Role of Prednisolone in Platelet Activation by Inhibiting TxA2 Generation through the Regulation of cPLA2 Phosphorylation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Platelet Preparation
2.4. Platelet Aggregation and Secretion Assay
2.5. Measurement of TxA2 Generation
2.6. Western Blot Analysis
2.7. Statistical Analysis
3. Results
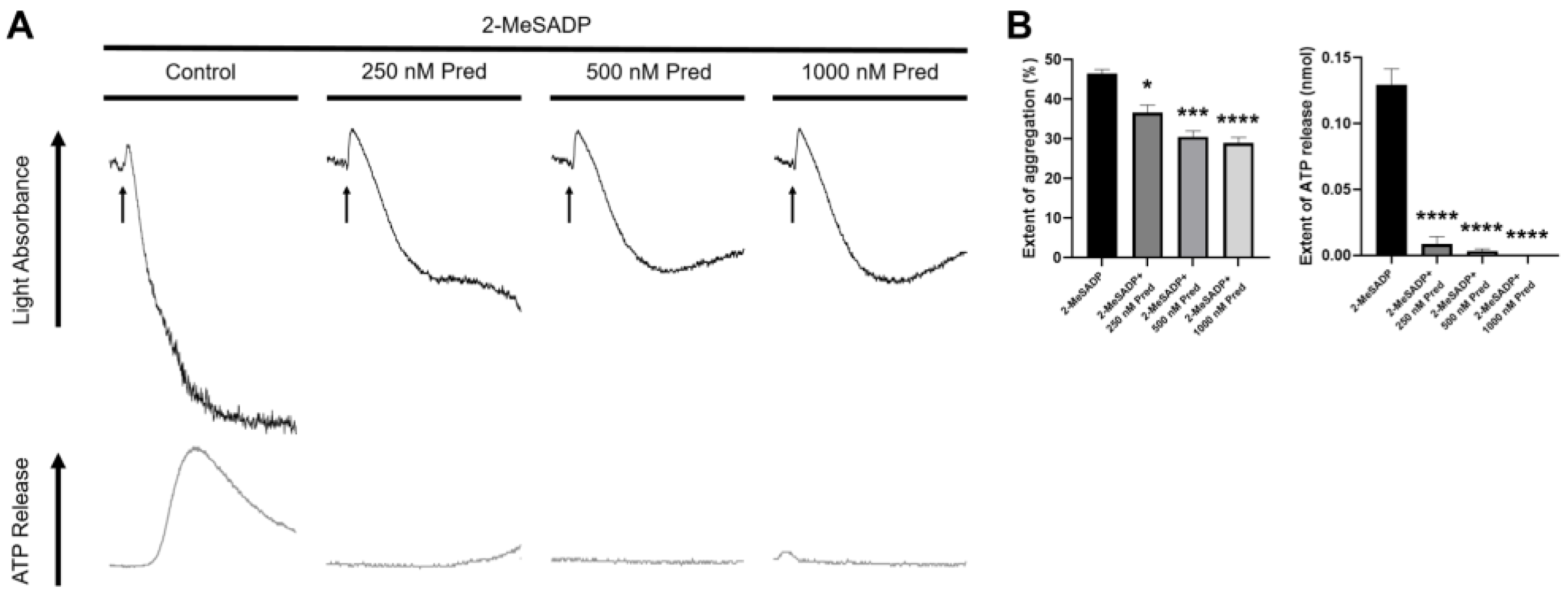
3.1. Prednisolone Regulates 2-MeSADP-Induced Secondary Wave of Platelet Aggregation and Secretion in Platelets
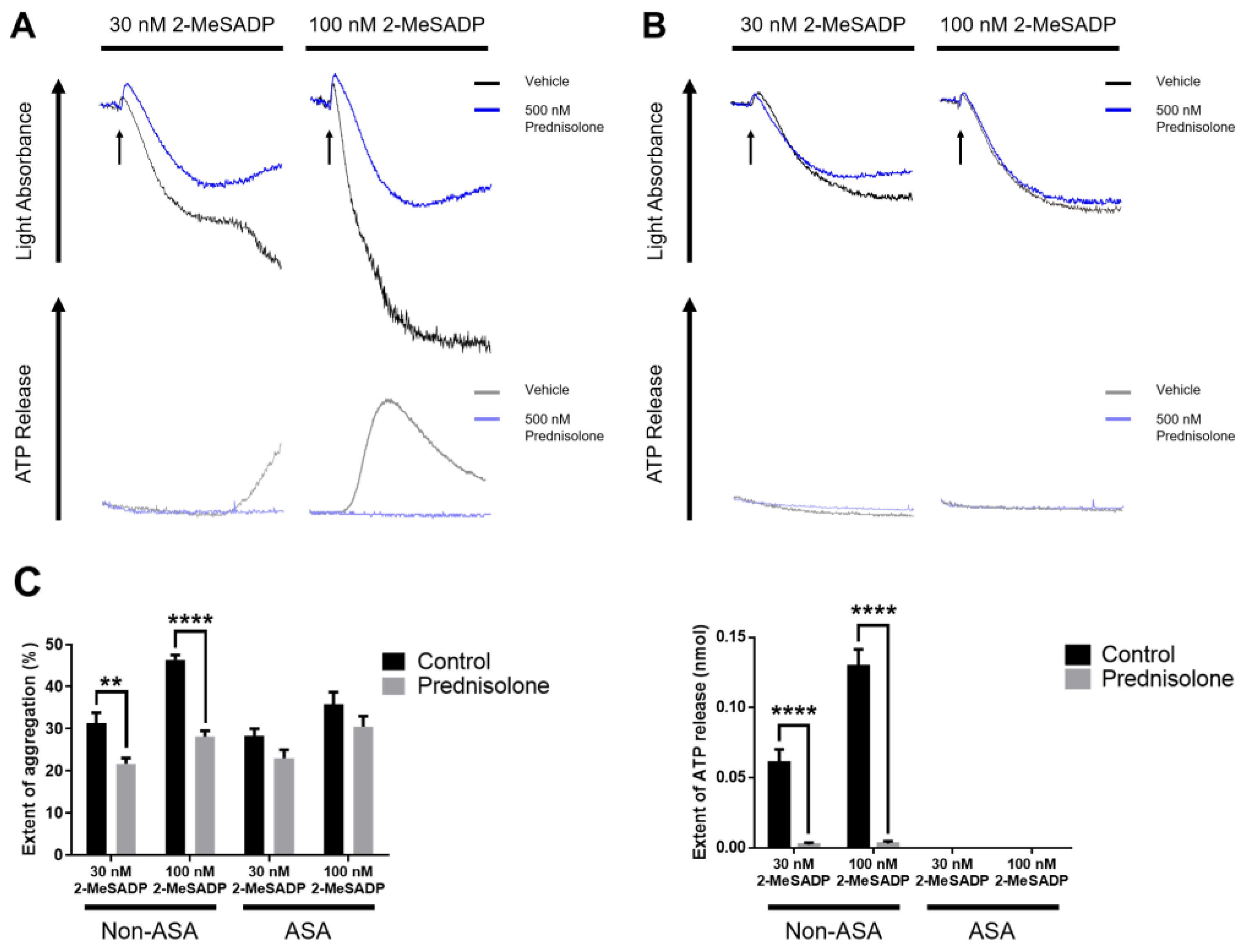
3.2. The Effect of Prednisolone in 2-MeSADP-Induced Platelet Function in Aspirinated Platelets
3.3. Prednisolone Regulates Only Low Concentrations of Thrombin-Induced Platelet Aggregation and Secretion
3.4. Prednisolone Inhibits 2-MeSADP- and Thrombin-Induced TxA2 Generation in Platelets
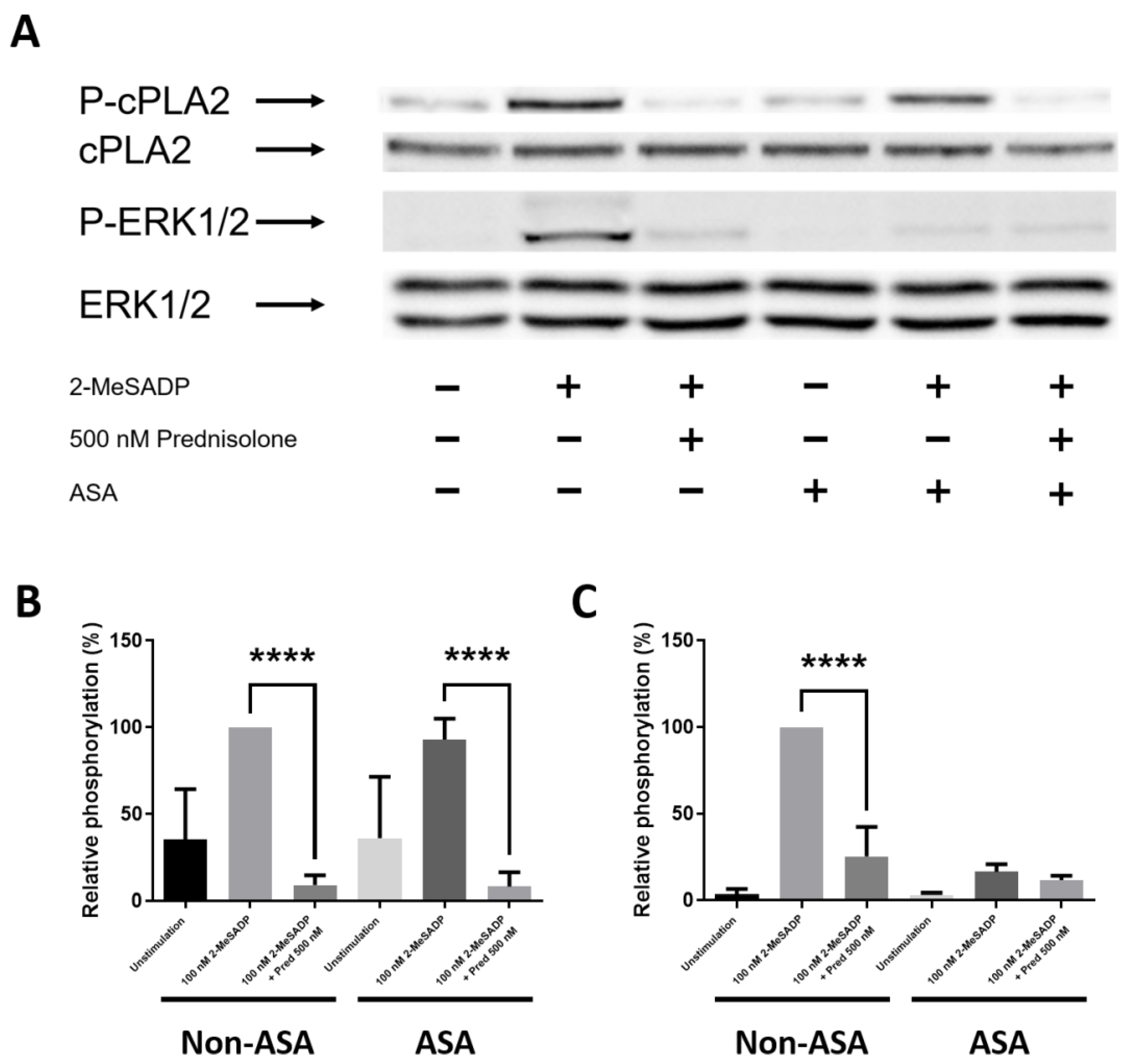
3.5. Prednisolone Inhibits 2-MeSADP-Induced cPLA2 and ERK Phosphorylation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barnes, P.J. Molecular mechanisms and cellular effects of glucocorticosteroids. Immunol. Allergy Clin. North Am. 2005, 25, 451–468. [Google Scholar] [CrossRef] [PubMed]
- Fardet, L.; Petersen, I.; Nazareth, I. Prevalence of long-term oral glucocorticoid prescriptions in the UK over the past 20 years. Rheumatology 2011, 50, 1982–1990. [Google Scholar] [CrossRef] [PubMed]
- Bénard-Laribière, A.; Pariente, A.; Pambrun, E.; Bégaud, B.; Fardet, L.; Noize, P. Prevalence and prescription patterns of oral glucocorticoids in adults: A retrospective cross-sectional and cohort analysis in France. BMJ Open 2017, 7, e015905. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Shang, N.; Zhang, Y.; Wang, D. Evaluation of Off-label Use of Oral Glucocorticoids in Outpatients. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2015, 37, 430–434. [Google Scholar] [PubMed]
