Sternal Abnormalities on Thoracic Radiographs of Dogs and Cats
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
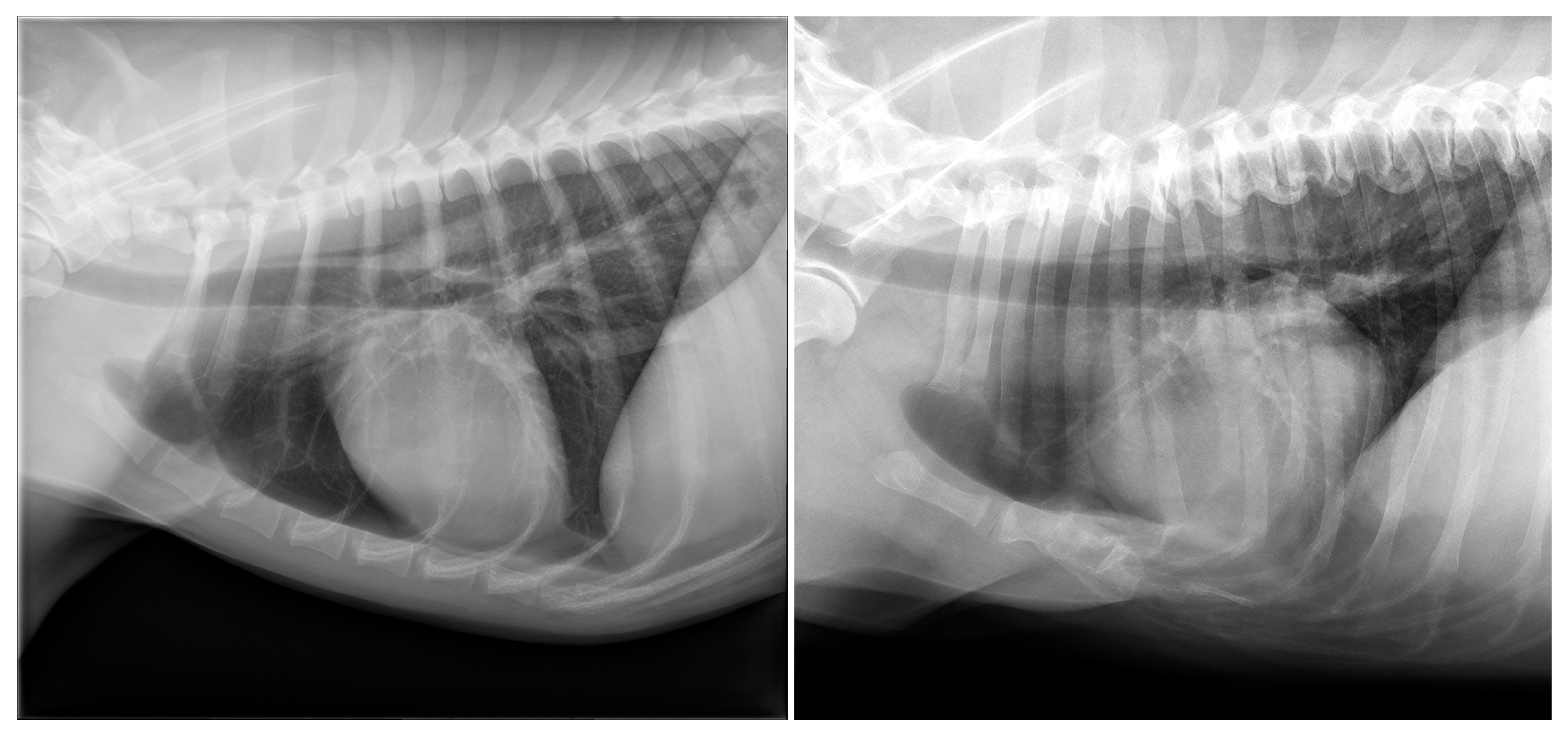
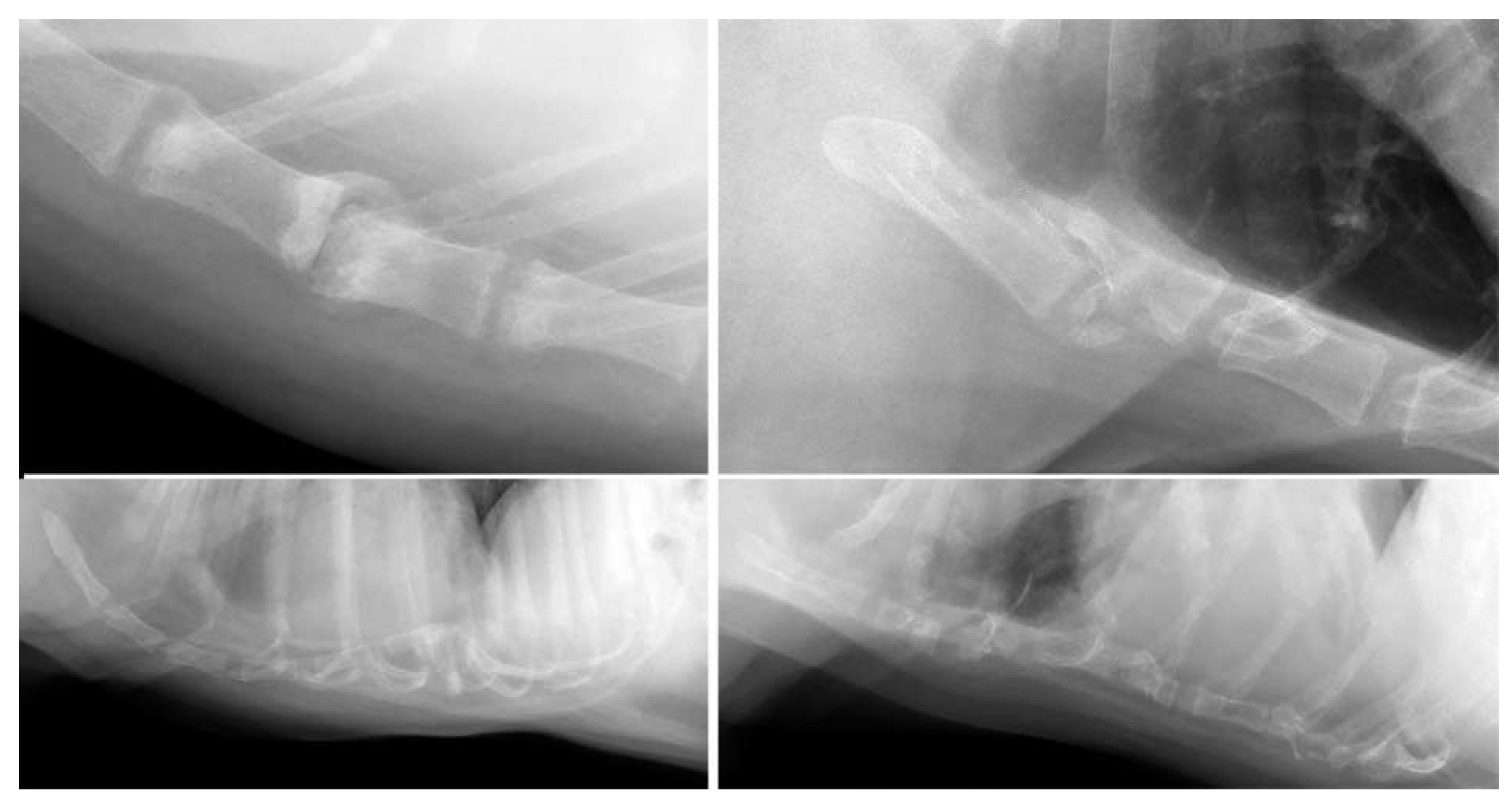
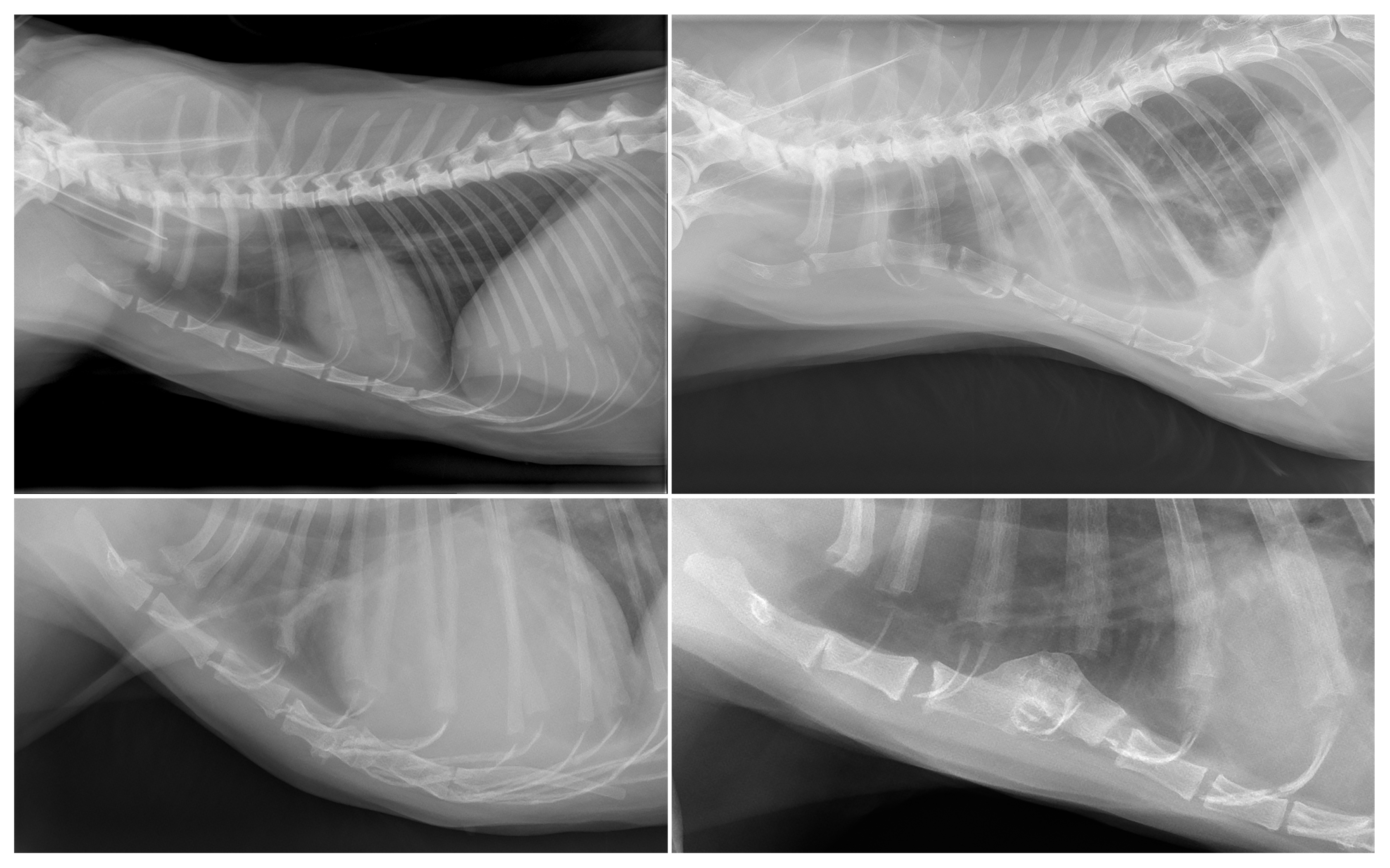
3.1. Dogs
3.2. Cats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lahunta, A.d.; Evans, H.E.; Hermanson, J.W. Miller and Evans’ Anatomy of the Dog, 5th ed.; Elsevier: St. Louis, MO, USA, 2019; pp. 44, 140. [Google Scholar]
- Singh, B. Dyce, Sack, and Wensing’s Textbook of Veterinary Anatomy, 5th ed.; Elsevier: St. Louis, MO, USA, 2018; pp. 38–39. [Google Scholar]
- Hudson, L.; Hamilton, W. Atlas of Feline Anatomy For Veterinarians; Teton NewMedia: Jackson, MS, USA, 2017; p. 31. [Google Scholar]
- König, H.E.; Liebich, H.G. Veterinary Anatomy of Domestic Animals, 7th ed.; Thieme: Stuttgart, Germany, 2020; pp. 125–128. [Google Scholar]
- Schwarz, T.; Johnson, V. BSAVA Manual of Canine and Feline Thoracic Imaging; BSAVA: Quedgeley, UK, 2008; p. 340. [Google Scholar]
- Thrall, D.E.; Robertson, I.D. Atlas of Normal Radiographic Anatomy & Anatomic Variants in the Dog and Cat, 2nd ed.; Elsevier: St. Louis, MO, USA, 2016; pp. 203–205. [Google Scholar]
- Schwarzkopf, I.; Bavegems, V.C.A.; Vandekerckhove, P.M.F.P.; Melis, S.M.; Cornillie, P.; de Rooster, H. Surgical Repair of a Congenital Sternal Cleft in a Cat. Vet. Surg. 2014, 43, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Benlloch-Gonzalez, M.; Poncet, C. Sternal Cleft Associated with Cantrell’s Pentalogy in a German Shepherd Dog. J. Am. Anim. Hosp. Assoc. 2015, 51, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Eiger, S.N.; Mison, M.B.; Aronson, L.R. Congenital sternal defect repair in an adult cat with incomplete pentalogy of Cantrell. J. Am. Vet. Med. Assoc. 2019, 254, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Komsta, R.; Osiński, Z.; Dębiak, P.; Twardowski, P.; Lisiak, B. Prevalence of pectus excavatum (PE), pectus carinatum (PC), tracheal hypoplasia, thoracic spine deformities and lateral heart displacement in thoracic radiographs of screw-tailed brachycephalic dogs. PLoS ONE 2019, 14, e0223642. [Google Scholar] [CrossRef]
