Impact of Red Sea Bream Iridovirus Infection on Rock Bream (Oplegnathus fasciatus) and Other Fish Species: A Study of Horizontal Transmission
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Fish
2.2. Virus
2.3. qPCR and Virus Concentration in Seawater
2.4. Histopathological Scoring and Grading of RSIV Infection
2.5. Experimental Immersion Infection of Rock Bream
2.6. Interspecies Cohabitation Challenge
2.7. Statistical Analysis
3. Results
3.1. Pathogenicity Assessment of RSIV Immersion Infection
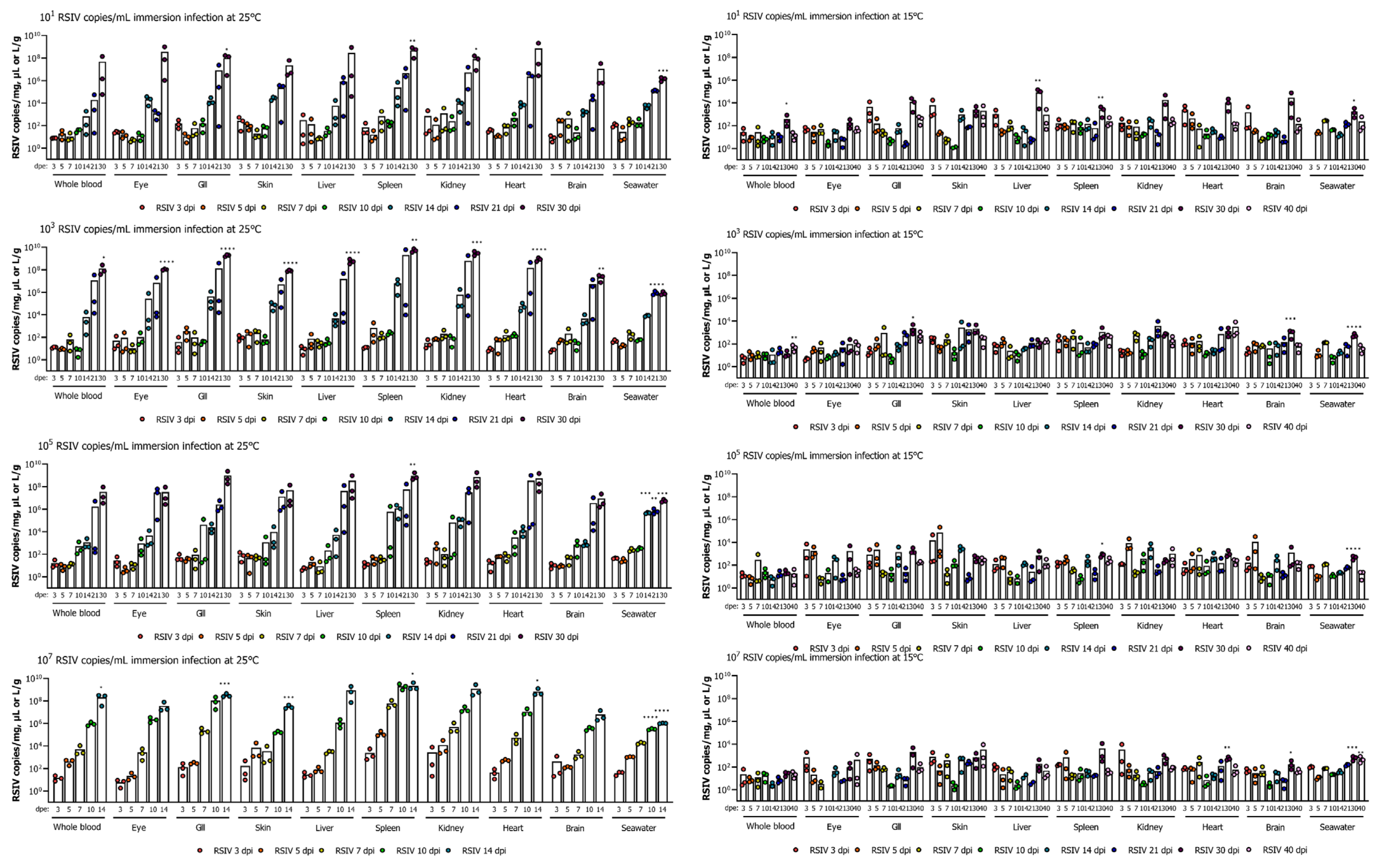
3.2. Viral Load Kinetics in Rock Bream and Viral Shedding Ratio
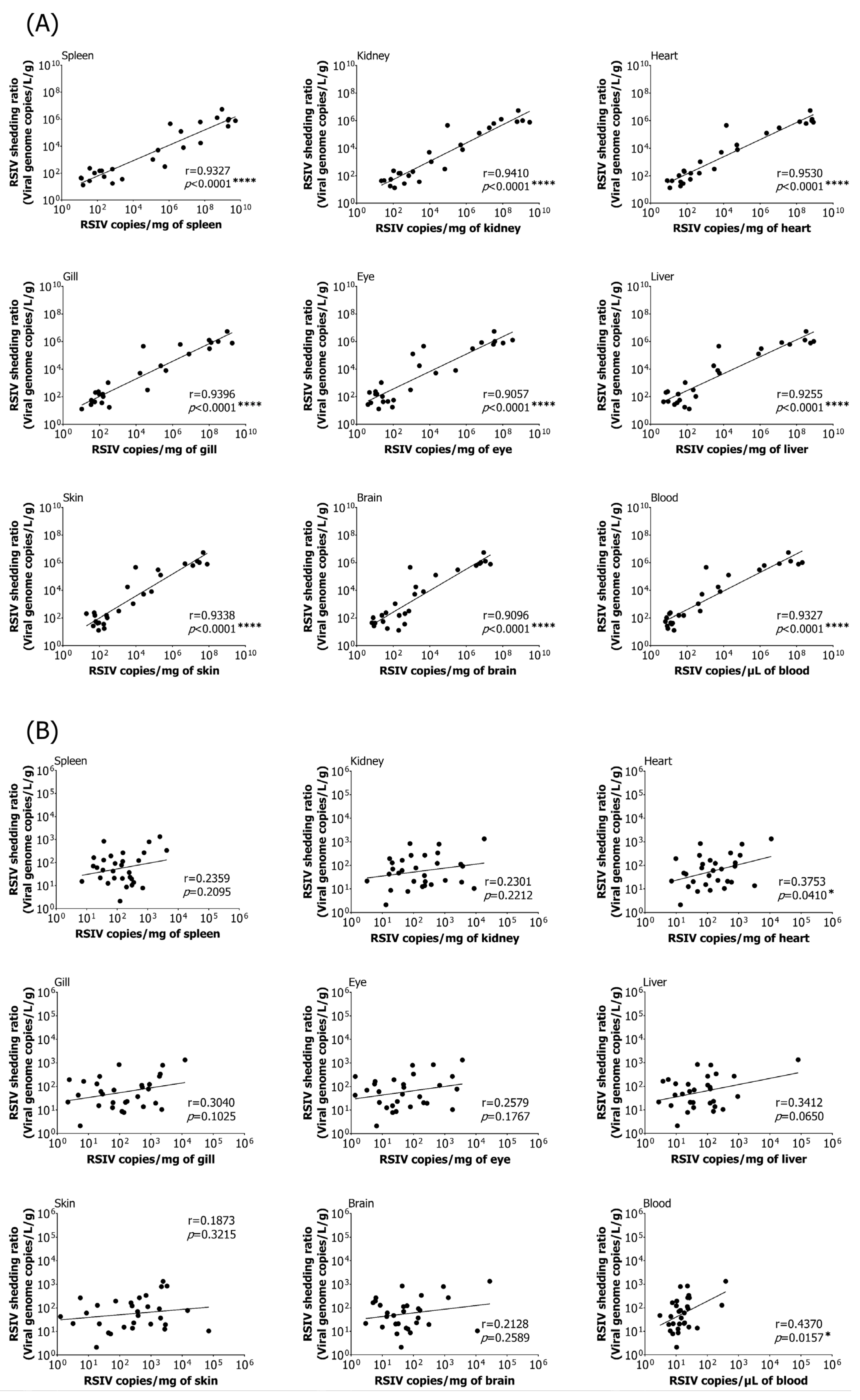