- Chen, G.; Hu, W.; Xuan, N.; Fan, H.; Zhu, J.; Cui, W.; Zhang, G. Survey on the use of glucocorticoids in severe community-acquired pneumonia in intensive care unit of forty-five hospitals in Zhejiang Province. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2019, 31, 488–492. [Google Scholar]
- Gilor, C.; Graves, T.K. Interpretation of laboratory tests for canine Cushing’s syndrome. Top. Companion Anim. Med. 2011, 26, 98–108. [Google Scholar] [CrossRef]
- Graversen, D.; Vestergaard, P.; Stochholm, K.; Gravholt, C.; Jørgensen, J. Mortality in Cushing’s syndrome: A systematic review and meta-analysis. Eur. J. Intern. Med. 2012, 23, 278–282. [Google Scholar] [CrossRef]
- Wei, L.; MacDonald, T.M.; Walker, B.R. Taking glucocorticoids by prescription is associated with subsequent cardiovascular disease. Ann. Intern. Med. 2004, 141, 764–770. [Google Scholar] [CrossRef]
- Fardet, L.; Fève, B. Systemic glucocorticoid therapy: A review of its metabolic and cardiovascular adverse events. Drugs 2014, 74, 1731–1745. [Google Scholar] [CrossRef]
- Park, F.M.; Blois, S.L.; Abrams-Ogg, A.C.; Wood, R.D.; Allen, D.G.; Nykamp, S.G.; Downie, A. Hypercoagulability and ACTH-dependent hyperadrenocorticism in dogs. J. Vet. Intern. Med. 2013, 27, 1136–1142. [Google Scholar] [CrossRef]
- Casonato, A.; Pontara, E.; Boscaro, M.; Sonino, N.; Sartorello, F.; Ferasin, S.; Girolami, A. Abnormalities of von Willebrand factor are also part of the prothrombotic state of Cushing’s syndrome. Blood Coagul. Fibrinolysis 1999, 10, 145–151. [Google Scholar] [CrossRef]