- Komsta, R.; Łojszczyk, A.; Dębiak, P.; Twardowski, P.; Lisiak, B. Computed tomographic evaluation of pectus excavatum in 14 cats. PLoS ONE 2022, 17, e0262866. [Google Scholar] [CrossRef]
- Hassan, E.A.; Hassan, M.H.; Torad, F.A. Correlation between clinical severity and type and degree of pectus excavatum in twelve brachycephalic dogs. J. Vet. Med. Sci. 2018, 80, 766–771. [Google Scholar] [CrossRef] [Green Version]
- Thrall, D.E. Textbook of Veterinary Diagnostic Radiology; Saunders: St. Louis, MO, USA, 2017; pp. 618–626. [Google Scholar]
- Trost, M.E.; Inkelmann, M.A.; Galiza, G.J.N.; Silva, T.M.; Kommers, G.D. Occurrence of tumours metastatic to bones and multicentric tumours with skeletal involvement in dogs. J. Comp. Pathol. 2014, 150, 8–17. [Google Scholar] [CrossRef]
- Serra, C.I.; Soler, C.; Moratalla, V.; Sifre, V.; Redondo, J.I. Surgical management of a traumatic dislocation of the sternum in an English bulldog. J. Small Anim. Pract. 2015, 56, 407–410. [Google Scholar] [CrossRef]
- Pietra, M.; Pisoni, L.; Linta, N.; Pinna, S.; Romagnoli, N.; Diana, A. Endoscopy-assisted tracheal reconstruction of post-traumatic obstruction in a cat: A case report. Vet. Med. 2016, 60, 341–344. [Google Scholar] [CrossRef] [Green Version]
- Lam, L. Acute sternal subluxation in an indoor cat. Can. Vet. J. 2018, 59, 82–84. [Google Scholar]
- Morabito, S.; Specchi, S.; Auriemma, E.; Ferro, S.; Kuhnert, P.; Zini, E. Computed tomographic and ultrasonographic findings of abdominal arterial pseudoaneurysms caused by systemic mycosis in dogs. J. Small Anim. Pract. 2020, 61, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Blondel, M.; Gros, L.; Lucas, M.; Delverdier, M.; Palierne, S. Multifocal haematogenous osteomyelitis and septic physitis in a dog. Vet. Rec. Case Rep. 2020, 8, e001207. [Google Scholar] [CrossRef]
