Prevalence of Chronic Progressive Lymphedema in the Rhenish German Draught Horse
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Sample Collection
2.3. Data Collection
2.4. Examination of the Horses
2.5. Statistical Analysis
b2(age2 * sex)m + eijklmn
3. Results
3.1. Overall Prevalence by Age and Sex
3.2. Signalment Data of Enrolled Horses
3.3. Generalized Linear Multivariable Model for CPL-Scores
3.4. Testing Stud-Related Factors for CPL-Scores Using Generalized Linear Multivariable Models
3.5. Testing Animal-Related Variables for CPL-Scores Using Generalized Linear Multivariable Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wallraf, A.; Hamann, H.; Deegen, E.; Ohnesorge, B.; Distl, O. Analysis of the prevalence of pastern dermatitis in German Coldblood horse breeds. Berl. Munch. Tierarztl. Wochenschr. 2004, 117, 148–152. [Google Scholar] [PubMed]
- De Keyser, K.; Janssens, S.; Peeters, L.; Gasthuys, F.; Oosterlinck, M.; Buys, N. Chronic progressive lymphedema in the Belgian draft horse in Belgium: Clinical phenotyping, prevalence and risk factor analysis. Vlaams Diergeneeskd. Tijdschr. 2014, 83, 119–124. [Google Scholar] [CrossRef]
- Affolter, V.K. Chronic progressive lymphedema in draft horses. Vet. Clin. N. Am. Equine Pract. 2013, 29, 589–605. [Google Scholar] [CrossRef] [PubMed]
- Affolter, V.K.; Dalley, B.; Kass, P.H.; Brown, E.A.; Sonder, C.; Bannasch, D.L. Chronic progressive lymphoedema in Friesian horses: Suggestive phenotype of affected horses and genome-wide association study. Vet. Dermatol. 2020, 31, 234-e51. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, G.L. Pastern dermatitis in Shires and Clydesdales. J. Equine Vet. Sci. 2001, 11, 524–527. [Google Scholar] [CrossRef]
- Gustine, G. Die Sogenannte Warzenmauke des Pferdes “Dermatitis Chronica Verrucosa”. Ph.D. Thesis, Vereinigte Medizinische Fakultät der Großherzöglichen Hessischen Ludwigs-Universität zu Gießen, Gießen, Germany, 1910. [Google Scholar]
- Schäper, W. Konstitutionelle Hauterkrankungen beim Pferd. Z. Tierzüchtg. Züchtgsbiol. 1937, 37, 295–330. [Google Scholar]
- Wussow, W.; Hartwig, W. Die Zuchtverwendungsdauer der Beschäler des Landgestüt Kreuz und ihre Abgangsursachen. Tierzucht 1954, 10, 336–342. [Google Scholar]
- Duclos, P. Les Eaux-aux-Jambes du Cheval, Dermatose Hyperplasique des Membres. Ph.D. Thesis, Ecole Nationale l’Universite Claude Bernand de Lyon, Lyon, France, 1972. [Google Scholar]
- Wallraf, A.; Hamann, H.; Ohnesorge, B.; Deegen, E.; Distl, O. Populationsgenetische Untersuchung zum Auftreten von Mauke beim Süddeutschen Kaltblut. Züchtungskunde 2004, 76, 246–261. [Google Scholar]