- Erem, C.; Nuhoglu, I.; Yilmaz, M.; Kocak, M.; Demirel, A.; Ucuncu, O.; Ersoz, H.O. Blood coagulation and fibrinolysis in patients with Cushing’s syndrome: Increased plasminogen activator inhibitor-1, decreased tissue factor pathway inhibitor, and unchanged thrombin-activatable fibrinolysis inhibitor levels. J. Endocrinol. Investig. 2009, 32, 169–174. [Google Scholar] [CrossRef]
- Rose, L.; Dunn, M.E.; Bedard, C. Effect of canine hyperadrenocorticism on coagulation parameters. J. Vet. Intern. Med. 2013, 27, 207–211. [Google Scholar] [CrossRef]
- Jacoby, R.C.; Owings, J.T.; Ortega, T.; Gosselin, R.; Feldman, E.C. Biochemical basis for the hypercoagulable state seen in Cushing syndrome. Arch. Surg. 2001, 136, 1003–1007. [Google Scholar] [CrossRef]
- Wu, Y.-P.; Vink, T.; Schiphorst, M.; van Zanten, G.H.; IJsseldijk, M.J.; de Groot, P.G.; Sixma, J.J. Platelet thrombus formation on collagen at high shear rates is mediated by von Willebrand factor–glycoprotein Ib interaction and inhibited by von Willebrand factor–glycoprotein IIb/IIIa interaction. Arter. Thromb. Vasc. Biol. 2000, 20, 1661–1667. [Google Scholar] [CrossRef]
- Jin, J.; Kunapuli, S.P. Coactivation of two different G protein-coupled receptors is essential for ADP-induced platelet aggregation. Proc. Natl. Acad. Sci. USA 1998, 95, 8070–8074. [Google Scholar] [CrossRef]
- Offermanns, S. Activation of platelet function through G protein–coupled receptors. Circ. Res. 2006, 99, 1293–1304. [Google Scholar] [CrossRef]
- Knezevic, I.; Borg, C.; Le Breton, G.C. Identification of Gq as one of the G-proteins which copurify with human platelet thromboxane A2/prostaglandin H2 receptors. J. Biol. Chem. 1993, 268, 26011–26017. [Google Scholar] [CrossRef]