- Wainberg, S.H.; Brisson, B.A.; Hayes, G.M.; Mackenzie, S. Use of gentamicin sulfate-impregnated sponges as adjuvant therapy for the treatment of chronic foreign body associated sternal osteomyelitis in a dog. Can. Vet. J. 2015, 56, 1161–1165. [Google Scholar] [PubMed]
- Weber, W.J.; Berry, C.R.; Kramer, R.W. Vacuum phenomenon in twelve dogs. Vet. Radiol. Ultrasound 1995, 36, 493–498. [Google Scholar] [CrossRef]
- Ellison, G.; Halling, K.B. Atypical pectus excavatum in two Welsh terrier littermates. J. Small Anim. Pract. 2004, 45, 311–314. [Google Scholar] [CrossRef]
- Fossum, T.W. Small Animal Surgery, 5th ed.; Elsevier Mosby: St. Louis, MO, USA, 2018; pp. 907–912. [Google Scholar]
- Knospe, C. Periods and Stages of the Prenatal Development of the Domestic Cat. Anat. Histol. Embryol. 2002, 31, 37–51. [Google Scholar] [CrossRef]
- Banz, A.C.; Gottfried, S.D. Peritoneopericardial diaphragmatic hernia: A retrospective study of 31 cats and eight dogs. J Am Anim Hosp Assoc 2010, 46, 398–404. [Google Scholar] [CrossRef]
- Burns, C.G.; Bergh, M.S.; McLoughlin, M.A. Surgical and nonsurgical treatment of peritoneopericardial diaphragmatic hernia in dogs and cats: 58 cases (1999–2008). J. Am. Vet. Med. Assoc. 2013, 242, 643–650. [Google Scholar] [CrossRef]
- Charlesworth, T.M.; Schwarz, T.; Sturgess, C.P. Pectus excavatum: Computed tomography and medium-term surgical outcome in a prospective cohort of 10 kittens. J. Feline Med. Surg. 2016, 18, 613–619. [Google Scholar] [CrossRef] [Green Version]
- Kurosawa, T.A.; Ruth, J.D.; Steurer, J.; Austin, B.; Heng, H.G. Acquired pectus excavatum secondary to laryngeal paralysis in a dog. Vet. Radiol. Ultrasound 2012, 53, 329–332. [Google Scholar] [CrossRef]
- Kornder, J.; Platt, S.R.; Eagleson, J.; Kent, M.; Holmes, S.P. Vertebral polyostotic lymphoma in a geriatric dog. Vet. Radiol. Ultrasound 2016, 57, E42–E45. [Google Scholar] [CrossRef] [PubMed]
