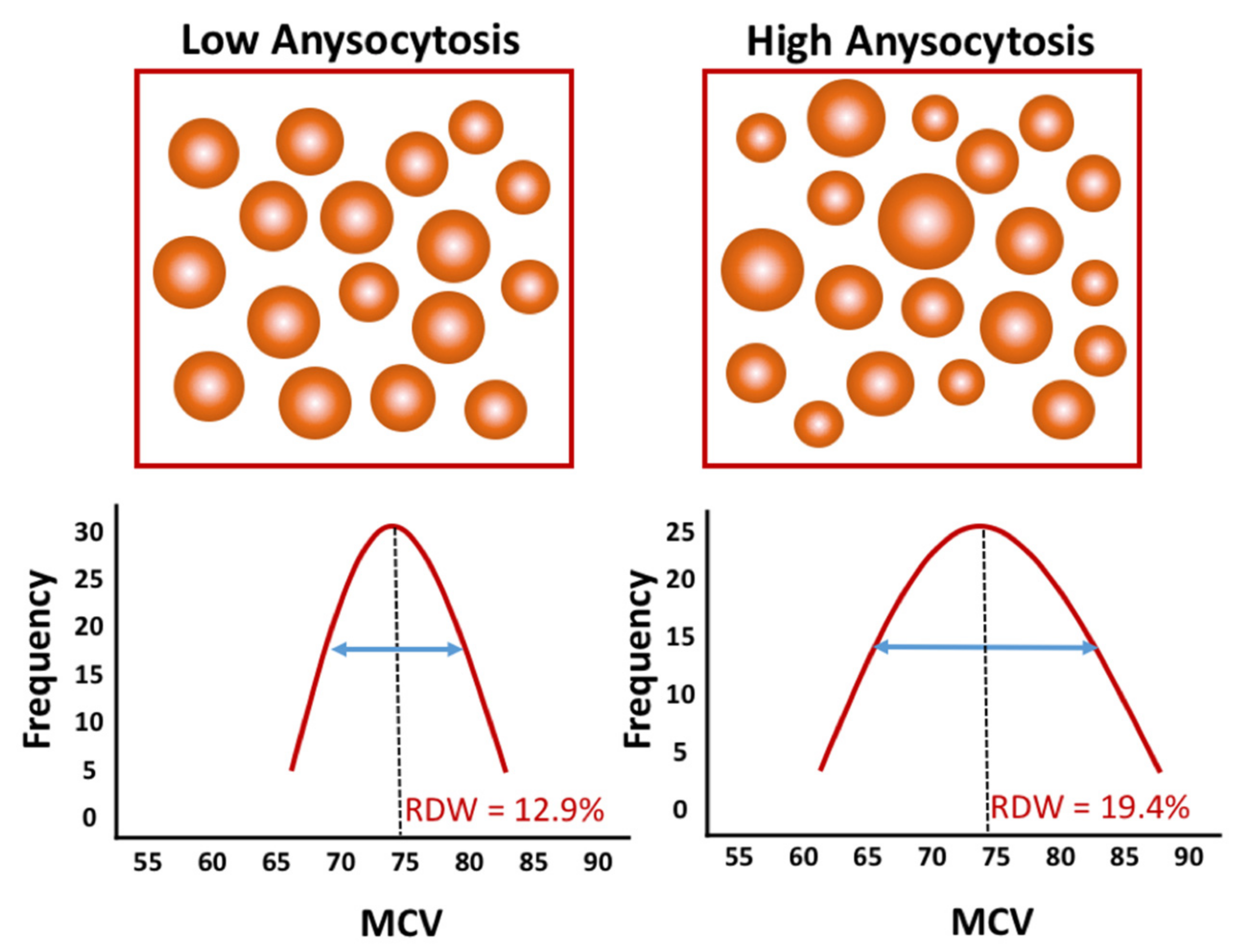
2. Red Blood Cell Distribution Width, Hematological Analyzers, and Patient Characteristics
3. Red Blood Cell Distribution Width and Cardiovascular Disease
3.1. RDW in Mitral Valve Disease
3.2. RDW in Pulmonary Arterial Hypertension and Heartworm Disease
4. Red Blood Cell Distribution Width and Pancreatitis
5. Red Blood Cell Distribution Width and Acute Trauma
6. Red Blood Cell Distribution Width and Critical Ill Dogs/Hospitalized Dogs/Dogs Admitted to Intensive Care Unit
7. Red Blood Cell Distribution Width and Miscellaneous Diseases
8. Pathophysiological Mechanisms of Increased RDW
8.1. Anemia (Erythropoietin Synthesis and Responsiveness) and Iron Metabolism
8.2. Inflammatory State
8.3. Oxidative Stress
8.4. Deformability and Fragmentation Properties of RBCs
9. Conclusions and Future Prospective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Neiger, R.; Hadley, J.; Pfeiffer, D.U. Differentiation of Dogs with Regenerative and Non-Regenerative Anaemia on the Basis of Their Red Cell Distribution Width and Mean Corpuscular Volume. Vet. Rec. 2002, 150, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Salvagno, G.L.; Sanchis-Gomar, F.; Picanza, A.; Lippi, G. Red Blood Cell Distribution Width: A Simple Parameter with Multiple Clinical Applications. Crit. Rev. Clin. Lab. Sci. 2015, 52, 86–105. [Google Scholar] [CrossRef] [PubMed]
- Arkew, M.; Gemechu, K.; Haile, K.; Asmerom, H. Red Blood Cell Distribution Width as Novel Biomarker in Cardiovascular Diseases: A Literature Review. J. Blood Med. 2022, 13, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Nanjarapalle, S.; Samantaray, A.; Vishnubhotla, S. Red Cell Distribution Width as a Severity Marker on the Outcome of Patients with Acute Kidney Injury on Renal Replacement Therapy. Indian J. Crit. Care Med. 2020, 24, 95–98. [Google Scholar] [CrossRef]
- Xanthopoulos, A.; Giamouzis, G.; Dimos, A.; Skoularigki, E.; Starling, R.C.; Skoularigis, J.; Triposkiadis, F. Red Blood Cell Distribution Width in Heart Failure: Pathophysiology, Prognostic Role, Controversies and Dilemmas. J. Clin. Med. 2022, 11, 1951. [Google Scholar] [CrossRef]
- Yousefi, B.; Sanaie, S.; Ghamari, A.A.; Soleimanpour, H.; Karimian, A.; Mahmoodpoor, A. Red Cell Distribution Width as a Novel Prognostic Marker in Multiple Clinical Studies. Indian J. Crit. Care Med. 2020, 24, 49–54. [Google Scholar] [CrossRef]
- O’Connell, R.M.; Boland, M.R.; O’Driscoll, J.; Salih, A.; Arumugasamy, M.; Walsh, T.N.; Allen, M.J.; Beddy, D.J. Red Cell Distribution Width and Neutrophil to Lymphocyte Ratio as Predictors of Outcomes in Acute Pancreatitis: A Retrospective Cohort Study. Int. J. Surg. 2018, 55, 124–127. [Google Scholar] [CrossRef]
- Gravito-Soares, M.; Gravito-Soares, E.; Gomes, D.; Almeida, N.; Tomé, L. Red Cell Distribution Width and Red Cell Distribution Width to Total Serum Calcium Ratio as Major Predictors of Severity and Mortality in Acute Pancreatitis. BMC Gastroenterol. 2018, 18, 108. [Google Scholar] [CrossRef]