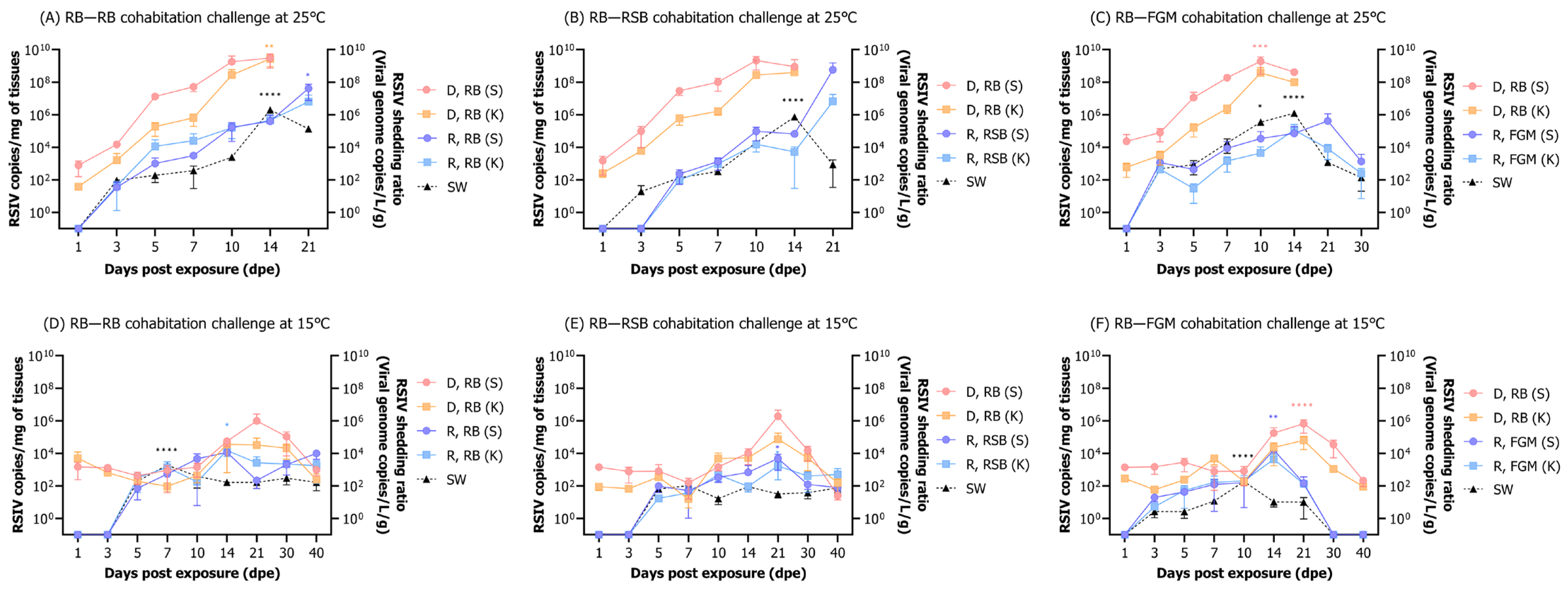
3.3. Correlation between Viral Load in Various Tissues and RSIV Shedding Ratio
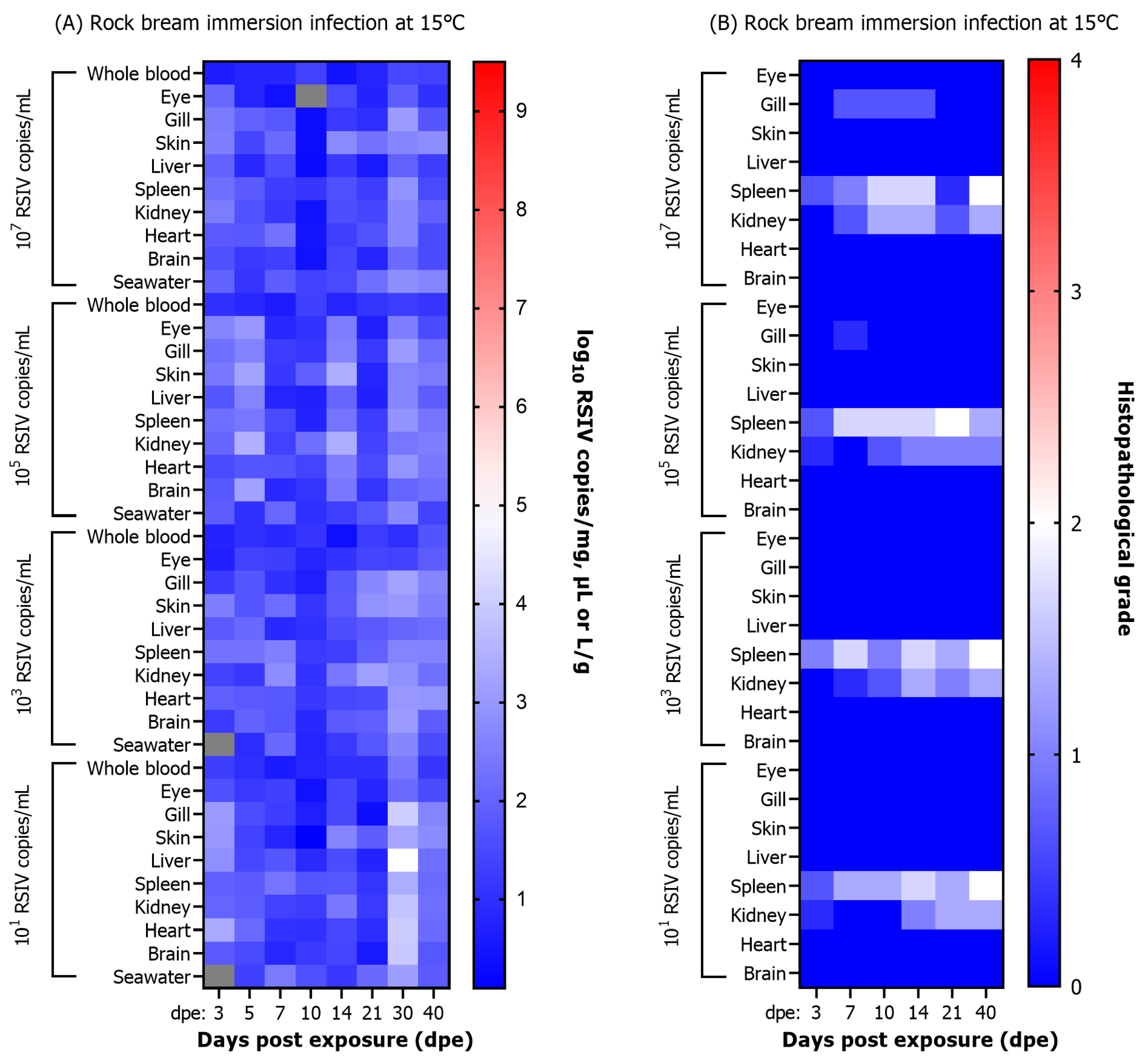
3.4. Analysis of Histopathological Grade of RSIV Infection
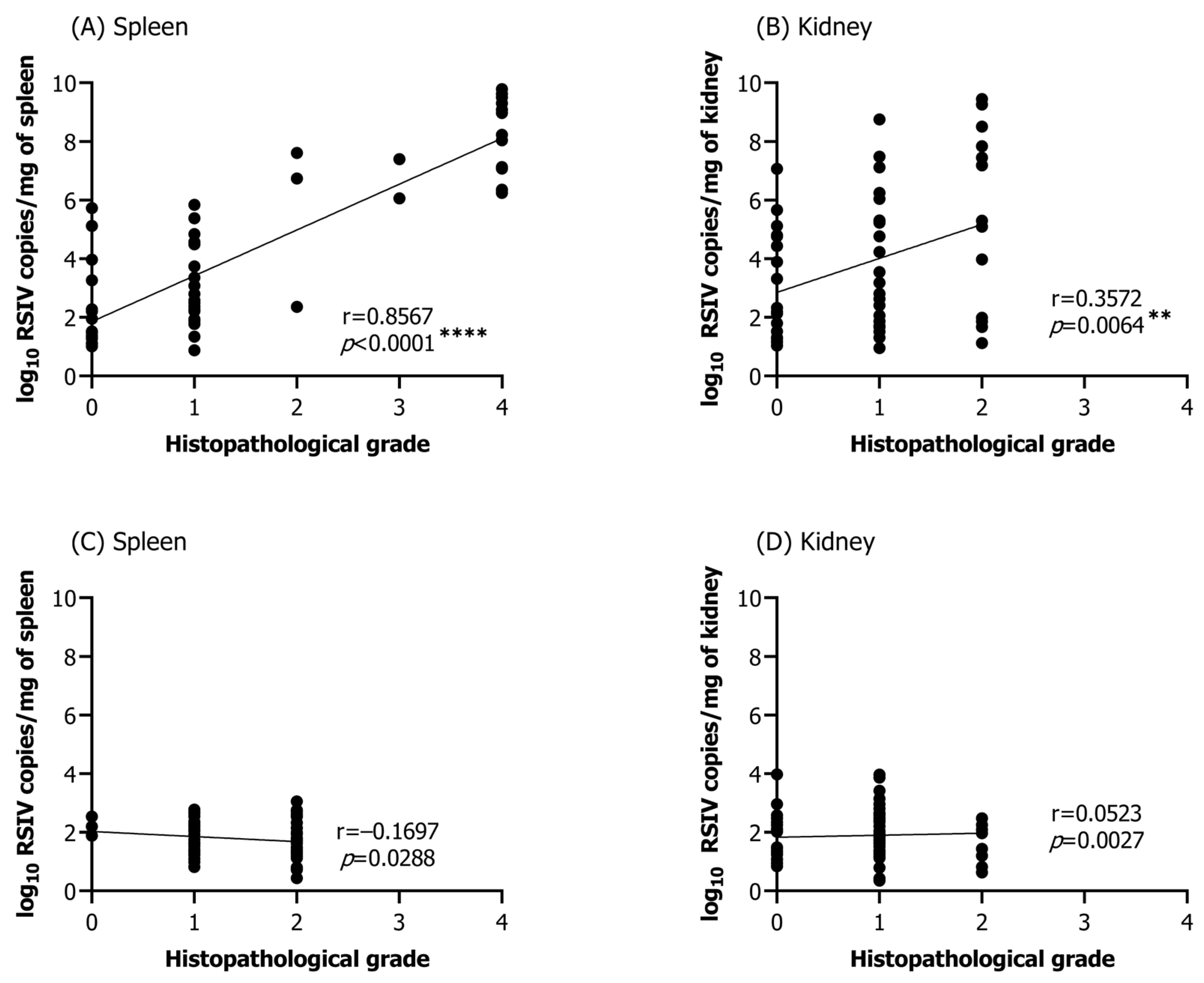
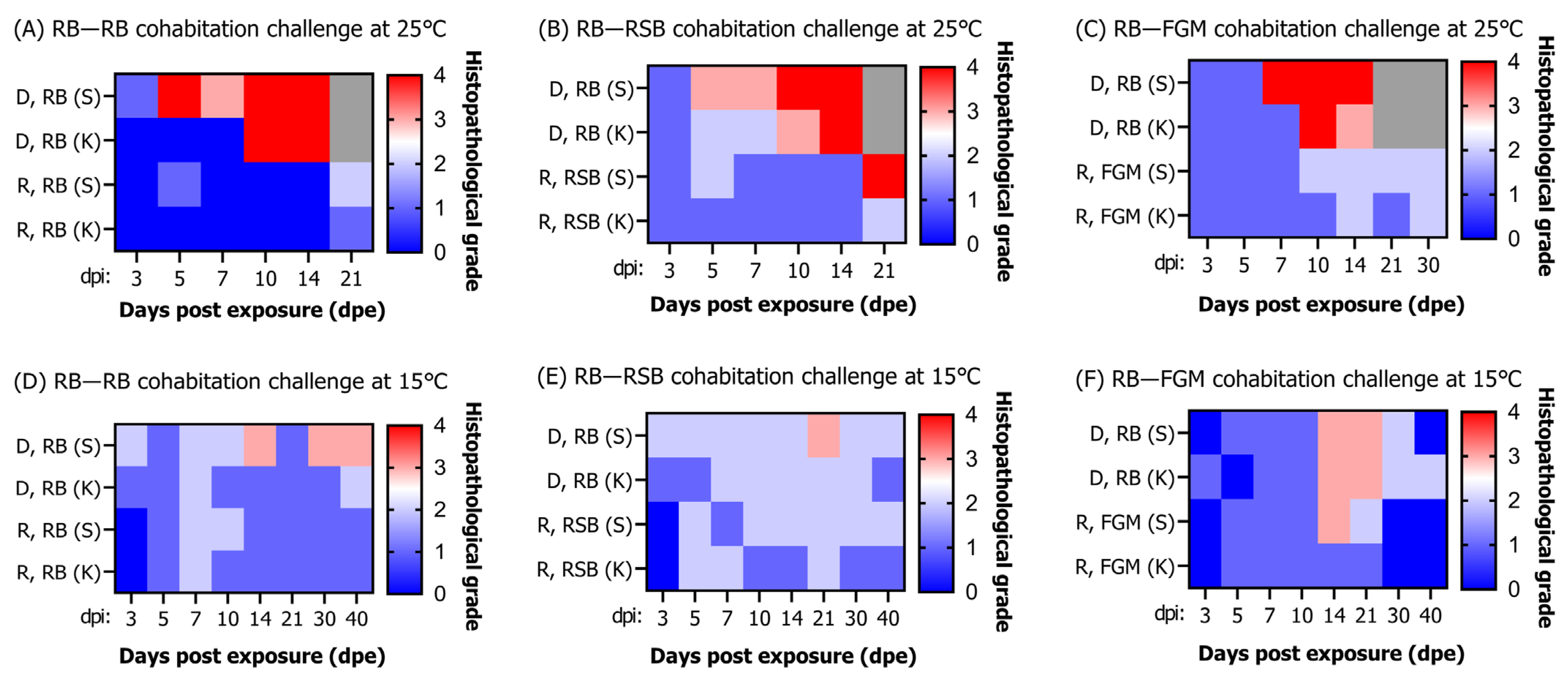