- Dhaliwal, R.S.; Reed, A.L.; Kitchell, B.E. Multicentric lymphoma in a dog with multiple-site skeletal involvement. Vet. Radiol. Ultrasound 2001, 42, 38–41. [Google Scholar] [CrossRef]
- Cornell, K.K.; Bostwick, D.G.; Cooley, D.M.; Hall, G.; Harvey, H.J.; Hendrick, M.J.; Pauli, B.U.; Render, J.A.; Stoica, G.; Sweet, D.C.; et al. Clinical and pathologic aspects of spontaneous canine prostate carcinoma: A retrospective analysis of 76 cases. Prostate 2000, 45, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Ros, C.; Fernandez-Flores, F.; de la Fuente, C.; Pi, D.; Anor, S.; Planellas, M.; Pumarola, M. Tumour-to-Tumour Metastasis Phenomenon: Metastatic Prostatic Adenocarcinoma within an Anaplastic Oligodendroglioma in the Brain of a Dog. J. Comp. Pathol. 2018, 165, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Kawalilak, L.T.; Chen, A.V.; Roberts, G.R. Imaging characteristics of disseminated Geosmithia argillacea causing severe diskospondylitis and meningoencephalomyelitis in a dog. Clin. Case Rep. 2015, 3, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Burton, C.A.; White, R.N. Review of the technique and complications of median J sternotomy in the dog and cat. J. Small Anim. Pract. 1996, 37, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Ford, L.T.; Gilula, L.A.; Murphy, W.A.; Gado, M. Analysis of gas in vacuum lumbar disc. Am. J. Roentgenol. 1977, 128, 1056–1057. [Google Scholar] [CrossRef] [Green Version]
- Müller, M.K.; Ludewig, E.; Oechtering, G.; Scholz, M.; Flegel, T. The vacuum phenomenon in intervertebral disc disease of dogs based on computed tomography images. J. Small Anim. Pract. 2013, 54, 253–257. [Google Scholar] [CrossRef]
- Suwankong, N.; Meij, B.P.; Voorhout, G.; Boer, A.H.d.; Hazewinkel, H.A.W. Review and retrospective analysis of degenerative lumbosacral stenosis in 156 dogs treated by dorsal laminectomy. Vet. Comp. Orthop. Traumatol. 2008, 21, 285–293. [Google Scholar] [CrossRef] [Green Version]
| Group | Findings | Breeds * |
|---|---|---|