- Goyal, H.; Awad, H.; Hu, Z.-D. Prognostic Value of Admission Red Blood Cell Distribution Width in Acute Pancreatitis: A Systematic Review. Ann. Transl. Med. 2017, 5, 342. [Google Scholar] [CrossRef] [Green Version]
- Guglielmini, C.; Poser, H.; Pria, A.D.; Drigo, M.; Mazzotta, E.; Berlanda, M.; Luciani, A. Red Blood Cell Distribution Width in Dogs with Chronic Degenerative Valvular Disease. J. Am. Vet. Med. Assoc. 2013, 243, 858–862. [Google Scholar] [CrossRef]
- Mazzotta, E.; Guglielmini, C.; Menciotti, G.; Contiero, B.; Baron Toaldo, M.; Berlanda, M.; Poser, H. Red Blood Cell Distribution Width, Hematology, and Serum Biochemistry in Dogs with Echocardiographically Estimated Precapillary and Postcapillary Pulmonary Arterial Hypertension. J. Vet. Intern. Med. 2016, 30, 1806–1815. [Google Scholar] [CrossRef]
- Swann, J.W.; Sudunagunta, S.; Covey, H.L.; English, K.; Hendricks, A.; Connolly, D.J. Evaluation of Red Cell Distribution Width in Dogs with Pulmonary Hypertension. J. Vet. Cardiol. 2014, 16, 227–235. [Google Scholar] [CrossRef] [Green Version]
- Guglielmini, C.; Valentini, C.M.; Contiero, B.; Valente, C.; Poser, H. Red Cell Distribution Width Has a Negative Prognostic Role in Dogs with Myxomatous Mitral Valve Disease. Animals 2021, 11, 778. [Google Scholar] [CrossRef]
- Roderick, K.V.; Abelson, A.L.; Nielsen, L.; Price, L.L.; Quinn, R. Evaluation of Red Blood Cell Distribution Width as a Prognostic Indicator in Cats with Acquired Heart Disease, with and without Congestive Heart Failure. J. Feline Med. Surg. 2017, 19, 648–656. [Google Scholar] [CrossRef]
- Stanzani, G.; Cowlam, R.; English, K.; Connolly, D.J. Evaluation of Red Blood Cell Distribution Width in Cats with Hypertrophic Cardiomyopathy. J. Vet. Cardiol. 2015, 17 (Suppl. 1), S233–S243. [Google Scholar] [CrossRef] [Green Version]
- Guglielmini, C.; Crisi, P.E.; Tardo, A.M.; Di Maggio, R.; Contiero, B.; Boari, A.; Fracassi, F.; Miglio, A. Prognostic Role of Red Cell Distribution Width and Other Routine Clinico-Pathological Parameters in Dogs with Acute Pancreatitis. Animals 2022, 12, 3483. [Google Scholar] [CrossRef]
- Kim, S.-J.; Suh, S.-I.; Hyun, C. Evaluation of Red Blood Cell Profiles in Dogs with Heartworm Disease. Can. J. Vet. Res. 2020, 84, 265–271. [Google Scholar]
- Martinez, C.; Mooney, C.T.; Shiel, R.E.; Tang, P.K.; Mooney, L.; O’Neill, E.J. Evaluation of Red Blood Cell Distribution Width in Dogs with Various Illnesses. Can. Vet. J. 2019, 60, 964–971. [Google Scholar]
- Garcia-Arce, M.; Gow, A.G.; Handel, I.; Ngoi, W.; Thomas, E. Retrospective Evaluation of Red Blood Cell Distribution Width as a Prognostic Factor in Critically Ill Dogs (December 2016 to April 2017): 127 Cas. J. Vet. Emerg. Crit. Care 2022, 32, 405–412. [Google Scholar] [CrossRef]
- Ludwik, T.M.; Heinrich, D.A.; Rendahl, A.; Friedenberg, S.G. Red Cell Distribution Width Is a Predictor of All-Cause Mortality in Hospitalized Dogs. J. Vet. Emerg. Crit. Care 2021, 32, 9–17. [Google Scholar] [CrossRef]