3.5. Correlation between Viral Load and Histopathological Infection Grade
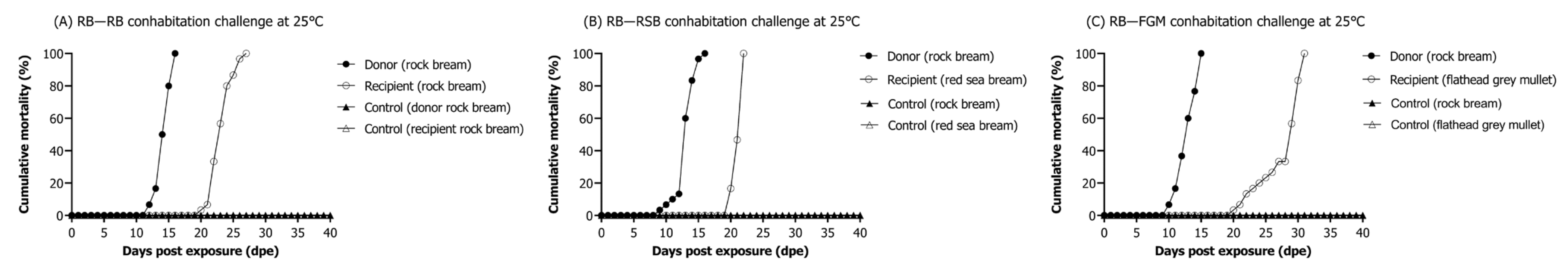
3.6. Evaluation of Risk Associated with RSIV Cohabitation Challenge
3.6.1. Interspecies Mortality
3.6.2. RSIV Dynamics in Fish and Rearing Seawater
3.6.3. Histopathological Infection Grade
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WOAH (World Organisation for Animal Health). Red Sea Bream Iridoviral Disease. In Manual of Diagnostic Tests for Aquatic Animals; WOAH: Paris, France, 2012; Available online: https://www.woah.org/fileadmin/Home/eng/Health_standards/aahm/current/2.3.07_RSIVD.pdf (accessed on 29 March 2022).