- De Cock, H.E.; Affolter, V.K.; Wisner, E.R.; Ferraro, G.L.; MacLachlan, N.J. Progressive swelling, hyperkeratosis, and fibrosis of distal limbs in Clydesdales, Shires, and Belgian draft horses, suggestive of primary lymphedema. Lymphat. Res. Biol. 2003, 1, 191–199. [Google Scholar] [CrossRef]
- Geburek, F.; Ohnesorge, B.; Deegen, E.; Doeleke, R.; Hewicker-Trautwein, M. Alterations of epidermal proliferation and cytokeratin expression in skin biopsies from heavy draught horses with chronic pastern dermatitis. Vet. Dermatol. 2005, 16, 373–384. [Google Scholar] [CrossRef]
- De Keyser, K.; Janssens, S.; Buys, N. Chronic progressive lymphoedema in draught horses. Equine Vet. J. 2015, 47, 260–266. [Google Scholar] [CrossRef] [PubMed]
- De Cock, H.E.; Affolter, V.K.; Wisner, E.R.; Larson, R.F.; Ferraro, G.L. Lymphoscintigraphy of draught horses with chronic progressive lymphoedema. Equine Vet. J. 2006, 38, 148–151. [Google Scholar] [CrossRef] [PubMed]
- De Cock, H.E.; Affolter, V.K.; Farver, T.B.; Van Brantegem, L.; Scheuch, B.; Ferraro, G.L. Measurement of skin desmosine as an indicator of altered cutaneous elastin in draft horses with chronic progressive lymphedema. Lymphat. Res. Biol. 2006, 4, 67–72. [Google Scholar] [CrossRef] [PubMed]
- De Cock, H.E.; Van Brantegem, L.; Affolter, V.K.; Oosterlinck, M.; Ferraro, G.L.; Ducatelle, R. Quantitative and qualitative evaluation of dermal elastin of draught horses with chronic progressive lymphoedema. J. Comp. Pathol. 2009, 140, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Van Brantegem, L.; De Cock, H.; Affolter, V.K.; Duchateau, L.; Hoogewijs, M.; Govaere, J.; Ferraro, G.; Ducatelle, R. Antibodies to elastin peptides in sera of Belgian Draught horses with chronic progressive lymphoedema. Equine Vet. J. 2007, 39, 418–421. [Google Scholar] [CrossRef] [PubMed]
- De Keyser, K.; Berth, M.; Christensen, N.; Willaert, S.; Janssens, S.; Ducatelle, R.; Goddeeris, B.M.; De Cock, H.; Buys, N. Assessment of plasma anti-elastin antibodies for use as a diagnostic aid for chronic progressive lymphoedema in Belgian Draught Horses. Vet. Immunol. Immunopathol. 2015, 163, 16–22. [Google Scholar] [CrossRef]
- Powell, H.; Affolter, V.K. Combined decongestive therapy including equine manual lymph drainage to assist management of chronic progressive lymphoedema in draught horses. Equine Vet. Educ. 2012, 24, 81–89. [Google Scholar] [CrossRef]
- Rüfenacht, S.; Roosje, P.J.; Sager, H.; Doherr, M.G.; Straub, R.; Goldinger-Müller, P.; Gerber, V. Combined moxidectin and environmental therapy do not eliminate Chorioptes bovis infestation in heavily feathered horses. Vet. Dermatol. 2011, 22, 17–23. [Google Scholar] [CrossRef]