- Pfeifer, M.E.; Prittie, J.E.; Zollo, A.M.; Weltman, J.G. Red Cell Distribution Width, Illness Severity, and All-Cause Mortality in Dogs Admitted to the ICU. J. Vet. Emerg. Crit. Care 2022, 32, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Fish, E.J.; Hansen, S.C.; Spangler, E.A.; Gaillard, P.R.; Fan, S.; Bacek, L.M. Retrospective Evaluation of Serum/Plasma Iron, Red Blood Cell Distribution Width, and Nucleated Red Blood Cells in Dogs with Acute Trauma (2009-2015): 129 Cases. J. Vet. Emerg. Crit. Care 2019, 29, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Pavesi, F.; Bardi, M.; Pipitone, S. Lack of Harmonization of Red Blood Cell Distribution Width (RDW). Evaluation of Four Hematological Analyzers. Clin. Biochem. 2014, 47, 1100–1103. [Google Scholar] [CrossRef]
- Welles, E.G.; Hall, A.S.; Carpenter, D.M. Canine Complete Blood Counts: A Comparison of Four in-Office Instruments with the ADVIA 120 and Manual Differential Counts. Vet. Clin. Pathol. 2009, 38, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Moritz, A.; Fickenscher, Y.; Meyer, K.; Failing, K.; Weiss, D.J. Canine and Feline Hematology Reference Values for the ADVIA 120 Hematology System. Vet. Clin. Pathol. 2004, 33, 32–38. [Google Scholar] [CrossRef]
- Miglio, A.; Gavazza, A.; Siepi, D.; Bagaglia, F.; Misia, A.; Antognoni, M.T. Hematological and Biochemical Reference Intervals for 5 Adult Hunting Dog Breeds Using a Blood Donor Database. Animals 2020, 10, 1212. [Google Scholar] [CrossRef]
- Patel, K.V.; Semba, R.D.; Ferrucci, L.; Newman, A.B.; Fried, L.P.; Wallace, R.B.; Bandinelli, S.; Phillips, C.S.; Yu, B.; Connelly, S.; et al. Red Cell Distribution Width and Mortality in Older Adults: A Meta-Analysis. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 258–265. [Google Scholar] [CrossRef]
- Lippi, G.; Targher, G.; Montagnana, M.; Salvagno, G.L.; Zoppini, G.; Guidi, G.C. Relation between Red Blood Cell Distribution Width and Inflammatory Biomarkers in a Large Cohort of Unselected Outpatients. Arch. Pathol. Lab. Med. 2009, 133, 628–632. [Google Scholar] [CrossRef]
- Majercik, S.; Fox, J.; Knight, S.; Horne, B.D. Red Cell Distribution Width Is Predictive of Mortality in Trauma Patients. J. Trauma Acute Care Surg. 2013, 74, 1021–1026. [Google Scholar] [CrossRef]
- Lippi, G.; Bovo, C.; Buonocore, R.; Mitaritonno, M.; Cervellin, G. Red Blood Cell Distribution Width in Patients with Limb, Chest and Head Trauma. Arch. Med. Sci. 2017, 13, 606–611. [Google Scholar] [CrossRef]
- Shehata, H.A.; Ali, M.M.; Evans-Jones, J.C.; Upton, G.J.; Manyonda, I.T. Red Cell Distribution Width (RDW) Changes in Pregnancy. Int. J. Gynaecol. Obstet. 1998, 62, 43–46. [Google Scholar] [CrossRef]
- Rørtveit, R.; Saevik, B.K.; Eggertsdóttir, A.V.; Skancke, E.; Lingaas, F.; Thoresen, S.I.; Jansen, J.H. Age-Related Changes in Hematologic and Serum Biochemical Variables in Dogs Aged 16–60 Days. Vet. Clin. Pathol. 2015, 44, 47–57. [Google Scholar] [CrossRef]
