Simple Summary
Yellow River Carp (Cyprinus carpio haematopterus) is a major commercial farmed species belonging to Cyprinus carpio, which has been cultivated widely, especially in Northern China. However, no relative literature focused on appropriate dietary protein level for large-sized Cyprinus carpio haematopterus. The present study investigated the effects of different dietary protein levels on the growth performance, physicochemical indexes, quality, and molecular expression of Cyprinus carpio haematopterus and aimed to determine which dietary protein level was most appropriate for Cyprinus carpio haematopterus. It was found that the optimal dietary protein level for Cyprinus carpio haematopterus (160.24 ± 15.56 g) is 250–280 g/kg based on different aspect analysis. This study is also helpful in laying the foundations for the breeding of new carp varieties with low dietary protein levels.
Abstract
A 12-week rearing trial was carried out to estimate effects on the growth performance, physicochemical indexes, quality, and the molecular expression of Yellow River Carp (Cyprinus carpio haematopterus) using five practical diets, including dietary protein levels of 220, 250, 280, 310, and 340 g/kg. The results illustrated that the fish’s weight gain (WG) and specific growth rate (SGR) were significantly influenced, with an ascending dietary protein level of up to 250 g/kg (p < 0.05). The carp muscle contents of total saturated fatty acids (∑SFA), monounsaturated fatty acids (∑MUFA), polyunsaturated fatty acids (∑PUFA), and fatty acids (∑FA) decreased significantly with the ascending dietary protein levels, except for the 250 g/kg protein diet (p < 0.05). Only the glutamic acid and total essential amino acid (∑EAA) contents were significantly influenced by the ascending dietary protein levels (p < 0.05). The relative GH expression of the carp muscle significantly decreased with the increase in the dietary protein level up to 310 g/kg, and then it significantly increased (p < 0.05). In the intestines, the peak relative TOR expression was observed on the 220 g/kg protein diet, while the relative 4EBP1 expression was significantly influenced by the dietary protein level up to 250 g/kg (p < 0.05). In the muscle, the peak relative TOR and 4EBP1 expression levels were observed on the 250 g/kg protein diet. In gills, the lowest relative Rhag, Rhbg, and Rhcg1 expression levels were observed on the 250 g/kg protein diet. Based on all of the aforementioned results, the optimal dietary protein level for Cyprinus carpio haematopterus (160.24 ± 15.56 g) is 250–280 g/kg.
1. Introduction
As mentioned in the State of the World Fisheries and Aquaculture report, aquatic food is making increasingly important contributions to food security and nutrition in the 21st century [1]. Fish account for more than 40% of the total production [1]. The contribution of fish, as a protein source, is very important for the global population [2]. Protein in feed is an essential factor for increasing the growth performance and production of fish, because protein is the major constituent of the fish body [3]. The protein levels of fish vary according to the species, body size, protein source, and other rearing conditions [4]. Due to their high price, the proteins account for a major proportion of the feed cost. Therefore, an appropriate dietary protein level not only maintains the optimal growth of fish but also reduces the feed cost [5]. Moreover, excessive protein intake from feed will increase amino acid metabolism pressure on the fish body and negatively influence the environment [6,7]. Therefore, the appropriate dietary protein level is an important measure not only for ensuring that fish are in the most suitable growth state but also for reducing costs and expanding the economic benefits.
In 2021, the annual yield of common carp (Cyprinus carpio) was 2.83 million tons, accounting for 10.73% of the total freshwater fish production in China [8]. The culture pattern includes ponds, reservoirs, lakes, and rice fields. Yellow River Carp (Cyprinus carpio haematopterus) is a major commercial farmed species belonging to Cyprinus carpio, which has been cultivated widely, especially in Northern China, because of its delicious flesh, high disease resistance, and elegant appearance, with golden scales and red or orange tail fins [9]. As previous studies have mentioned, the dietary protein levels decrease with the increase in the fish body size [10]. Previous studies on the dietary protein levels of Cyprinus carpio mainly focused on juvenile Cyprinus carpio with weights below 20 g [9,11] and juvenile Cyprinus carpio haematopterus with weights of 21.56 ± 0.46 g [12]. Song et al. [9] demonstrated that appropriate dietary hydroxyproline supplementation can promote myogenesis and collagen synthesis in the muscle of Cyprinus carpio haematopterus, and this ultimately improves the raw- and cooked-fillet texture characteristics. Yun et al. [12] posited that different dietary methionine supplementations can affect the growth performance and fillet texture characteristics of Cyprinus carpio haematopterus. However, limited information is available for large-sized Cyprinus carpio haematopterus. At the same time, feed enterprises excessively publicize the dietary protein contents of feeds to raise their price, which is unfavorable to industrial development. Therefore, there is an urgent need to identify an appropriate dietary protein level for large-sized Cyprinus carpio haematopterus. Hence, the present study investigated the effects of different dietary protein levels on the growth performance, physicochemical indexes, quality, and molecular expression of Cyprinus carpio haematopterus and aimed to determine which dietary protein level was most appropriate for Cyprinus carpio haematopterus. This study is also helpful in laying the foundations for the breeding of new carp varieties with low dietary protein levels.
2. Materials and Methods
2.1. Feed Formulation and Preparation
The feed formulation was designed according to the feed formula for common carp (GB/T 36782-2018, 2019) [13], and the proximate composition of the practical diets is specified in the Supplementary Materials, Table S1. Five different protein diets with 220, 250, 280, 310, and 340 g/kg of protein and a similar crude lipid level of 60 g/kg were fed to Cyprinus carpio haematopterus, respectively. Soybean meal was the main protein source used to regulate the different protein levels. Wheat meal was equally decreased as the dietary protein level increased to ensure that the available dietary energy was similar between all the formulations. The ingredients of the diets were thoroughly mixed together and used to produce 3.0 mm diameter feed pellets. All the diets were air-dried, sieved into pellets, and stored at −20 °C until use.
2.2. Fish Management and Feeding
Large-sized Cyprinus carpio haematopterus were obtained from the Kuandian Experimental Station (Dandong, China) of Heilongjiang Fisheries Research Institute, Chinese Academy of Fishery Sciences, China. Subsequently, the carp were marked with electronic labels and fed with a commercial diet containing a crude protein level of 280 g/kg twice each day during indoor acclimatization for two weeks. After that, the healthy fish (n = 75) with a mean initial body weight of (160.24 ± 15.56) g were randomly assigned to 15 indoor glass tanks (125 × 55 × 70 cm3), with each diet group containing a total of 15 individuals in 3 replicates.