| Degeneration (n = 98) | New bone formation (n = 98); collapse (n = 13); vacuum phenomenon (n = 13); sclerosis (n = 9); dislocation (n = 5) | Labrador Retriever (16/62); mixed (13/145); Boxer (7/13); Flatcoated Retriever (7/15); Cavalier King Charles Spaniel (5/11); German Shepherd (5/21); Bernese Mountain Dog (4/26); Belgian Shepherd (3/8); Dachshund (3/19); English Cocker Spaniel (3/13); American Bulldog (2/6); Drentsche Patrijshond (2/6); Golden Retriever (2/23); Irish Setter (2/3); Labradoodle (2/22); Welsh Springer Spaniel (2/3); White Swiss Shepherd Dog (2/14); Anatolian Shepherd Dog (1/1); Border Collie (1/10); Border Terrier (1/4); Bouvier des Flandres (1/2); Chow Chow (1/3); English Springer Spaniel (1/4); Frisian Water Dog (1/2); Hovawart (1/2); Irish Wolfhound (1/2); Jack Russell Terrier (1/15); Kooikerhondje (1/5); Newfoundland (1/5); Dobermann (1/2); Rottweiler (1/10); Spanish Water Dog (1/3); Stabyhoun (1/12); West Highland White Terrier (1/3); Wirehaired Pointing Griffon (1/1) |
| Abnormal number (n = 62) | 9 sternebrae (n = 16) | Labrador Retriever (7/62); Dachshund (2/19); Beagle (1/7); Australian shepherd (1/4); English Cocker Spaniel (1/13); Irish Wolfhound (1/2); mixed (1/145); Rhodesian Ridgeback (1/9); Whippet (1/5) |
| <8 sternebrae (n = 46) | Mixed (10/145); Chihuahua (6/28); Pomeranian (5/14); Labrador Retriever (4/62); Pug (4/7); French Bulldog (3/23); Staffordshire Bull Terrier (2/10); West Highland White Terrier (2/3); American Staffordshire Terrier (1/8); Australian Shepherd (1/4); Bearded Collie (1/2); Boxer (1/13); Drentsche Patrijshond (1/6); Great Dane (1/2); Miniature Pinscher (1/1); Dobermann (1/2); Shih Tzu (1/6); Spanish Water dog (1/3) | |
| Shape deformity (n = 24) | Pectus excavatum (n = 6) | Pug (2/7); Boxer (1/13); Dachshund (1/19); French Bulldog (1/23); mixed (1/145) |
| Pectus carinatum (n = 18) | French Bulldog (8/23); Chihuahua (5/28); mixed (3/145); Pug (1/7); Welsh Springer Spaniel (1/3) | |