- Allen, L.A.; Felker, G.M.; Mehra, M.R.; Chiong, J.R.; Dunlap, S.H.; Ghali, J.K.; Lenihan, D.J.; Oren, R.M.; Wagoner, L.E.; Schwartz, T.A.; et al. Validation and Potential Mechanisms of Red Cell Distribution Width as a Prognostic Marker in Heart Failure. J. Card. Fail. 2010, 16, 230–238. [Google Scholar] [CrossRef] [Green Version]
- Reinero, C.; Visser, L.C.; Kellihan, H.B.; Masseau, I.; Rozanski, E.; Clercx, C.; Williams, K.; Abbott, J.; Borgarelli, M.; Scansen, B.A. ACVIM Consensus Statement Guidelines for the Diagnosis, Classification, Treatment, and Monitoring of Pulmonary Hypertension in Dogs. J. Vet. Intern. Med. 2020, 34, 549–573. [Google Scholar] [CrossRef]
- Rhodes, C.J.; Wharton, J.; Howard, L.S.; Gibbs, J.S.R.; Wilkins, M.R. Red Cell Distribution Width Outperforms Other Potential Circulating Biomarkers in Predicting Survival in Idiopathic Pulmonary Arterial Hypertension. Heart 2011, 97, 1054–1060. [Google Scholar] [CrossRef]
- Wang, W.; Liu, J.; Yang, Y.; Zhai, Z.; Wang, C.; Wang, J. Red Cell Distribution Width Is Increased in Chronic Thromboembolic Pulmonary Hypertension. Clin. Respir. J. 2016, 10, 54–60. [Google Scholar] [CrossRef]
- Lemos, N.M.D.O.; Alberigi, B.; Labarthe, N.; Knacfuss, F.B.; Baldani, C.D.; da Silva, M.F.A. How Does Dirofilaria Immitis Infection Impact the Health of Dogs Referred to Cardiology Care. Braz. J. Vet. Med. 2022, 44, e002622. [Google Scholar] [CrossRef]
- Cetinkaya, E.; Senol, K.; Saylam, B.; Tez, M. Red Cell Distribution Width to Platelet Ratio: New and Promising Prognostic Marker in Acute Pancreatitis. World J. Gastroenterol. 2014, 20, 14450–14454. [Google Scholar] [CrossRef]
- Fabrès, V.; Dossin, O.; Reif, C.; Campos, M.; Freiche, V.; Maurey, C.; Pilot-Storck, F.; Desquilbet, L.; Benchekroun, G. Development and Validation of a Novel Clinical Scoring System for Short-Term Prediction of Death in Dogs with Acute Pancreatitis. J. Vet. Intern. Med. 2019, 33, 499–507. [Google Scholar] [CrossRef] [Green Version]
- Gori, E.; Pierini, A.; Lippi, I.; Meucci, V.; Perondi, F.; Marchetti, V. Evaluation of Asymmetric Dimethylarginine as an Inflammatory and Prognostic Marker in Dogs with Acute Pancreatitis. J. Vet. Intern. Med. 2020, 34, 1144–1149. [Google Scholar] [CrossRef]
- Jeon, T.J.; Park, J.Y. Clinical Significance of the Neutrophil-Lymphocyte Ratio as an Early Predictive Marker for Adverse Outcomes in Patients with Acute Pancreatitis. World J. Gastroenterol. 2017, 23, 3883–3889. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Y.; Han, J.; Shen, H.; Zhao, M.; Cai, S. Neutrophil-Lymphocyte Ratio, Gamma-Glutamyl Transpeptidase, Lipase, High-Density Lipoprotein as a Panel of Factors to Predict Acute Pancreatitis in Pregnancy. Medicine 2018, 97, e11189. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Tao, J.; Zhu, Z.; Wang, W. The Early Prognostic Value of Inflammatory Markers in Patients with Acute Pancreatitis. Clin. Res. Hepatol. Gastroenterol. 2019, 43, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Gülen, B.; Sonmez, E.; Yaylaci, S.; Serinken, M.; Eken, C.; Dur, A.; Turkdogan, F.T.; Söğüt, Ö. Effect of Harmless Acute Pancreatitis Score, Red Cell Distribution Width and Neutrophil/Lymphocyte Ratio on the Mortality of Patients with Nontraumatic Acute Pancreatitis at the Emergency Department. World J. Emerg. Med. 2015, 6, 29–33. [Google Scholar] [CrossRef] [Green Version]