The rearing trial lasted for three months, from 12 June to 12 September 2021. The carp were hand-fed four times each day with a ration of 40–50 g/kg of the fish’s body weight (05:30 a.m., 10:00 a.m., 02:00 p.m. and 05:00 p.m.). One-third of the total water volume was replaced twice each day (07:00 a.m. and 03:30 p.m.). Meanwhile, the remaining diet was removed at the time of water replacement to refresh the water. During the entire rearing period, the light was maintained in a natural photoperiod, the water temperature was 22.34 ± 0.56 °C, the dissolved oxygen was 6.87 ± 0.76, the total ammonia was 0.15 ± 0.03 mg/L, and the pH was 7.98 ± 0.23.
2.3. Sample Collection
Upon the termination of the feeding trial, all carp were fasted for 24 h. Subsequently, all carp were counted and weighed to determine the weight gain (WG), specific growth rate (SGR), and survival rate (SR). After the carp were anaesthetized with MS-222 (Sigma, St Louis, MO, USA, 0.1 g/L), a total of 15 carp, with 5 carp contained in each indoor glass tank in 3 replicates, were collected to obtain blood samples via tail vein bleeding, and the samples were prepared via centrifugation (3000× g rpm/min, 15 min, 4 °C) for hematological parameter detection. The separated plasma was stored at −80 °C until use. After blood sampling, the gills, intestines, and muscles of fifteen carp within each diet group were dissected and frozen immediately in liquid nitrogen and then stored at −80 °C until the analysis of the gene expression and intestinal enzyme activities [14]. Approximately 2 g of midgut tissues was removed and washed in 0.9% physiological saline that was pre-cooled at 4 °C. Intestinal tissue homogenates were collected and stored at −80 °C for the determination of intestinal digestive enzymes and antioxidant enzymes. Five individual muscles in each replicate were mixed to calculate the proximate composition and fatty acid profile and perform amino acid analysis.
2.4. The Growth Performance
The percentage of weight gain (WG, %) and specific growth rate (SGR, %/d) were calculated based on the initial body weight and final body weight. The percentage of survival rate (SR, %) was calculated based on the difference between the numbers of initial and final individuals. The formulae are presented in the following Equations (1)–(3):
where Wt is the initial body weight and Wt−1 is the final body weight; D is the interval (days) between the two sampling periods; NF is the number of final individuals; and NI is the number of initial individuals.
2.5. The Analysis of the Plasma Hematological Parameters and Enzyme Activities
All indices of the plasma hematological parameters and enzyme activities [14] were determined using commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), including alanine aminotransferase (ALT), aspartate aminotransferase (AST), globulin (GLO), alkaline phosphatase (ALP), total protein (TP), albumin (ALB), blood urea nitrogen (BUN), total cholesterol (TC), triglyceride (TG), high-density cholesterol (HDL-C), low-density cholesterol (LDL-C), uric acid (UA), total bile acid (TBA), α-amylase (α-AMS), lipase (LPS), trypsin (TPS), catalase (CAT), superoxide dismutase (SOD), and malondialdehyde (MDA).
2.6. Proximate Composition
The moisture of the carp muscle was measured using a vacuum freeze dryer (FD-1A-50, Biocoll, Beijing, China) at −50 °C, with vacuum freezing to constant weight. The five freeze-dried samples of each muscle were randomly selected and combined into one sample and then combined into three duplicate samples. The AOAC [15] method was used to determine the crude protein (Kjeldahl method) and ash heated at 550 °C to a constant weight. According to the GB 5009.6-2016 determination of fat in foods [16], the crude fat in the freeze-dried samples was extracted using the Soxhlet extraction method, and its content was determined. Based on the moisture, the dry weight of crude protein, crude fat, and ash were converted into the wet weight.
2.7. Fatty Acid Profile and Evaluation
The composition and content of carp muscle fatty acids were determined according to the peak area percentage method in the GB 5009.168-2016 determination of fatty acids in foods [17]. The results are presented as the percentage of each fatty acid with respect to the total fatty acids (%). The evaluation of the fatty acid quality was performed using the hypocholesterolemic/hypercholesterolemic ratio (h/H), index of atherogenicity (AI), and index of thrombogenicity (TI) [18], using Equations (4)–(6):
2.8. Amino Acid Analysis and Evaluation
The composition and content of carp muscle amino acids were analyzed according to the GB 5009.124-2016 method of determination of amino acids in foods [19]. The protein quality was evaluated using the amino acid score (AAS) and chemical score (CS) of essential amino acids (EAA). Meanwhile, the essential amino acid index (EAAI) was calculated. The AAS and CS were calculated in relation to a reference scoring pattern suggested by FAO/WHO/UNU [20]. The EAAI was calculated according to the equation described by Shahidi and Synowiecki [21]. The formulae are presented in the following Equations (7)–(9):
where A, B, … G refer to the contents of Ile, Leu, Lys, Thr, Val, sulfur-containing amino acids (Met + Cys), and aromatic amino acids (Phe + Tyr) in the tested protein (mg/g N, dry weight); AE, BE, … GE refer to the Ile, Leu, Lys, Thr, Val, sulfur-containing amino acids (Met + Cys), and aromatic amino acids (Phe + Tyr) in the egg protein pattern (mg/g N, dry weight); and n refers to the number of amino acids.
2.9. Molecular Expression Analysis
Trizol reagent was used to extract total RNA from the muscle and intestine samples. Subsequently, the extracted total RNA was quantified via spectrophotometry at 260/280 nm and verified using agarose gel electrophoresis. The first-strand cDNA was synthesized using the TaKaRa PrimeScript™ RT reagent Kit with the gDNA Eraser (RR047A, Bao bio engineering Co., Ltd., Dalian, China). Real-time quantitative PCR (qPCR) was conducted with the cDNA template and the TaKaRa SYBR® Premix Ex Taq™ (Tli RNaseH Plus) (RR0420A, Bao bio engineering Co., Ltd., Dalian, China), following the manufacturer’s protocol. The primers are listed in the Supplementary Materials, Table S2.
2.10. Statistical Analysis
The results are presented as the mean values ± standard error (SE). SPSS 22.0 software (SPSS Inc, Chicago, IL, USA) was used for the statistical analysis. The normality and homogeneity of the results were checked with Levene’s test. When necessary, arcsine square root or logarithmic transformation was performed prior to analysis. A one-way analysis of variance (ANOVA) was used to determine the differences between these treatments, using Duncan’s multiple range tests. p < 0.05 was regarded as the level of statistical significance.