- Fedele, C.; von Rautenfeld, D.B. Manual lymph drainage for equine lymphoedema-treatment strategy and therapist training. Equine Vet. Educ. 2007, 19, 26–31. [Google Scholar] [CrossRef]
- Poore, L.A.; Else, R.W.; Licka, T.L. The clinical presentation and surgical treatment of verrucous dermatitis lesions in a draught horse. Vet. Dermatol. 2012, 23, 71–75, e17. [Google Scholar] [CrossRef]
- De Keyser, K.; Janssens, S.; Peeters, L.; Foqué, N.; Gasthuys, F.; Oosterlinck, M.; Buys, N. Genetic parameters for chronic progressive lymphedema in Belgian Draught Horses. J. Anim. Breed. Genet. 2014, 131, 522–528. [Google Scholar] [CrossRef] [PubMed]
- De Keyser, K.; Peeters, L.; Buys, N.; Janssens, S. Assessment of skinfold thickness as a factor related to chronic progressive lymphoedema in Belgian draught horses. Commun. Agric. Appl. Biol. Sci. 2010, 76, 189–192. [Google Scholar]
- Wallraf, A. Populationsgenetische Untersuchung zum Auftreten von Mauke bei den Deutschen Kaltblutpferderassen. Ph.D. Thesis, Tierärztliche Hochschule Hannover, Hannover, Germany, 2003. [Google Scholar]
- Kumpf, J. Beitrag zur Behandlung von Warzenmauke. Ph.D. Thesis, Humboldt Universität zu Berlin, Berlin, Germany, 1953. [Google Scholar]
- Straiton, E.C.; Ross-Rahte, R. Pferdekrankheiten Erkennen und Behandeln: Praxisbuch mit über 300 Fotos, 11th ed.; BLV-Verlag: Munich, Germany, 1988; pp. 164–167. [Google Scholar]
- Mittmann, E.H.; Mömke, S.; Distl, O. Whole-genome scan identifies quantitative trait loci for chronic pastern dermatitis in German draft horses. Mamm. Genome 2010, 21, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Aberle, K.S.; Hamann, H.; Drögemüller, C.; Distl, O. Genetic diversity in German draught horse breeds compared with a group of primitive, riding and wild horses by means of microsatellite DNA markers. Anim. Genet. 2004, 35, 270–277. [Google Scholar] [CrossRef]
- Weischer, F. Zur Klärung der Erb-und Umweltbeziehungen der Warzenmauke des Pferdes. Tierärztl. Umschau 1949, 4, 318–320. [Google Scholar]
- Wussow, W.; Hartwig, W. Erbbiologische Untersuchungen über die Mauke beim Kaltblutpferd. Tierzucht 1955, 9, 195–198. [Google Scholar]
- Kerchner, K.; Fleischer, A.; Yosipovitch, G. Lower extremity lymphedema: Update: Pathophysiology, diagnosis, and treatment guidelines. J. Am. Acad. Dermatol. 2008, 59, 324–331. [Google Scholar] [CrossRef]
| Region (Breeding Association) | Studs | Horses | Proportion by Sex (Male/Gelding/Female) | ||
|---|---|---|---|---|---|
| Westphalia (Westphalian) | 75 | 268 | 0.21 | 0.15 | 0.64 |