- Ganji, A.; Esmaeilzadeh, A.; Ghanaei, O.; Saberi, A.; Taherzadeh, D.; Sazgarnia, S.; Mayabi Joghal, Z.; Zirak, M.; AbdolahRamazani, S.; Zarifmahmoudi, L. Predictive Value of Red Blood Cell Distribution Width for Mortality in Patients with Acute Pancreatitis: A Systematic Review and Meta-Analysis. Med. J. Islam. Repub. Iran 2017, 31, 124. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Fuentes, H.E.; Attar, B.M.; Jaiswal, P.; Demetria, M. Evaluation of the Prognostic Value of Neutrophil to Lymphocyte Ratio in Patients with Hypertriglyceridemia-Induced Acute Pancreatitis. Pancreatology 2017, 17, 893–897. [Google Scholar] [CrossRef]
- Kong, T.; Park, J.E.; Park, Y.S.; Lee, H.S.; You, J.S.; Chung, H.S.; Park, I.; Chung, S.P. Usefulness of Serial Measurement of the Red Blood Cell Distribution Width to Predict 28-Day Mortality in Patients with Trauma. Am. J. Emerg. Med. 2017, 35, 1819–1827. [Google Scholar] [CrossRef]
- Loveday, S.; Sinclair, L.; Badrick, T. Does the Addition of RDW Improve Current ICU Scoring Systems? Clin. Biochem. 2015, 48, 569–574. [Google Scholar] [CrossRef]
- Bazick, H.S.; Chang, D.; Mahadevappa, K.; Gibbons, F.K.; Christopher, K.B. Red Cell Distribution Width and All-Cause Mortality in Critically Ill Patients. Crit. Care Med. 2011, 39, 1913–1921. [Google Scholar] [CrossRef] [Green Version]
- Förhécz, Z.; Gombos, T.; Borgulya, G.; Pozsonyi, Z.; Prohászka, Z.; Jánoskuti, L. Red Cell Distribution Width in Heart Failure: Prediction of Clinical Events and Relationship with Markers of Ineffective Erythropoiesis, Inflammation, Renal Function, and Nutritional State. Am. Heart J. 2009, 158, 659–666. [Google Scholar] [CrossRef]
- Zorlu, A.; Bektasoglu, G.; Guven, F.M.K.; Dogan, O.T.; Gucuk, E.; Ege, M.R.; Altay, H.; Cinar, Z.; Tandogan, I.; Yilmaz, M.B. Usefulness of Admission Red Cell Distribution Width as a Predictor of Early Mortality in Patients with Acute Pulmonary Embolism. Am. J. Cardiol. 2012, 109, 128–134. [Google Scholar] [CrossRef]
- Von Haehling, S.; Anker, M.S.; Jankowska, E.A.; Ponikowski, P.; Anker, S.D. Anemia in Chronic Heart Failure: Can We Treat? What to Treat? Heart Fail. Rev. 2012, 17, 203–210. [Google Scholar] [CrossRef]
- Kuzi, S.; Mazaki-Tovi, M.; Suchodolski, J.S.; Rimer, D.; Lidbury, J.A.; Steiner, J.M.; Buono, A.; Nivy, R.; Segev, G.; Aroch, I. Protease Inhibitors, Inflammatory Markers, and Their Association with Outcome in Dogs with Naturally Occurring Acute Pancreatitis. J. Vet. Intern. Med. 2020, 34, 1801–1812. [Google Scholar] [CrossRef]
- Mansfield, C.S.; James, F.E.; Robertson, I.D. Development of a Clinical Severity Index for Dogs with Acute Pancreatitis. J. Am. Vet. Med. Assoc. 2008, 233, 936–944. [Google Scholar] [CrossRef]