3. Results
3.1. The Growth Performance
The effects on the growth performance of carp with different dietary protein levels are shown in Table 1. The SR was 100% among these five treatments, and no mortality was observed. The FBW increased with the increase in the dietary protein level up to 280 g/kg (p < 0.05), whereas the WG and SGR were significantly influenced and increased with the ascending dietary protein level up to 250 g/kg, beyond which no further increase was detected (p < 0.05).

Table 1.
Effects of different dietary protein levels on the growth performance of Cyprinus carpio haematopterus.
3.2. The Plasma Hematological Parameters and Enzyme Activities
The plasma hematological parameters and intestinal enzyme activities of the carp are presented in Table 2. No significant differences were observed in the contents of ALT, AST, GLO, TP, ALB, BUN, TC, TG, HDL-C, LDL-C, or TBA between any of the treatments (p > 0.05). A descending tendency of the ALP parameter, decreased from (281.58 ± 6.59) U/L to (115.12 ± 6.70) U/L, was detected via the increase in the dietary protein levels. Carp that were fed on the 340 g/kg protein diet presented the highest UA contents (28.89 ± 2.38 μmol/L), with a significant difference when compared with the other treatments (p < 0.05).

Table 2.
Effects of different dietary protein levels on the plasma hematological parameters of Cyprinus carpio haematopterus.
The intestinal digestive enzyme activity contents, including α-AMS, LPS, and TPS, showed a significant upward trend with the increase in the dietary protein level up to 280 g/kg, and then no further increase was detected (p < 0.05) (Table 3). The intestinal antioxidant enzyme activities of CAT and SOD first rose significantly with the increasing dietary protein level and then reduced when the dietary protein level increased above 280 g/kg (p < 0.05). Nevertheless, the MDA contents were significantly influenced, reducing with the ascending dietary protein level up to 280 g/kg, and then no further reduction was observed (p < 0.05).

Table 3.
Effects of different dietary protein levels on the intestinal enzyme activities of Cyprinus carpio haematopterus.
3.3. Proximate Composition
The results of the proximate composition illustrated that the dietary protein levels had significant effects on the contents of moisture, crude protein, and crude lipids in the carp muscle (p < 0.05) but not on the ash content (p > 0.05) (Table 4). The protein content maintained a high level with the increasing dietary protein level, but it significantly dropped when the dietary protein was 340 g/kg. A similar tendency was also observed in the crude lipid contents, which decreased significantly when the dietary protein increased to 250 and 310 g/kg (p < 0.05).

Table 4.
Effects of different dietary protein levels on the proximate composition of Cyprinus carpio haematopterus (%, wet weight).
3.4. Fatty Acids Profiles
The fatty acid compositions and contents of carp muscle with different dietary protein levels are presented in Table 5. Overall, the carp muscle contents of total saturated fatty acids (∑SFA), monounsaturated fatty acids (∑MUFA), polyunsaturated fatty acids (∑PUFA), and fatty acids (∑FA) decreased significantly with the ascending dietary protein levels, except for the 250 g/kg protein diet (p < 0.05). C16:0 was the dominant fatty acid within the SFA, while C18:1n9c was the most abundant fatty acid within the MUFA. C18:2n6c was the dominant fatty acid within the PUFA. A significant decrease in the ∑n-6 PUFA contents was noted for protein diets between 280 g/kg and 310 g/kg (p < 0.05). There was a significant decreasing tendency of the h/H parameters for the protein diets between the 280 g/kg and 310 g/kg (p < 0.05), whereas there was a significant increasing tendency of the AI parameter with the increasing dietary protein level (p < 0.05).

Table 5.
Effects of different dietary protein levels on the fatty acids and evaluation of Cyprinus carpio haematopterus (g/100g, dry weight).
3.5. Amino Acids Analysis
Table 6 illustrates the amino acid compositions and contents of carp muscle with different dietary protein levels. Only the glutamic acid and ∑EAA contents were significantly influenced by the ascending dietary protein levels (p < 0.05). However, the other 22 amino acid parameters were not significantly influenced by the increase in dietary protein levels among any of the treatments (p > 0.05). The results of the protein quality evaluation of the essential amino acids demonstrated that the dietary protein levels had no significant effect on the AAS, CS, or EAAI (p > 0.05) (Table 7). Meanwhile, the approximate values of the parameters were observed with an increase in the dietary protein level.

Table 6.
Effects of different dietary protein levels on the amino acid profile of the muscle of Cyprinus carpio haematopterus (g/100 g, dry weight).

Table 7.
Effects of different dietary protein levels on the protein quality evaluation of essential amino acids in Cyprinus carpio haematopterus.