| Post-trauma (n = 12) | Dislocation (n = 5); collapse (n = 8); fracture (n = 2) | Labrador Retriever (3/62); mixed (2/145); Alaskan Malamute (1/4); Boxer (1/13); Chihuahua (1/28); Chow Chow (1/3); German Shepherd (1/21); Labradoodle (1/22); Welsh Springer Spaniel (1/3) |
| Aggressive bone lesion (n = 2) | Osteolysis; pathological fracture | Chihuahua (n = 1); Labradoodle (n = 1) |
| Group | Findings | Breeds * |
|---|---|---|
| Intersternebral cartilage (n = 15) | New bone formation (n = 15); sclerosis (n = 5) | Domestic Shorthair (10/112); Bengal (1/3); British Shorthair (1/10); mixed (1/11); Norwegian Forest (1/4); Siamese (1/4) |
| Abnormal number (n = 27) | 9 sternebrae (n = 7) | Domestic Shorthair (4/112); Bengal (1/3); mixed (1/11); Siamese (1/4) |
| < 8 sternebrae (n = 20) | Domestic Shorthair (13/112); British Shorthair (2/10); Ragdoll (2/7); Oriental Shorthair (1/1); Persian (1/1); Sphynx (1/2) | |
| Shape deformity (n = 8) | Pectus excavatum (n = 6) | Domestic Shorthair (6/112) |
| Pectus carinatum (n = 2) | Domestic Shorthair (1/112); Norwegian Forest (1/4) | |
| Post-trauma (n = 3) | Dislocation (n = 3) | Domestic Shorthair (3/112) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van den Broek, D.H.N.; Vester, S.C.; Tobón Restrepo, M.; Veraa, S. Sternal Abnormalities on Thoracic Radiographs of Dogs and Cats. Animals 2023, 13, 1233. https://doi.org/10.3390/ani13071233
van den Broek DHN, Vester SC, Tobón Restrepo M, Veraa S. Sternal Abnormalities on Thoracic Radiographs of Dogs and Cats. Animals. 2023; 13(7):1233. https://doi.org/10.3390/ani13071233
Chicago/Turabian Stylevan den Broek, Dirk H. N., Siemone C. Vester, Mauricio Tobón Restrepo, and Stefanie Veraa. 2023. "Sternal Abnormalities on Thoracic Radiographs of Dogs and Cats" Animals 13, no. 7: 1233. https://doi.org/10.3390/ani13071233
APA Stylevan den Broek, D. H. N., Vester, S. C., Tobón Restrepo, M., & Veraa, S. (2023). Sternal Abnormalities on Thoracic Radiographs of Dogs and Cats. Animals, 13(7), 1233. https://doi.org/10.3390/ani13071233