- Neumann, S. Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte Ratios in Dogs and Cats with Acute Pancreatitis. Vet. Clin. Pathol. 2021, 50, 45–51. [Google Scholar] [CrossRef]
- Martinelli, E.; Locatelli, C.; Bassis, S.; Crosara, S.; Paltrinieri, S.; Scarpa, P.; Spalla, I.; Zanaboni, A.; Quintavalla, C.; Brambilla, P. Preliminary Investigation of Cardiovascular–Renal Disorders in Dogs with Chronic Mitral Valve Disease. J. Vet. Intern. Med. 2016, 30, 1612–1618. [Google Scholar] [CrossRef]
- Solak, Y.; Yilmaz, M.I.; Saglam, M.; Caglar, K.; Verim, S.; Unal, H.U.; Gok, M.; Demirkaya, E.; Gaipov, A.; Kayrak, M.; et al. Red Cell Distribution Width Is Independently Related to Endothelial Dysfunction in Patients with Chronic Kidney Disease. Am. J. Med. Sci. 2014, 347, 118–124. [Google Scholar] [CrossRef]
- Lippi, G.; Targher, G.; Montagnana, M.; Salvagno, G.L.; Zoppini, G.; Guidi, G.C. Relationship between Red Blood Cell Distribution Width and Kidney Function Tests in a Large Cohort of Unselected Outpatients. Scand. J. Clin. Lab. Investig. 2008, 68, 745–748. [Google Scholar] [CrossRef]
- Gori, E.; Pierini, A.; Lippi, I.; Ceccherini, G.; Perondi, F.; Marchetti, V. Evaluation of C-Reactive Protein/Albumin Ratio and Its Relationship with Survival in Dogs with Acute Pancreatitis. N. Z. Vet. J. 2020, 68, 345–348. [Google Scholar] [CrossRef]
- Man, M.-A.; Davidescu, L.; Motoc, N.-S.; Rajnoveanu, R.-M.; Bondor, C.-I.; Pop, C.-M.; Toma, C. Diagnostic Value of the Neutrophil-to-Lymphocyte Ratio (NLR) and Platelet-to-Lymphocyte Ratio (PLR) in Various Respiratory Diseases: A Retrospective Analysis. Diagnostics 2021, 12, 81. [Google Scholar] [CrossRef]
- Nivy, R.; Kuzi, S.; Yochai, A.; Aroch, I.; Bruchim, Y. Evaluation of Serum Histone Concentrations and Their Associations with Hemostasis, Markers of Inflammation, and Outcome in Dogs with Naturally Occurring Acute Pancreatitis. Am. J. Vet. Res. 2021, 82, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Pierini, A.; Gori, E.; Lippi, I.; Ceccherini, G.; Lubas, G.; Marchetti, V. Neutrophil-to-Lymphocyte Ratio, Nucleated Red Blood Cells and Erythrocyte Abnormalities in Canine Systemic Inflammatory Response Syndrome. Res. Vet. Sci. 2019, 126, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Montagnana, M.; Cervellin, G.; Meschi, T.; Lippi, G. The Role of Red Blood Cell Distribution Width in Cardiovascular and Thrombotic Disorders. Clin. Chem. Lab. Med. 2011, 50, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Morceau, F.; Dicato, M.; Diederich, M. Pro-Inflammatory Cytokine-Mediated Anemia: Regarding Molecular Mechanisms of Erythropoiesis. Mediat. Inflamm. 2009, 2009, 405016. [Google Scholar] [CrossRef] [Green Version]
- Vayá, A.; Alis, R.; Suescún, M.; Rivera, L.; Murado, J.; Romagnoli, M.; Solá, E.; Hernandez-Mijares, A. Association of Erythrocyte Deformability with Red Blood Cell Distribution Width in Metabolic Diseases and Thalassemia Trait. Clin. Hemorheol. Microcirc. 2015, 61, 407–415. [Google Scholar] [CrossRef]