| Thuringia (Saxon-Thuringian) | 3 | 106 | 0.17 | 0.03 | 0.80 |
| Brandenburg (Brandenburg-Anhalt) | 2 | 67 | 0.40 | 0.08 | 0.52 |
| Rhineland (Rhenish) | 14 | 43 | 0.12 | 0.19 | 0.70 |
| Lower Saxony (Lower Saxon) | 2 | 9 | 0.67 | 0.00 | 0.33 |
| Total | 96 | 493 | 0.23 | 0.12 | 0.66 |
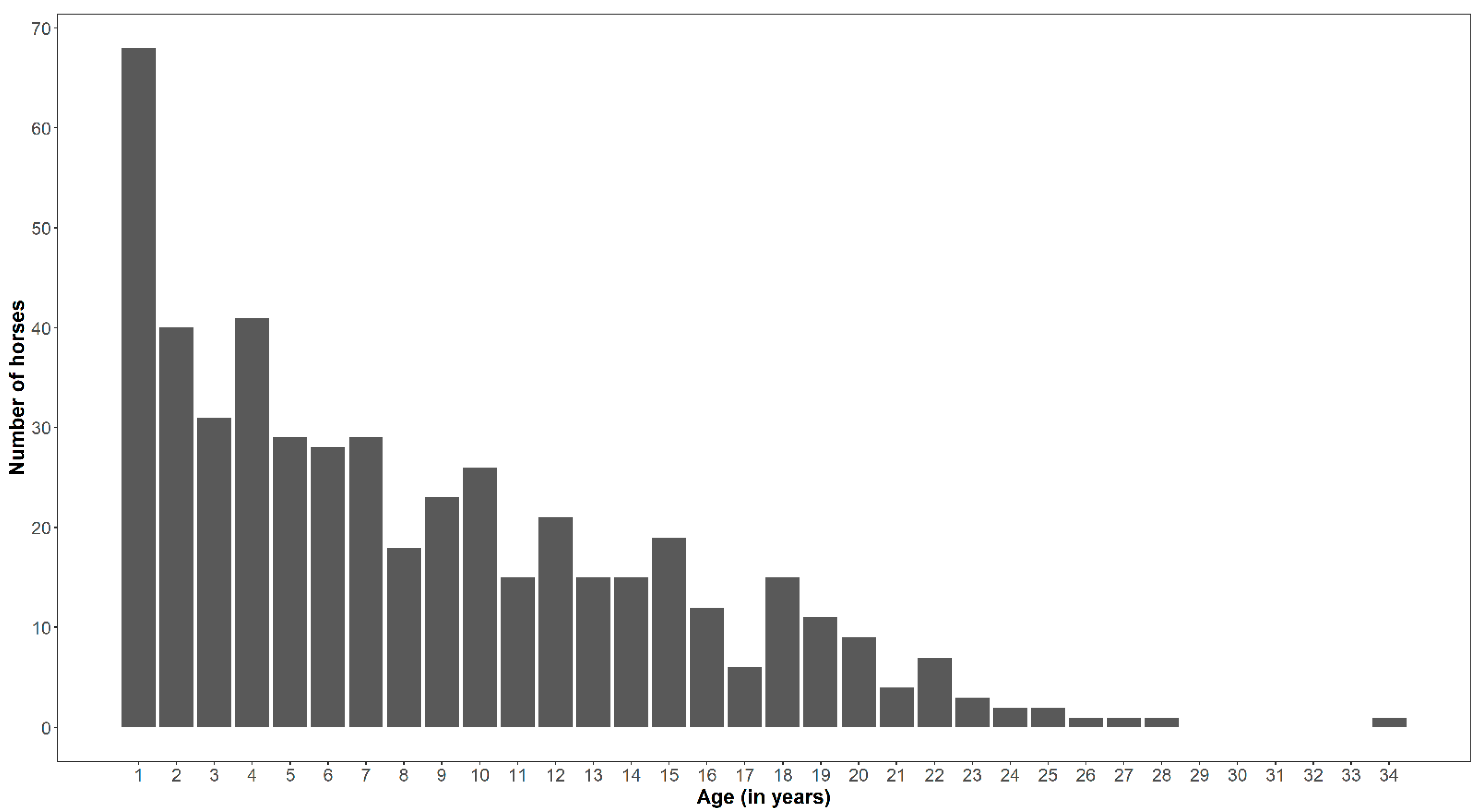
| Age Group (Years) | No of Horses | CPL-Score | Overall CPL- Score (Mean ± SD) | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | |||
| <1 | 68 | 40.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 1–3 | 71 | 27.0 | 29.0 | 12.9 | 2.5 | 0.0 | 0.0 | 0.648 ± 0.927 |
| 3–6 | 98 | 20.6 | 19.4 | 34.7 | 18.3 | 0.0 | 0.0 | 1.337 ± 1.130 |
| 6–9 | 70 | 5.9 | 22.6 | 20.8 | 17.5 | 16.9 | 0.0 | 2.114 ± 1.325 |
| 9–12 | 62 | 2.4 | 19.4 | 12.9 | 20.8 | 18.5 | 33.3 | 2.516 ± 1.127 |
| 12–18 | 82 | 3.5 | 6.5 | 13.9 | 23.3 | 44.6 | 50.0 | 2.805 ± 1.222 |
| >17 | 42 | 0.6 | 3.2 | 5.0 | 17.5 | 20.0 | 16.7 | 2.976 ± 0.999 |
| Total (n) | 493 | 170 | 31 | 101 | 120 | 65 | 6 | 1.696 ± 1.464 |
| Percentage | 100 | 34.5 | 6.3 | 20.5 | 24.3 | 13.2 | 1.2 | |
| CI | 30.3–38.7 | 4.2–8.8 | 16.9–24.1 | 20.1–28.1 | 10.2–16.2 | 0.1–2.2 | ||
| CPL-Score | No of Males | Percentage (CI) | No of Females | Percentage (CI) | No of Geldings | Percentage (CI) |
|---|---|---|---|---|---|---|
| 0 | 48 | 43.2 (34.1–52.5) | 117 | 36.0 (30.7–41.2) | 5 | 8.8 (3.0–19.6) |
| 1 | 5 | 4.5 (1.5–10.2) | 23 | 7.1 (4.5–10.4) | 3 | 5.3 (1.1–14.6) |
| 2 | 19 | 17.1 (10.6–25.4) | 69 | 21.2 (16.9–26.1) | 13 | 21.8 (12.7–35.8) |
| 3 | 19 | 17.1 (10.6–25.4) | 76 | 23.4 (18.9–28.4) | 25 | 43.9 (30.7–57.6) |
| 4 | 16 | 14.4 (8.5–22.4) | 39 | 12.0 (8.7–16.0) | 10 | 17.5 (8.8–29.9) |
| 5 | 4 | 3.6 (1.0–9.0) | 1 | 0.3 (0–1.7) | 1 | 1.8 (0–9.4) |
| Total | 111 | 100 | 325 | 100 | 57 | 100 |
| CPL-score (average ± SD) | 1.577 ± 1.593 | 1.603 ± 1.429 | 2.456 ± 1.166 | |||
| Source of Variation | DF | Normal Distribution | Multinomial Distribution | ||
|---|---|---|---|---|---|
| F-Value | p-Value | F-Value | p-Value | ||
| Breeding association | 3 | 21.31 | <0.0001 | 21.31 | <0.0001 |
| Sex | 2 | 5.84 | 0.0031 | 6.48 | 0.0017 |
| Coat colour | 2 | 3.06 | 0.0479 | 4.89 | 0.0079 |
| Age (linear) by sex | 3 | 92.87 | <0.0001 | 56.99 | <0.0001 |
| Age (quadratic) by sex | 3 | 33.83 | <0.0001 | 29.79 | <0.0001 |
| Age | Regression Coefficients with Their Standard Errors | p-Value | ||
|---|---|---|---|---|
| Male | Gelding | Female | ||
| All horses (n = 493) | ||||
| - linear | 0.5795 ± 0.0523 | 0.1416 ± 0.0785 | 0.3146 ± 0.0254 | <0.0001 |
| - quadratic | −0.0187 ± 0.0028 | −0.0023 ± 0.0030 | −0.0083 ± 0.0011 | <0.0001 |