3.6. Molecular Expression
3.6.1. Relative GH Expression
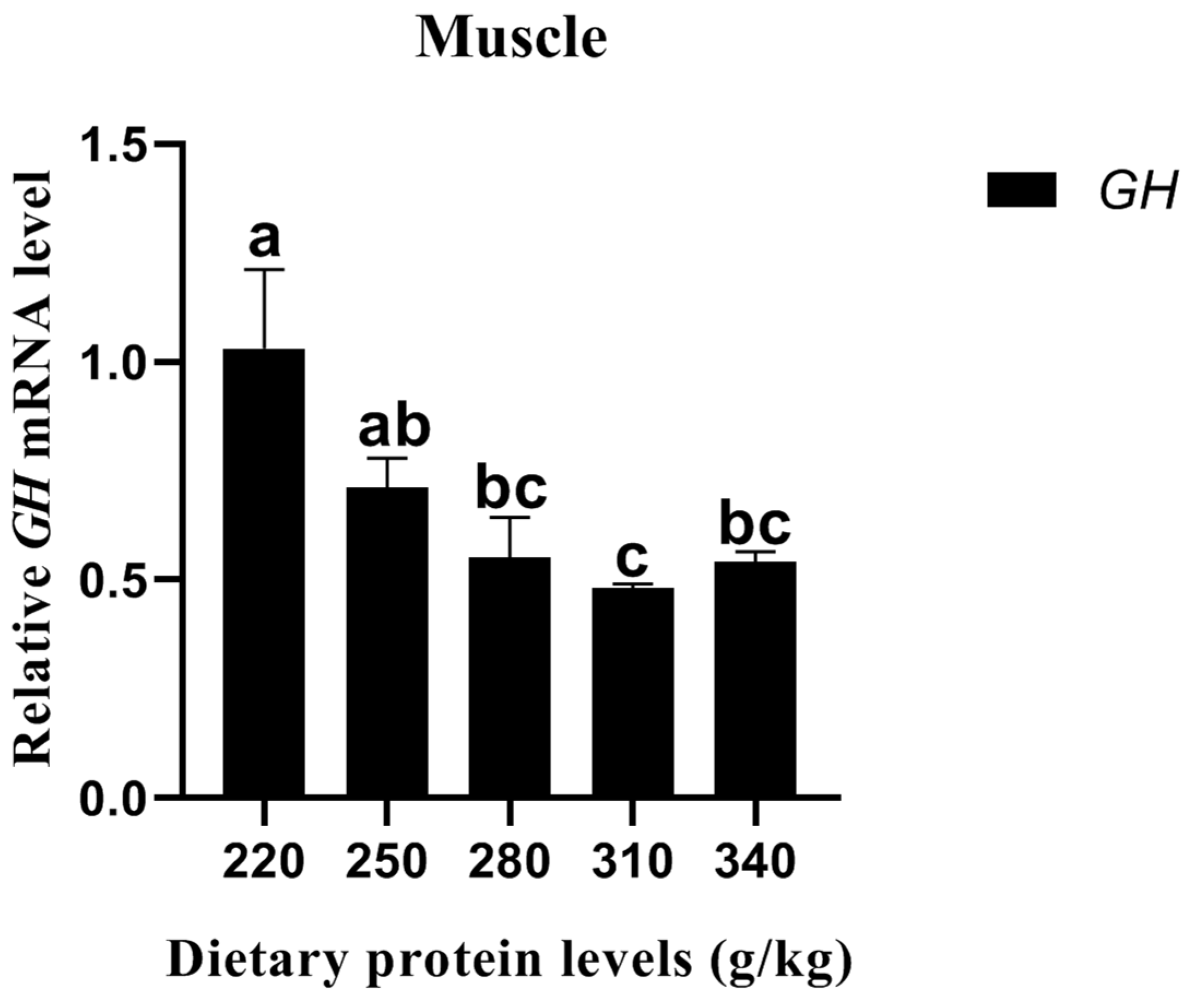
The relative GH expression of carp muscle significantly decreased with the increasing dietary protein level up to 310 g/kg, and then it significantly increased (p < 0.05) (Figure 1). No significant difference in relative GH expression was detected between the 280 g/kg and 340 g/kg protein diets (p > 0.05).

Figure 1.
Effects of different dietary protein levels on the GH gene expression of Cyprinus carpio haematopterus. Notes: Data are presented as means ± standard error (SE). Values with different superscripts (a, b and c) are significantly different (p < 0.05). Abbreviations: GH, growth hormone.
3.6.2. Relative TOR and 4EBP1 Expression of Protein Synthesis
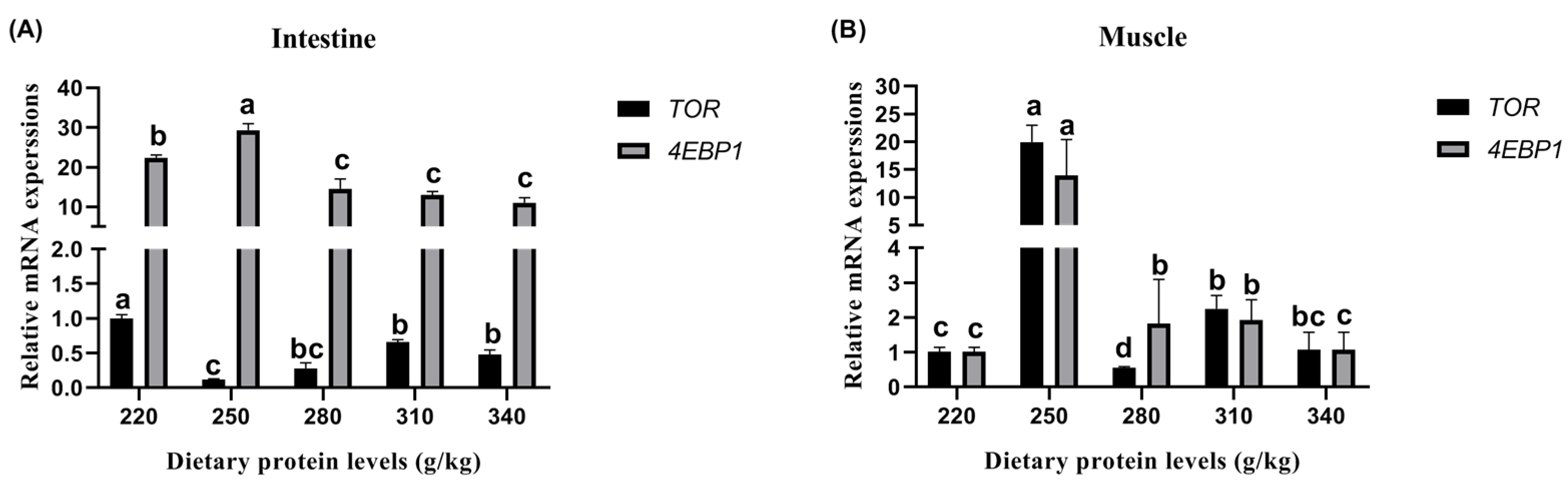
The results for the relative TOR and 4EBP1 expression of protein synthesis in the intestines and muscle are illustrated in Figure 2. In the intestines, the peak relative TOR expression was observed on the 220 g/kg protein diet (Figure 2A). Similarly, the relative 4EBP1 expression was significantly influenced by the increase in the dietary protein level up to a 250 g/kg (p < 0.05), and then no further increase was detected among the 280 g/kg, 310 g/kg, and 340 g/kg protein diets (p > 0.05). In the muscle, significant changes in TOR and 4EBP1 expression were observed with the increase in the dietary protein level from 220 g/kg to 250 g/kg (p < 0.05) (Figure 2B), and then the values dropped after the dietary protein level reached beyond 280 g/kg. The peak relative TOR and 4EBP1 expression levels were observed in the fish on the 250 g/kg protein diet.

Figure 2.
Effects of different dietary protein levels on the TOR and 4EBP1 gene expression of Cyprinus carpio haematopterus. Note: Data are presented as means ± standard error (SE). Values with different superscripts (a, b and c) are significantly different (p < 0.05). Abbreviations: TOR, target of rapamycin; 4EBP1, eukaryotic translation initiation factor 4E binding protein 1.