- Caimi, G.; Carlisi, M. The Unpredictable Erythrocyte Deformability Alteration in Some Hematological Disorders: How the Classification of Primary Hyperviscosity Syndromes Could Change. Clin. Hemorheol. Microcirc. 2023. [Google Scholar] [CrossRef]
- Varga, A.; Matrai, A.A.; Barath, B.; Deak, A.; Horvath, L.; Nemeth, N. Interspecies Diversity of Osmotic Gradient Deformability of Red Blood Cells in Human and Seven Vertebrate Animal Species. Cells 2022, 11, 1351. [Google Scholar] [CrossRef]
- Rubio, A.; López, M.; Rodrigues, T.; Campo-Deaño, L.; Vega, E.J. A Particulate Blood Analogue Based on Artificial Viscoelastic Blood Plasma and RBC-like Microparticles at a Concentration Matching the Human Haematocrit. Soft Matter 2022, 18, 7510–7523. [Google Scholar] [CrossRef]
- Loyer, X.; Vion, A.-C.; Tedgui, A.; Boulanger, C.M. Microvesicles as Cell-Cell Messengers in Cardiovascular Diseases. Circ. Res. 2014, 114, 345–353. [Google Scholar] [CrossRef] [Green Version]
- Agouni, A.; Andriantsitohaina, R.; Martinez, M.C. Microparticles as Biomarkers of Vascular Dysfunction in Metabolic Syndrome and Its Individual Components. Curr. Vasc. Pharmacol. 2014, 12, 483–492. [Google Scholar] [CrossRef] [Green Version]
- Herring, J.M.; Smith, S.A.; McMichael, M.A.; O’Brien, M.; Ngwenyama, T.R.; Corsi, R.; Galligan, A.; Beloshapka, A.N.; Deng, P.; Swanson, K.S. Microparticles in Stored Canine RBC Concentrates. Vet. Clin. Pathol. 2013, 42, 163–169. [Google Scholar] [CrossRef]
- McEntire, M.C.; Wardrop, K.J.; Davis, W.C. Comparison of Established and Novel Methods for the Detection and Enumeration of Microparticles in Canine Stored Erythrocyte Concentrates for Transfusion. Vet. Clin. Pathol. 2017, 46, 54–63. [Google Scholar] [CrossRef]


| Author, Year | Study Design | Study Population | Major Findings | References |
|---|---|---|---|---|
| Neiger et al., 2002 | Retrospective cohort | 74 dogs without hematological abnormalities, 51 dogs with regenerative anemia, and 92 dogs with non-regenerative anemia | For each 1% RDW increase, there could be an increase in the odds by a factor of 1.3 that an anemic dog can be affected by regenerative anemia | [1] |
| Guglielmini et al., 2013 | Retrospective case-control study | 27 healthy dogs and 135 dogs with mitral valve disease (MVD) with or without heart failure (HF) | Mean RDW in dogs with MVD was not significantly different from that of healthy dogs. The RDW of dogs with MVD without HF was not significantly different from that of dogs with MVD and HF | [10] |
| Swann et al., 2014 | Retrospective case-control study | 79 healthy dogs and 44 dogs with pre-capillary pulmonary hypertension (PH) | Median RDW was significantly increased in dogs with pre-capillary PH compared to that of dogs of the control group | [12] |
| Mazzotta et al., 2016 | Prospective study | 19 healthy dogs, 82 dogs with MVD with or without PH, and 26 dogs with pre-capillary PH | Median RDW in dogs with pre-capillary PH and post-capillary PH was significantly higher compared to that of control dogs. Positive association | [11] |