| Horses ≥ 1 year (n = 425) | ||||
| - linear | 0.5418 ± 0.0705 | 0.1360 ± 0.0819 | 0.3242 ± 0.0306 | <0.0001 |
| - quadratic | −0.0171 ± 0.0034 | −0.0020 ± 0.0031 | −0.0086 ± 0.0013 | <0.0001 |
| Horses ≥ 2 years (n = 385) | ||||
| - linear | 0.5255 ± 0.0917 | 0.1566 ± 0.0879 | 0.3029 ± 0.0364 | <0.0001 |
| - quadratic | −0.0164 ± 0.0042 | −0.0027 ± 0.0033 | −0.0079 ± 0.0014 | <0.0001 |
| Horses ≥ 3 years (n = 354) | ||||
| - linear | 0.4627 ± 0.1122 | 0.1633 ± 0.0882 | 0.3229 ± 0.0419 | <0.0001 |
| - quadratic | −0.0140 ± 0.0048 | −0.0030 ± 0.0033 | −0.0086 ± 0.0016 | <0.0001 |
| Horses ≥ 4 years (n = 313) | ||||
| - linear | 0.4539 ± 0.1417 | 0.1126 ± 0.1069 | 0.2795 ± 0.0502 | <0.0001 |
| - quadratic | −0.0136 ± 0.0057 | −0.0014 ± 0.0038 | −0.0073 ± 0.0018 | <0.0001 |
| Horses ≥ 5 years (n = 284) | ||||
| - linear | 0.4600 ± 0.1998 | 0.1179 ± 0.1265 | 0.2916 ± 0.0577 | <0.0001 |
| - quadratic | −0.0137 ± 0.0078 | −0.0016 ± 0.0043 | −0.0076 ± 0.0020 | 0.0006 |
| Horses ≥ 6 years (n = 256) | ||||
| - linear | 0.3829 ± 0.2454 | 0.2088 ± 0.1592 | 0.2793 ± 0.0664 | 0.0001 |
| - quadratic | −0.01134 ± 0.0089 | −0.0041 ± 0.0051 | −0.0073 ± 0.0022 | 0.0054 |
| Horses ≥ 7 years (n = 227) | ||||
| - linear | 0.4051 ± 0.2946 | 0.2061 ± 0.1707 | 0.2540 ± 0.0829 | 0.0063 |
| - quadratic | −0.0122 ± 0.0104 | −0.0042 ± 0.0053 | −0.0066 ± 0.0026 | 0.0394 |
| Horses ≥ 8 years (n = 209) | ||||
| - linear | 0.5193 ± 0.4228 | 0.2649 ± 0.1878 | 0.2189 ± 0.0914 | 0.0295 |
| - quadratic | −0.0160 ± 0.0141 | −0.0056 ± 0.0057 | −0.0056 ± 0.0028 | 0.1040 |
| Effect/ Level | Level | LSM ± SE | p-Values | OR (95%-CI) | p-Values |
|---|---|---|---|---|---|
| LSM | C-Log | ||||
| HBA | |||||
| Brandenburg-Anhalt | 1.51 ± 0.12 | 0.01/0.61/0.001 | 8.16 (4.25–15.67) | 0.001/0.43/0.001 | |
| Rhenish | 2.06 ± 0.16 | -/0.01/0.06 | 1.87 (0.97–3.61) | -/0.003/0.06 | |
| Saxon-Thuringian | 1.59 ± 0.13 | -/-/0.001 | 6.05 (3.47–10.57) | -/-/0.001 | |
| Westphalian | 2.36 ± 0.08 | - | - | - | |
| Sex | |||||
| Female | 1.45 ± 0.07 | 0.001/0.001 | 5.08 (2.99–8.63) | 0.006/0.001 | |
| Gelding | 1.97 ± 0.16 | -/0.188 | 1.92 (0.94–3.95) | -/0.07 | |
| Male | 1.99 ± 0.11 | - | - | - | |
| Coat colour | |||||
| Chestnut | 1.84 ± 0.10 | 0.6303/0.0376 | 1.77 (1.16–2.71) | 0.57/0.008 | |
| Black | 1.76 ± 0.16 | -/0.0828 | 2.19 (1.08–4.45) | -/0.03 | |
| Bay | 2.04 ± 0.08 | - | - | - |
| Source of Variation with Horse Farm-Related Factors | DF | Normal Distribution | Multinomial Distribution | ||
|---|---|---|---|---|---|
| F-Value | p-Value | F-Value | p-Value | ||