3.6.3. Relative Rhesus Glycoprotein Expression
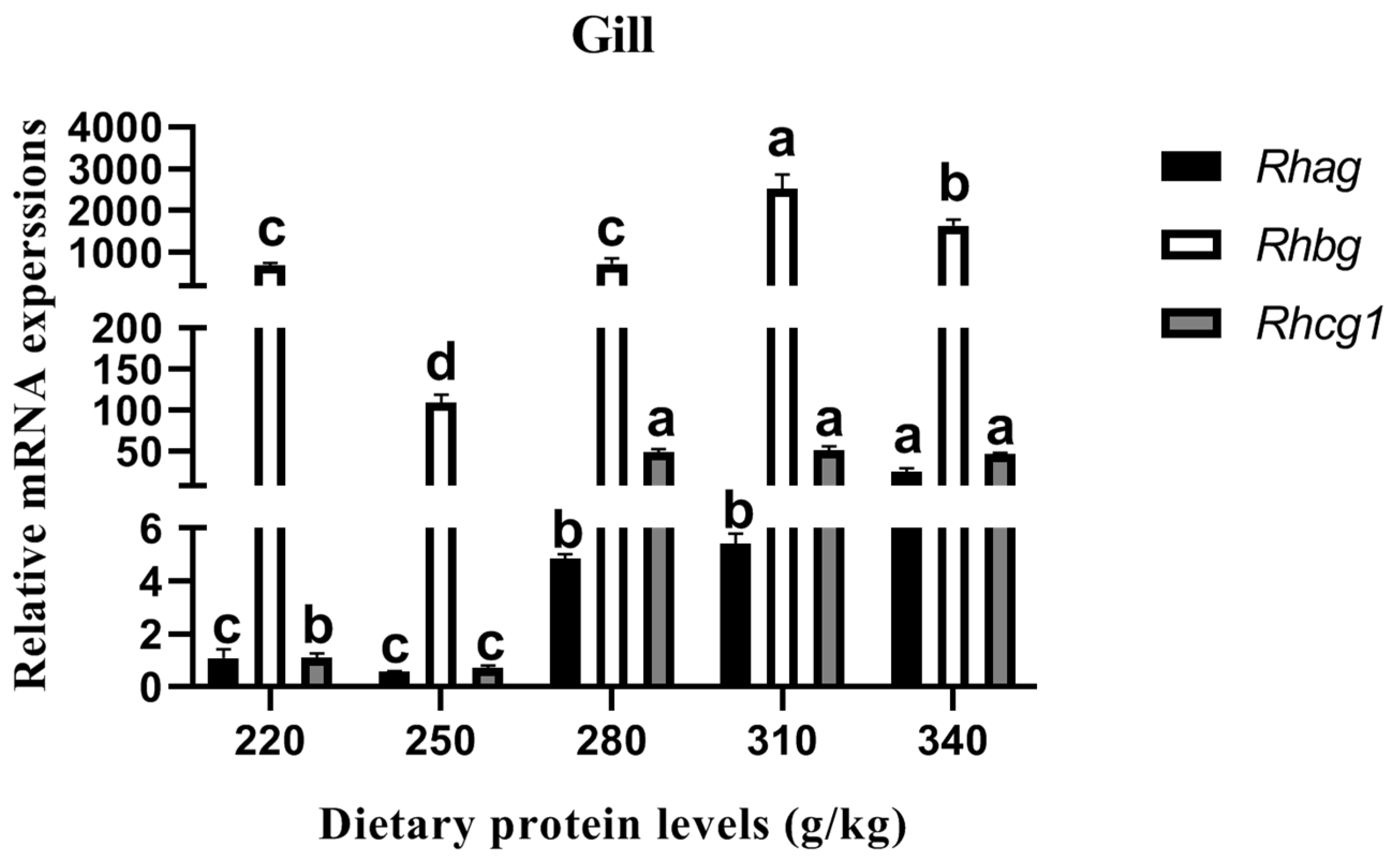
The relative Rhesus glycoproteins (RH) expression in the gills is shown in Figure 3. The relative Rhag, Rhbg, and Rhcg1 expression was downregulated with the increase in the dietary protein level from 220 g/kg to 250 g/kg, albeit that no significant difference in Rhag between the two treatments was found (p > 0.05). However, significantly upregulated relative Rhag, Rhbg, and Rhcg1 expression levels were observed with the increase in the dietary protein level from 250 g/kg to 310 g/kg (p < 0.05). Among these treatments, the relative Rhag expression on the 340 g/kg protein diet was higher than that of the other treatments (p < 0.05). Meanwhile, the maximum values of relative Rhbg and Rhcg1 expression were detected in the fish on the 310 g/kg protein diet.

Figure 3.
Effects of different dietary protein levels on the RH gene expression of Cyprinus carpio haematopterus. Note: Data are presented as means ± standard error (SE). Values with different superscripts (a, b and c) are significantly different (p < 0.05). Abbreviations: Rhag, Rhesus a glycoprotein; Rhbg, Rhesus b glycoprotein; Rhcg1, Rhesus c glycoprotein 1.
4. Discussion
The relatively low WG and SGR obtained in the present study were compared with the findings of published papers [14], which illustrated that there are some differences in growth performance between different carp varieties. In comparison with the same carp varieties [9,12], the IBM of large-sized Cyprinus carpio haematopterus was selected and found to affect the SGR and WG in the present study. The WG and SGR were first significantly enhanced with the increase in the dietary protein up to an optimal level, while thereafter, they slightly decreased, which illustrated that excessive dietary protein levels affect the nutrient utilization and feed efficiency [3], further resulting in the clear growth inhibition of fish [14]. Reductions in the WG and SGR with dietary protein levels beyond the optimal level have been observed in Ictalurus punctatus [22], Mystus nemurus [23], Tor putitora Hamilton [24], Scophthalmus maximus L. [25], Puntius gonionotus [26], Pagrus [27], and Acipenser baerii ♀ × A. gueldenstaedtii ♂ [28]. Carp may expend large amounts of energy to metabolize excess dietary protein, while the amount of energy for growth is substantially reduced, resulting in the disruption of growth [29]. Lower relative GH expression in the muscle may be another reason for the growth inhibition of Cyprinus carpio haematopterus. The present study illustrated that higher relative GH expression in the muscle was found in the fish on the 220–250 g/kg protein diet. A similar tendency was also observed in Carassius auratus gibelio [30]. The findings of the current study demonstrated that GH expression was significantly influenced by the ascending dietary protein level and significantly influenced the WG and SGR.