- Inouye, K.; Yamano, K.; Maeno, Y.; Nakajima, K.; Matsuoka, M.; Wada, Y.; Sorimachi, M. Iridovirus Infection of Cultured Red Sea Bream, Pagrus major. Fish Pathol. 1992, 27, 19–27. [Google Scholar] [CrossRef]
- Sohn, S.G.; Choi, D.L.; Do, J.W.; Hwang, J.Y.; Park, J.W. Mass Mortalities of Cultured Striped Beakperch, Oplegnathus fasciatus by iridoviral infection. J. Fish Pathol. 2000, 13, 121–127. [Google Scholar]
- Kim, Y.J.; Jung, S.J.; Choi, T.J.; Kim, H.R.; Rajendran, K.V.; Oh, M.J. PCR Amplification and Sequence Analysis of Irido-Like Virus Infecting Fish in Korea. J. Fish Dis. 2002, 25, 121–124. [Google Scholar] [CrossRef]
- Kurita, J.; Nakajima, K. Megalocytiviruses. Viruses 2012, 4, 521–538. [Google Scholar] [CrossRef]
- Wang, C.S.; Shih, H.H.; Ku, C.C.; Chen, S.N. Studies on Epizootic Iridovirus Infection Among Red Sea Bream, Pagrus major (Temminck Schlegel), Cultured in Taiwan. J. Fish Dis. 2003, 26, 127–133. [Google Scholar] [CrossRef]
- Shi, C.Y.; Wang, Y.G.; Yang, S.-L.; Huang, J.; Wang, Q.Y. The First Report of an Iridovirus-Like Agent Infection in Farmed Turbot, Scophthalmus maximus, in China. Aquaculture 2004, 236, 11–25. [Google Scholar] [CrossRef]
- Kim, K.H.; Choi, K.M.; Joo, M.S.; Kang, G.; Woo, W.S.; Sohn, M.Y.; Son, H.J.; Kwon, M.G.; Kim, J.O.; Kim, D.H.; et al. Red Sea Bream Iridovirus (RSIV) Kinetics in Rock Bream (Oplegnathus fasciatus) at Various Fish-Rearing Seawater Temperatures. Animals 2022, 12, 1978. [Google Scholar] [CrossRef]
- Kawato, Y.; Mekata, T.; Inada, M.; Ito, T. Application of Environmental DNA for Monitoring Red Sea Bream Iridovirus at a Fish Farm. Microbiol. Spectr. 2021, 9, e00796-e21. [Google Scholar] [CrossRef]
- Min, J.G.; Jeong, Y.J.; Jeong, M.A.; Kim, J.O.; Hwang, J.Y.; Kwon, M.G.; Kim, K.I. Experimental Transmission of Red Sea Bream Iridovirus (RSIV) between Rock Bream (Oplegnathus fasciatus) and Rockfish (Sebastes schlegelii). J. Fish Pathol. 2021, 34, 1–7. [Google Scholar] [CrossRef]
- NFQS (National Fishery Products Quality Management Service). Aquatic Life Disease Outbreak List in Korea; NFQS: Busan, Korea, 2023; Available online: https://www.nfqs.go.kr/PreventionMgM/fishguard/fishguard01.jsp (accessed on 10 February 2023).
- Gibson-Kueh, S.; Ngoh-Lim, G.H.; Netto, P.; Kurita, J.; Nakajima, K.; Ng, M.L. A Systemic Iridoviral Disease in Mullet, Mugil cephalus L., and Tiger Grouper, Epinephelus fuscoguttatus Forsskal: A First Report and Study. J. Fish Dis. 2004, 27, 693–699. [Google Scholar] [CrossRef]
- Lee, W.L.; Kim, S.R.; Yun, H.M.; Kitamura, S.I.; Jung, S.J.; Oh, M.J. Detection of Red Sea Bream Iridovirus (RSIV) from Marine Fish in the Southern Coastal Area and East China Sea. Fish Pathol. 2007, 20, 211–220. [Google Scholar]
- Kim, K.H.; Choi, K.M.; Kang, G.; Woo, W.S.; Sohn, M.Y.; Son, H.J.; Yun, D.; Kim, D.H.; Park, C.I. Development and Validation of a Quantitative Polymerase Chain Reaction Assay for the Detection of Red Sea Bream Iridovirus. Fishes 2022, 7, 236. [Google Scholar] [CrossRef]
- Kwon, W.J.; Yoon, M.J.; Jin, J.W.; Kim, K.I.; Kim, Y.C.; Hong, S.; Jeong, H.D. Development and Characterization of Megalocytivirus Persistently-Infected Cell Cultures for High Yield of Virus. Tissue Cell 2020, 66, 101387. [Google Scholar] [CrossRef]
- Kawato, Y.; Ito, T.; Kamaishi, T.; Fujiwara, A.; Ototake, M.; Nakai, T.; Nakajima, K. Development of Red Sea Bream Iridovirus Concentration Method in Seawater by Iron Flocculation. Aquaculture 2016, 450, 308–312. [Google Scholar] [CrossRef]
- Meza, K.; Inami, M.; Dalum, A.S.; Lund, H.; Bjelland, A.M.; Sørum, H.; Løvoll, M. Comparative Evaluation of Experimental Challenge by Intraperitoneal Injection and Cohabitation of Atlantic Salmon (Salmo salar L.) after Vaccination Against Piscirickettsia salmonis (EM90-like). J. Fish Dis. 2019, 42, 1713–1730. [Google Scholar] [CrossRef] [PubMed]
- Torres-Corral, Y.; González-Barreiro, O.; Riaza, A.; Santos, Y. Establishment of Different Challenge Models for Aeromonas salmonicida Subsp. Achromogenes in Turbot and Sole. Aquaculture 2022, 555, 738261. [Google Scholar] [CrossRef]