| Breeding association | 3 | 12.44 | <0.0001 | 10.16 | <0.0001 |
| Sex | 2 | 7.90 | 0.0004 | 7.28 | 0.0008 |
| Coat colour | 2 | 4.81 | 0.0090 | 2.96 | 0.0530 |
| Age (linear) by sex | 3 | 49.49 | <0.0001 | 74.11 | <0.0001 |
| Age (quadratic) by sex | 3 | 24.54 | <0.0001 | 26.82 | <0.0001 |
| Outdoor facilities for horses in summer (OUTS) | 2 | 4.06 | 0.0073 | 4.22 | 0.0059 |
| Bedding type (BED) | 7 | 3.37 | 0.0016 | 2.37 | 0.0221 |
| Time interval for cleaning out the stable (CLEAN) | 3 | 8.22 | <0.0001 | 6.60 | 0.0002 |
| Type of roughage fed in winter months (ROUW) | 4 | 2.54 | 0.0394 | 3.28 | 0.0115 |
| Type of concentrate fed in winter months (CONW) | 1 | 9.65 | 0.0020 | 7.53 | 0.0063 |
| Length of hoof trimming intervals (HOFT) | 4 | 4.16 | 0.0026 | 5.33 | 0.0003 |
| Age | Regression Coefficients with Their Standard Errors and p-Values | |||
|---|---|---|---|---|
| Front Limb | p-Value | Hind Limb | p-Value | |
| Horses ≥ 1 year (n = 396) | ||||
| - linear | 2.8298 ± 0.8884 | 0.0016 | 0.9506 ± 0.2368 | <0.0001 |
| - quadratic | −0.07092 ± 0.02838 | 0.0129 | −0.01157 ± 0.003839 | 0.0028 |
| - cubic | 0.000574 ± 0.000293 | 0.0506 | ||
| Horses ≥ 3 years (n = 336) | ||||
| - linear | 6.8489 ± 1.1279 | <0.0001 | 1.6400 ± 0.2869 | <0.0001 |
| - quadratic | −0.1946 ± 0.03596 | <0.0001 | −0.02225 ± 0.004575 | <0.0001 |
| - cubic | 0.001807 ± 0.000372 | <0.0001 | ||
| Horses ≥ 6 years (n = 243) | ||||
| - linear | 8.0452 ± 1.2289 | <0.0001 | 1.9710 ± 0.3001 | <0.0001 |
| - quadratic | −0.2304 ± 0.03888 | <0.0001 | −0.02706 ± 0.004732 | <0.0001 |
| - cubic | 0.002155 ± 0.000400 | <0.0001 | ||
| Horses ≥ 9 years (n = 176) | ||||
| - linear | 7.8459 ± 1.3991 | <0.0001 | 12.4234 ± 3.9350 | 0.0019 |
| - quadratic | −0.2248 ± 0.04413 | <0.0001 | −0.3488 ± 0.1220 | 0.0048 |
| - cubic | 0.002109 ± 0.000452 | <0.0001 | 0.003279 ± 0.001251 | 0.0096 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sievers, J.; Distl, O. Prevalence of Chronic Progressive Lymphedema in the Rhenish German Draught Horse. Animals 2023, 13, 999. https://doi.org/10.3390/ani13060999
Sievers J, Distl O. Prevalence of Chronic Progressive Lymphedema in the Rhenish German Draught Horse. Animals. 2023; 13(6):999. https://doi.org/10.3390/ani13060999
Chicago/Turabian StyleSievers, Johanna, and Ottmar Distl. 2023. "Prevalence of Chronic Progressive Lymphedema in the Rhenish German Draught Horse" Animals 13, no. 6: 999. https://doi.org/10.3390/ani13060999
APA StyleSievers, J., & Distl, O. (2023). Prevalence of Chronic Progressive Lymphedema in the Rhenish German Draught Horse. Animals, 13(6), 999. https://doi.org/10.3390/ani13060999