The TOR and 4EBP1 genes are involved in protein synthesis in the muscle and intestinal tissues of carp [14]. The present study demonstrated that a dietary protein level above 250 g/kg affected TOR and 4EBP1 expression and, in addition, protein synthesis. However, the crude protein and ∑TAA in the muscle were not significantly influenced by the dietary protein level of 220–310 g/kg. The aforementioned results may be explained by the following reasons: When the high protein diet was fed to Cyprinus carpio haematopterus, the exogenous food source of protein (their diet) was enough for the carp to accomplish the activities required for life. In contrast, when the low protein diet was fed to Cyprinus carpio haematopterus, the carp needed to improve their own endogenous protein synthesis in order to increase their protein deposition. Therefore, more endogenous biosynthetic pathways and proteins were promoted and accumulated via the high levels of TOR and 4EBP1 expression in the carp. A similar response pattern was also observed in Epinephelus lanceolatus [31] and Cyprinus carpio Songpu [14]. The ∑EAA contents showed an ascending tendency with the increase in dietary protein, indicating that the endogenous biosynthetic pathway can only synthesize the nonessential amino acids required by the carp, and the essential amino acids must be obtained via exogenous biological pathways such as the feed.
An interesting phenomenon was also noted, whereby the crude lipid and fatty acid contents of the muscle decreased significantly with the increase in dietary protein. These results were also observed in Bidyanus bidyanus [32], Epinephelus coioides [33], Pelteobagrus fulvidraco [34], and Sparus macrocephalus [35]. The important function of crude lipids is to supply energy. When the high protein diet was fed to the carp, the protein in the carp replaced the crude lipid oxidation energy supply. Therefore, the level of crude lipid accumulation gradually decreased.
For carp, one of the major functions of the gills is to excrete ammonia in active transport, which relies principally on the proteins expressed by the RH gene [36,37,38]. The present study demonstrated that the relative Rhag, Rhbg, and Rhcg1 expression was significantly upregulated with increase in the dietary protein level from 250 g/kg to 310 g/kg, implying that high amounts of dietary protein increased the ammonia metabolism of the carp. These results were also observed in Cyprinus carpio Songpu [14]. The plasma hematological parameters and intestinal enzyme activities of carp can reflect the state of ammonia metabolism in vivo. This study showed that the carp were in a relatively poor survival state when fed with a 340 g/kg protein diet. SOD and CAT can inhibit inflammatory factors [39], scavenge free radicals [40], improve immune function [41], and perform detoxification [42] in fish. Furthermore, MDA is a typical parameter that is used to reflect the oxidative damage of the body [43]. In this study, the activities of SOD and CAT in the serum of Cyprinus carpio haematopterus first increased and then decreased with an increase in the dietary protein level. The content of MDA first decreased and then increased. These results are consistent with findings on Cyprinus carpio [44,45] and Aristichthys nobilis [46], implying that the antioxidant capacity of Cyprinus carpio haematopterus will not be weakened by a reduction in dietary protein. The present study also illustrated that the α-AMS, LPS, and TPS activities of the Cyprinus carpio haematopterus intestine first increased and then decreased, results which were observed in previous studies [47,48]. These results demonstrated an increase in the digestive enzyme activities of the fish intestine with the appropriate dietary protein, and excessive protein intake damaged the fish digestive function [49].
5. Conclusions
Based on all of the aforementioned parameters, including the WG, SGR, plasma hematological parameters, intestinal enzyme activities, proximate composition, fatty acids, amino acids, and gene expression, the optimal dietary protein level for Cyprinus carpio haematopterus (160.24 ± 15.56 g) is 250–280 g/kg, which helps the fish to obtain a better growth performance, physicochemical indexes, and nutritional quality. Nevertheless, excessive protein intake from the diet will place an ammonia metabolic burden on carp and increase the cost of its culture. Additionally, this study is significant for the breeding of new carp varieties with low dietary protein levels.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani13071237/s1, Table S1: Formulation and proximate composition of the experimental diets (g/kg, dry matter); Table S2: The sequences of primers used in real-time quantitative PCR analysis.
Author Contributions
Conceptualization, S.W., L.S. and Z.J.; methodology, S.W., J.T. and Z.J.; software, S.W., X.J. and X.S.; validation, L.C., Y.G. and X.H.; formal analysis, J.T., X.J. and X.S.; investigation, S.W. and X.J.; resources, Z.J.; data curation, S.W., X.J. and C.L.; writing—original draft preparation, S.W.; writing—review and editing, S.W. and Z.J.; visualization, C.L., Y.G., X.H. and L.C.; supervision, L.S. and Z.J.; project administration, Z.J.; funding acquisition, L.S. and Z.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the earmarked fund of the China Agriculture Research System (CARS-45-07); Central Public Interest Scientific Institution Basal Research Fund, CAFS (2020TD31); and the Natural Science Foundation of Heilongjiang Province (TD2019C004).
Institutional Review Board Statement
The use of Cyprinus carpio haematopterus in the present study was reviewed and approved by the Committee for the Welfare and Ethics of Laboratory Animals of Heilongjiang River Fisheries Research Institute (CAFS) (approval number: 20210910-001 (approved on 5 September 2021)).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding authors.