| Fish et al., 2019 | Retrospective observational study | 129 dogs with acute traumatic injury | RDW was not associated with survival in dogs with traumatic injury | [22] |
| Martinez et al., 2019 | Retrospective study | 79 healthy dogs and 276 dogs with various disorders including endocrine, neurological, respiratory, hematological, cardiovascular, hepatic, and pancreatic disease | RDW of dogs with immune-mediated hemolytic anemia, immune-mediated thrombocytopenia, hyperadrenocorticism, hypothyroidism, hepatic vascular anomaly, pneumonia, chronic kidney disease, multi-centric lymphoma, and MVD was significantly higher compared to that of healthy dogs | [18] |
| Kim et al., 2019 | Prospective study | 20 healthy dogs and 86 dogs with heartworm disease (HWD) at different stage of disease severity | Higher RDW values were found in dogs with severe HWD compared to that of the control group. RDW was significantly correlated with presence of PH and anemia | [17] |
| Ludwik et al., 2021 | Retrospectivesingle-center study | 5183 dogs admitted to ICU | Higher RDW values on presentation to ICU were significantly associated with greater odds of all-cause in-hospital mortality compared to dogs with lower RDW values (OR = 2.1, 95% CI, 1.7–2.6) | [20] |
| Garcia-Arce et al., 2022 | Retrospective study | 127 critically ill dogs admitted to ICU | RDW was not associated with in-hospital mortality or length of hospitalization | [19] |
| Pfeifer et al., 2022 | Prospective observational study | 111 dogs admitted to intensive care unit (ICU) with different illness severity | RDW was not associated with illness severity nor did it predict in-hospital or 30-day mortality | [21] |
| Guglielmini et al., 2021 | Retrospective cohort study | 146 dogs with MVD at different stages of disease severity | RDW was independently associated with all-cause mortality at six months (HR: 1.203; 95% CI: 1.045–1.384, p = 0.010) | [13] |
| Guglielmini et al., 2022 | Retrospective cohort study | 70 dogs with acute pancreatitis | Increased RDW was an independent predictor of dead within 14 days (HR: 5.08; 95% CI: 1.14–22.67, p = 0.03) and showed good accuracy to predict negative outcome (AUC: 0.74; 95% CI: 0.63–0.84) | [16] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miglio, A.; Valente, C.; Guglielmini, C. Red Blood Cell Distribution Width as a Novel Parameter in Canine Disorders: Literature Review and Future Prospective. Animals 2023, 13, 985. https://doi.org/10.3390/ani13060985
Miglio A, Valente C, Guglielmini C. Red Blood Cell Distribution Width as a Novel Parameter in Canine Disorders: Literature Review and Future Prospective. Animals. 2023; 13(6):985. https://doi.org/10.3390/ani13060985
Chicago/Turabian StyleMiglio, Arianna, Carlotta Valente, and Carlo Guglielmini. 2023. "Red Blood Cell Distribution Width as a Novel Parameter in Canine Disorders: Literature Review and Future Prospective" Animals 13, no. 6: 985. https://doi.org/10.3390/ani13060985