- Garver, K.A.; Mahony, A.A.; Stucchi, D.; Richard, J.; Van Woensel, C.; Foreman, M. Estimation of Parameters Influencing Waterborne Transmission of Infectious Hematopoietic Necrosis Virus (IHNV) in Atlantic Salmon (Salmo salar). PLoS ONE 2013, 8, e82296. [Google Scholar] [CrossRef]
- Saksida, S.M. Infectious Haematopoietic Necrosis Epidemic (2001 to 2003) in Farmed Atlantic Salmon Salmo Salar in British Columbia. Dis. Aquat. Organ. 2006, 72, 213–223. [Google Scholar] [CrossRef]
- Matsuura, Y.; Nishioka, T.; Satoh, J.; Shimahara, Y.; Matsuyama, T.; Takano, T.; Kiryu, I.; Kawato, Y.; Terashima, S.; Masuma, S.; et al. Development of a Method for Experimental Infection of Pacific Bluefin Tuna with Red Seabream Iridoviral Disease. Aquaculture 2021, 539, 736627. [Google Scholar] [CrossRef]
- Jun, L.J.; Jeong, J.B.; Kim, J.H.; Nam, J.H.; Shin, K.W.; Kim, J.K.; Kang, J.C.; Jeong, H.D. Influence of Temperature Shifts on the Onset and Development of Red Sea Bream Iridoviral Disease in Rock Bream Oplegnathus fasciatus. Dis. Aquat. Organ. 2009, 84, 201–208. [Google Scholar] [CrossRef]
- Choi, S.K.; Kwon, S.R.; Nam, Y.K.; Kim, S.K.; Kim, K.H. Organ Distribution of Red Sea Bream Iridovirus (RSIV) DNA in Asymptomatic Yearling and Fingerling Rock Bream (Oplegnathus fasciatus) and Effects of Water Temperature on Transition of RSIV into Acute Phase. Aquaculture 2006, 256, 23–26. [Google Scholar] [CrossRef]
- Jung, M.H.; Kole, S.; Jung, S.J. Efficacy of Saponin-Based Inactivated Rock Bream Iridovirus (RBIV) Vaccine in Rock Bream (Oplegnathus fasciatus). Fish Shellfish Immunol. 2022, 121, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Bovo, G.; Nishizawa, T.; Maltese, C.; Borghesan, F.; Mutinelli, F.; Montesi, F.; De Mas, S. Viral Encephalopathy and Retinopathy of Farmed Marine Fish Species in Italy. Virus Res. 1999, 63, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.; Choi, K.M.; Joo, M.S.; Woo, W.S.; Kim, K.H.; Son, H.J.; Sohn, M.Y.; Kim, J.W.; Nam, B.H.; Park, C.I. A Case Report of Interspecies Transmission of Nervous Necrosis Virus (NNV) between Red Seabream Brood (Pagrus major) and Juvenile Pacific Cod (Gadus macrocephalus). Aquaculture 2023, 562, 738798. [Google Scholar] [CrossRef]
- Jarungsriapisit, J.; Moore, L.J.; Mæhle, S.; Skår, C.; Einen, A.C.; Fiksdal, I.U.; Morton, H.C.; Stefansson, S.O.; Taranger, G.L.; Patel, S. Relationship between Viral Dose and Outcome of Infection in Atlantic Salmon, Salmo Salar L., Post-Smolts Bath-Challenged with Salmonid Alphavirus Subtype 3. Vet. Res. 2016, 47, 102. [Google Scholar] [CrossRef]
- Coleman, D.J.; Camus, A.C.; Martínez-López, B.; Yun, S.; Stevens, B.; Soto, E. Effects of Temperature on Veronaea botryosa Infections in White Sturgeon Acipenser transmontanus and Fungal Induced Cytotoxicity of Fish Cell Lines. Vet. Res. 2018, 49, 11. [Google Scholar] [CrossRef]
- Oh, S.Y.; Nishizawa, T. Establishment of Rock Bream Oplegnathus fasciatus embryo (RoBE-4) cells with Cytolytic Infection of Red Seabream Iridovirus (RSIV). J. Virol. Methods 2016, 238, 1–5. [Google Scholar] [CrossRef]
- Jung, M.H.; Nikapitiya, C.; Vinay, T.N.; Lee, J.H.; Jung, S.J. Rock Bream Iridovirus (RBIV) Replication in Rock Bream (Oplegnathus fasciatus) Exposed for Different Time Periods to Susceptible Water Temperatures. Fish Shellfish Immunol. 2017, 70, 731–735. [Google Scholar] [CrossRef]
- Santos, D.M.; Melo, M.R.; Mendes, D.C.; Rocha, I.K.; Silva, J.P.; Cantanhêde, S.M.; Meletti, P.C. Histological Changes in Gills of Two Fish Species as Indicators of Water Quality in Jansen Lagoon (São Luís, Maranhão State, Brazil). Int. J. Environ. Res. Public Health 2014, 11, 12927–12937. [Google Scholar] [CrossRef]
- Shah, Z.U.; Parveen, S. Oxidative, Biochemical and Histopathological Alterations in Fishes from Pesticide Contaminated River Ganga, India. Sci. Rep. 2022, 12, 3628. [Google Scholar] [CrossRef]