Acknowledgments
We would like to thank all the students in our team for their help in the collection of samples.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAO. The State of World Fisheries and Aquaculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022; pp. 1–5. [Google Scholar]
- Yadav, A.K.; Mandal, S.C.; Patel, A.B.; Maurya, P.K. Evaluation of dietary protein requirement for the growth performance of minor carp, Cirrhinus reba (Hamilton, 1822) fingerlings. Aquac. Res. 2019, 50, 3343–3349. [Google Scholar] [CrossRef]
- Jin, Y.; Tian, L.X.; Xie, S.W.; Guo, D.Q.; Yang, H.J.; Liang, G.Y.; Liu, Y.J. Interactions between dietary protein levels, growth performance, feed utilization, gene expression and metabolic products in juvenile grass carp (Ctenopharyngodon idella). Aquaculture 2015, 437, 75–83. [Google Scholar] [CrossRef]
- Alam, M.S.; Watanabe, W.O.; Carroll, P.M. Dietary protein requirements of juvenile Black Sea Bass Centropristis striata. J. World Aquac. Soc. 2008, 39, 656–663. [Google Scholar] [CrossRef]
- Ye, W.; Han, D.; Zhu, X.; Yang, Y.; Jin, J.; Xie, S. Comparative study on dietary protein requirements for juvenile and pre-adult gible carp (Carassius auratus gibelio var. CASIII). Aquac. Nutr. 2016, 23, 755–765. [Google Scholar] [CrossRef]
- Wilson, R.P. Amino acids and proteins. In Fish Nutrition, 3rd ed.; Halver, J.E., Hardy, R.W., Eds.; Academic Press: Cambridge, MA, USA, 2002; pp. 143–179. [Google Scholar]
- Guo, L.; Jing, R.Z.; Cheng, Z.Y.; Sun, J.H.; Bai, D.Q.; Qiao, X.T. A preliminary study on decreasing feed protein of carp. Feed Ind. 2013, 34, 41–45. [Google Scholar]
- Bureau of Fisheries and Fishery Management, Ministry of Agriculture and Rural Affairs of China. 2021. China Fisheries Statistical Yearbook; Chinese Agricultural Press: Beijing, China, 2022; p. 25. [Google Scholar]
- Song, D.Y.; Yun, Y.H.; He, Z.J.; Mi, J.L.; Wang, L.M.; Jin, M.; Zhou, Q.C.; Nie, G.X. Fillet texture, physicochemical indexes, muscle cellularity and molecular expression in muscle of Yellow River carp (Cyprinus carpio haematopterus) in response to dietary hydroxyproline supplementation. Aquaculture 2022, 549, 737783. [Google Scholar] [CrossRef]
- National Research Council (NRC). Nutrient Requirements of Fish; National Academy Press: Washington, DC, USA, 1993; pp. 12–17. [Google Scholar]
- Tacon, A.G.J.; Metian, M. Global overview on the use of fish meal and fish oil in industrially compounded aquafeeds: Trends and future prospects. Aquaculture 2008, 285, 146–158. [Google Scholar] [CrossRef]
- Yun, Y.H.; Song, D.Y.; He, Z.J.; Mi, J.L.; Wang, L.M.; Nie, X.G. Effects of methionine supplementation in plant protein based diet on growth performance and fillet quality of juveniles Yellow River carp (Cyprinus carpio haematopterus). Aquaculture 2022, 549, 737810. [Google Scholar] [CrossRef]
- National Health Commission of the People’s Republic of China; State Administration for Market Regulation. GB/T 36782-2018. Formula Feed for Common Carp (Cyprinus carp), Standards Press of China: Beijing, China, 2018; pp. 1–6.
- Fan, Z.; Wu, D.; Li, J.N.; Zhang, Y.Y.; Xu, Q.Y.; Wang, L.S. Dietary protein requirement for large-size Songpu mirror carp (Cyprinus carpio Songpu). Aquac. Nutr. 2020, 26, 1748–1759. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of Association of Official Analytical Chemists, 16th ed.; Method: Arlington, VA, USA, 1995; p. 13. [Google Scholar]
- National Health Commission of the People’s Republic of China; State Administration for Market Regulation. GB 5009.6-2016. National Food Safety Standard-Determination of Fat in Food, Standards Press of China: Beijing, China, 2016; pp. 1–2.
- National Health Commission of the People’s Republic of China; State Administration for Market Regulation. GB 5009.168-2016. National Food Safety Standard-Determination of Fatty Acids in Food, Standards Press of China: Beijing, China, 2016; pp. 10–11.
- Śmietana, N.; Panicz, R.; Sobczak, M.; Śmietana, P.; Nędzarek, A. Spiny-Cheek Crayfish, Faxonius limosus (Rafinesque, 1817), as an alternative food source. Animals 2021, 11, 59. [Google Scholar] [CrossRef]
- National Health Commission of the People’s Republic of China; State Administration for Market Regulation. GB 5009.124-2016. National Food Safety Standard-Determination of Amino Acids in Food, Standards Press of China: Beijing, China, 2016; pp. 1–6.
- FAO/WHO/UNU. Protein and Amino Acid Requirements in Human Nutrition; WHO Technical Report Series; WHO: Geneva, Switzerland, 2020; Available online: https://www.who.int/nutrition/publications/nutrientrequirements/WHO_TRS_935/en/ (accessed on 8 August 2020).
- Shahidi, F.; Synowiecki, J. Nutrient composition of mechanically separated and surimi like seal meat. Food Chem. 1993, 47, 41–46. [Google Scholar] [CrossRef]
- Page, J.W. Interaction of dietary levels of protein and energy on channel catfish (Ictalurus punctatus). J. Nutr. 1973, 2, 1339–1346. [Google Scholar] [CrossRef]
- Khan, S.; Ang, K.J.; Ambak, M.A. The effect of varying dietary protein level on the growth, food conversion, protein utilization and body composition of tropical catfish Mystus nemurus (C. & V.) cultured in static pond water system. Aquac. Res. 1996, 27, 823–829. [Google Scholar]
- Islam, S.M.; Tanaka, M. Optimization of dietary protein requirement for pondreared masheer Tor putitora Hamilton (Cypriniformes: Cyprinidae). Aquac. Res. 2004, 35, 1270–1276. [Google Scholar] [CrossRef]
- Kim, K.; Wang, X.; Bai, S.C. Reevaluation of the dietary protein requirement of Japanese flounder Paralichthys olivaceus. J. World Aquac. Soc. 2003, 34, 133–139. [Google Scholar] [CrossRef]
- Mohanta, K.N.; Mohanty, S.N.; Jena, J.K.; Sahu, N.P. Protein requirement of silver barb, Puntius gonionotus fingerlings. Aquac. Nutr. 2008, 13, 143–152. [Google Scholar] [CrossRef]
- Schuchardt, D.; Vergara, J.M.; Fernández-Palacios, H.; Kalinowski, C.T.; Hernández-Cruz, C.M.; Izquierdo, M.S.; Robaina, L. Effects of different dietary protein and lipid levels on growth, feed utilization and body composition of red porgy (Pagrus pagrus) fingerlings. Aquac. Nutr. 2008, 14, 1–9. [Google Scholar] [CrossRef]