- Yang, S.; Li, D.; Feng, L.; Zhang, C.; Xi, D.; Liu, H.; Yan, C.; Xu, Z.; Zhang, Y.; Li, Y.; et al. Transcriptome Analysis Reveals the High Temperature Induced Damage is a Significant Factor Affecting the Osmotic Function of Gill Tissue in Siberian Sturgeon (Acipenser baerii). BMC Genom. 2023, 24, 2. [Google Scholar] [CrossRef]
- Ninawe, A.S.; Hameed, A.S.S.; Selvin, J. Advancements in Diagnosis and Control Measures of Viral Pathogens in Aquaculture: An Indian Perspective. Aquacult. Int. 2017, 25, 251–264. [Google Scholar] [CrossRef]
- Landmann, M.; Scheibner, D.; Graaf, A.; Gischke, M.; Koethe, S.; Fatola, O.I.; Raddatz, B.; Mettenleiter, T.C.; Beer, M.; Grund, C.; et al. A Semiquantitative Scoring System for Histopathological and Immunohistochemical Assessment of Lesions and Tissue Tropism in Avian Influenza. Viruses 2021, 13, 868. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.R.; Mu, Q.J.; Kong, W.G.; Qin, D.C.; Zhou, Y.; Wang, X.Y.; Cheng, G.F.; Luo, Y.Z.; Ai, T.S.; Xu, Z. Gut Mucosal Immune Responses and Protective Efficacy of Oral Yeast Cyprinid Herpesvirus 2 (CyHV-2) Vaccine in Carassius auratus gibelio. Front. Immunol. 2022, 13, 932722. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, C.; Pan, J.; Sun, C.; Zhou, H.; Meng, Y. Significance of the Viral Load of High-Risk HPV in the Diagnosis and Prediction of Cervical Lesions: A Retrospective Study. BMC Women’s Health 2021, 21, 353. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Shi, X.; Liu, J.; Zhang, L. Correlation between Human Papillomavirus Viral Load and Cervical Lesions Classification: A Review of Current Research. Front. Med. 2023, 10, 1111269. [Google Scholar] [CrossRef]
- Sasaki, M.; Tabata, K.; Kishimoto, M.; Itakura, Y.; Kobayashi, H.; Ariizumi, T.; Uemura, K.; Toba, S.; Kusakabe, S.; Maruyama, Y.; et al. S-217622, a SARS-CoV-2 Main Protease Inhibitor, Decreases Viral Load and Ameliorates COVID-19 Severity in Hamsters. Sci. Transl. Med. 2023, 15, eabq4064. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Chokkakula, S.; Min, S.C.; Kim, B.K.; Choi, W.S.; Oh, S.; Yun, Y.S.; Kang, D.H.; Lee, O.J.; Kim, E.G.; et al. Combination Therapy with Nirmatrelvir and Molnupiravir Improves the Survival of SARS-CoV-2 Infected Mice. Antiviral Res. 2022, 208, 105430. [Google Scholar] [CrossRef] [PubMed]
- Driouich, J.S.; Cochin, M.; Lingas, G.; Moureau, G.; Touret, F.; Petit, P.R.; Piorkowski, G.; Barthélémy, K.; Laprie, C.; Coutard, B.; et al. Favipiravir Antiviral Efficacy Against SARS-CoV-2 in a Hamster Model. Nat. Commun. 2021, 12, 1735. [Google Scholar] [CrossRef]
- Kim, K.H.; Kang, G.; Woo, W.S.; Sohn, M.Y.; Son, H.J.; Park, C.I. Development of a Propidium Monoazide-Based Viability Quantitative PCR Assay for Red Sea Bream Iridovirus Detection. Int. J. Mol. Sci. 2023, 24, 3426. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.-H.; Kang, G.; Woo, W.-S.; Sohn, M.-Y.; Son, H.-J.; Kwon, M.-G.; Kim, J.-O.; Park, C.-I. Impact of Red Sea Bream Iridovirus Infection on Rock Bream (Oplegnathus fasciatus) and Other Fish Species: A Study of Horizontal Transmission. Animals 2023, 13, 1210. https://doi.org/10.3390/ani13071210
Kim K-H, Kang G, Woo W-S, Sohn M-Y, Son H-J, Kwon M-G, Kim J-O, Park C-I. Impact of Red Sea Bream Iridovirus Infection on Rock Bream (Oplegnathus fasciatus) and Other Fish Species: A Study of Horizontal Transmission. Animals. 2023; 13(7):1210. https://doi.org/10.3390/ani13071210
Chicago/Turabian StyleKim, Kyung-Ho, Gyoungsik Kang, Won-Sik Woo, Min-Young Sohn, Ha-Jeong Son, Mun-Gyeong Kwon, Jae-Ok Kim, and Chan-Il Park. 2023. "Impact of Red Sea Bream Iridovirus Infection on Rock Bream (Oplegnathus fasciatus) and Other Fish Species: A Study of Horizontal Transmission" Animals 13, no. 7: 1210. https://doi.org/10.3390/ani13071210
APA StyleKim, K.-H., Kang, G., Woo, W.-S., Sohn, M.-Y., Son, H.-J., Kwon, M.-G., Kim, J.-O., & Park, C.-I. (2023). Impact of Red Sea Bream Iridovirus Infection on Rock Bream (Oplegnathus fasciatus) and Other Fish Species: A Study of Horizontal Transmission. Animals, 13(7), 1210. https://doi.org/10.3390/ani13071210