- Guo, Z.Q.; Zhu, X.M.; Liu, J.S.; Han, D.; Yang, Y.X.; Lan, Z.Q.; Xie, S.Q. Effects of dietary protein level on growth performance, nitrogen and energy budget of juvenile hybrid sturgeon, Acipenser baerii♀ × A. gueldenstaedtii♂. Aquaculture 2012, 338, 89–95. [Google Scholar] [CrossRef]
- Gan, L.; Liu, Y.J.; Tian, L.X.; Yang, H.J.; Yue, Y.R.; Chen, Y.J.; Liang, J.J.; Liang, G.Y. Effect of dietary protein reduction with lysine and methionine supplementation on growth performance, body composition and total ammonia nitrogen excretion of juvenile grass carp, Ctenopharyngodon idella. Aquac. Nutr. 2012, 18, 589–598. [Google Scholar] [CrossRef]
- Tu, Y.Q.; Xie, S.Q.; Han, D.; Yang, Y.X.; Jin, J.Y.; Liu, H.K.; Zhu, X.M. Growth performance, digestive enzyme, transaminase and GH-IGF-I axis gene responsiveness to different dietary protein levels in broodstock allogenogynetic gibel carp (Carassius auratus gibelio) CAS III. Aquaculture 2015, 446, 290–297. [Google Scholar] [CrossRef]
- Gao, Y.J.; Lu, S.D.; Wu, M.J.; Yao, W.; Jin, Z.B.; Wu, X.Y. Effects of dietary protein levels on growth, feed utilization and expression of growth related genes of juvenile giant grouper (Epinephelus lanceolatus). Aquaculture 2019, 504, 369–374. [Google Scholar] [CrossRef]
- Yang, S.; Liou, C.; Liu, F. Effects of dietary protein level on growth performance, carcass composition and ammonia excretion in juvenile silver perch (Bidyanus bidyanus). Aquaculture 2002, 213, 363–372. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, Y.; Mai, K.; Tian, L.; Liu, D.; Tan, X. Optimal dietary protein requirement of grouper Epinephelus coioides juveniles fed isoenergetic diets in floating net cages. Aquac. Nutr. 2004, 10, 247–252. [Google Scholar] [CrossRef]
- Ye, W.; Tan, X.; Chen, Y.; Luo, Z. Effects of dietary protein to carbohydrate ratios on growth and body composition of juvenile yellow catfish, Pelteobagrus fulvidraco (Siluriformes, Bagridae, Pelteobagrus). Aquac. Res. 2009, 40, 1410–1418. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, F.; Wang, L.L.; Shao, Q.; Xu, Z.; Xu, J. Dietary protein requirement of juvenile black sea bream, Sparus macrocephalus. J. World Aquac. Soc. 2010, 41, 151–164. [Google Scholar] [CrossRef]
- Nakada, T.; Westhoff, C.M.; Kato, A.; Hirose, S. Research communication ammonia secretion from fish gill depends on a set of Rh glycoproteins. FASEB J. 2007, 21, 1067–1074. [Google Scholar] [CrossRef]
- Weihrauch, D.; Wilkie, M.P.; Walsh, P.J. Ammonia and urea transporters in gills of fish and aquatic crustaceans. J. Exp. Biol. 2009, 212, 1716–1730. [Google Scholar] [CrossRef]
- Tu, H.Q.; Zhao, J.L.; Huang, S.Y.; Hao, Y.Y.; Cheng, Y.; Cao, X.Y. Ammonia transporter expression of Rh protein gen in gills of Nile Tilapia Oreochromis niloticus under stress of alkali. Fish. Sci. 2019, 38, 194–200. [Google Scholar]
- De Souza, R.R.; de Oliveira Paiva, P.D.; de Souza, A.R.; da Silva, R.R.; da Silva, D.P.C.; dos Reis, M.V.; Paiva, R. Morpho-anatomical changes and antioxidant enzyme activity during the acclimatization of Genipa americana. Acta Physiol. Plant. 2021, 43, 1–10. [Google Scholar] [CrossRef]
- Espinosa-Ruíz, C.; Esteban, M.Á. Wound-Induced Changes in Antioxidant Enzyme Activities in Skin Mucus and in Gene Expression in the Skin of Gilthead Seabream (Sparus aurata L.). Fishes 2021, 6, 15. [Google Scholar] [CrossRef]
- Heng, N.; Gao, S.; Chen, Y.; Wang, L.; Li, Z.; Guo, Y.; Sheng, X.H.; Wang, X.G.; Xing, K.; Xiao, L.F.; et al. Dietary supplementation with natural astaxanthin from Haematococcus pluvialis improves antioxidant enzyme activity, free radical scavenging ability, and gene expression of antioxidant enzymes in laying hens. Poultry Sci. 2021, 100, 101045. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, M.M.; Taha, N.M.; Lebda, M.A.; Elfeky, M.S.; Abdel-Latif, H.M. Effects of bovine lactoferrin and chitosan nanoparticles on serum biochemical indices, antioxidative enzymes, transcriptomic responses, and resistance of Nile tilapia against Aeromonas hydrophila. Fish Shellfish Immun. 2021, 111, 160–169. [Google Scholar] [CrossRef]
- Mu, Y.Y.; Shim, K.F.; Guo, J.Y. Effects of protein level in isocaloric diets on growth performance of the juvenile Chinese hairy crab, Eriocheir sinensis. Aquaculture 1998, 165, 139–148. [Google Scholar] [CrossRef]
- Sun, J.H.; Fan, Z.; Zhang, M.J.; Cheng, Z.Y.; Bai, D.Q.; Qiao, X.T. Effect of dietary protein level on hepatic function and antioxidant capacity of juvenile common carps (Cyprinus carpio). South China Fish. Sci. 2017, 13, 113–119. [Google Scholar]
- Ebrahimi, A.; Akrami, R.; Najdegerami, E.H.; Ghiasvand, Z.; Koohsari, H. Effects of different protein levels and carbon sources on water quality, antioxidant status and performance of common carp (Cyprinus carpio) juveniles raised in biofloc based system. Aquaculture 2020, 516, 734639. [Google Scholar] [CrossRef]
- Yu, H.; Ge, X.P.; Sun, S.M.; Zhu, J.; Ren, M.C.; Zhang, W.X.; Su, Y.L.; Mi, H.F. The effect of dietary protein level on the growth, digestive enzymes activities and antioxidant ability of the bighead carp (Aristichthys nobilis). J. Nanjing Agric. Univ. 2019, 42, 1158–1166. [Google Scholar]
- Ma, B.; Wang, L.; Lou, B.; Tan, P.; Xu, D.; Chen, R. Dietary protein and lipid levels affect the growth performance, intestinal digestive enzyme activities and related genes expression of juvenile small yellow croaker (Larimichthys polyactis). Aquac. Rep. 2020, 17, 100403. [Google Scholar] [CrossRef]
- Yue, H.; Huang, X.; Ruan, R.; Ye, H.; Li, Z.; Li, C. Effects of dietary protein levels on the growth, body composition, serum biochemistry and digestive enzyme activity in Chinese rice field eel (Monopterus albus) fingerlings. Aquac. Res. 2020, 51, 400–409. [Google Scholar] [CrossRef]
- Das, K.M.; Tripathi, S.D. Studies on the digestive enzymes of grass carp, Ctenopharyngodon idella (Val.). Aquaculture 1991, 92, 21–32. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).