- Djellas, Y.; Manganello, J.M.; Antonakis, K.; Le Breton, G.C. Identification of Gα13 as one of the G-proteins that couple to human platelet thromboxane A2 receptors. J. Biol. Chem. 1999, 274, 14325–14330. [Google Scholar] [CrossRef]
- Channon, J.; Leslie, C.C. A calcium-dependent mechanism for associating a soluble arachidonoyl-hydrolyzing phospholipase A2 with membrane in the macrophage cell line RAW 264.7. J. Biol. Chem. 1990, 265, 5409–5413. [Google Scholar] [CrossRef]
- Kramer, R.M.; Roberts, E.F.; Um, S.L.; Börsch-Haubold, A.G.; Watson, S.P.; Fisher, M.J.; Jakubowski, J.A. p38 mitogen-activated protein kinase phosphorylates cytosolic phospholipase A2 (cPLA2) in thrombin-stimulated platelets: Evidence that proline-directed phosphorylation is not required for mobilization of arachidonic acid by cPLA2. J. Biol. Chem. 1996, 271, 27723–27729. [Google Scholar] [CrossRef] [PubMed]
- Shankar, H.; Garcia, A.; Prabhakar, J.; Kim, S.; Kunapuli, S. P2Y12 receptor-mediated potentiation of thrombin-induced thromboxane A2 generation in platelets occurs through regulation of Erk1/2 activation. J. Thromb. Haemost. 2006, 4, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.Z.; Jin, J.; Kunapuli, S.P. Molecular Mechanism of Thromboxane A2-induced Platelet Aggregation: ESSENTIAL ROLE FOR P2TAC and α2ARECEPTORS. J. Biol. Chem. 1999, 274, 29108–29114. [Google Scholar] [CrossRef] [PubMed]
- Fatti, L.; Bottasso, B.; Invitti, C.; Coppola, R.; Cavagnini, F.; Mannucci, P. Markers of activation of coagulation and fibrinolysis in patients with Cushing’s syndrome. J. Endocrinol. Investig. 2000, 23, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Patrassi, G.; Zanon, R.D.B.; Boscaro, M.; Martinelli, S.; Girolami, A. Further studies on the hypercoagulable state of patients with Cushing’s syndrome. Thromb. Haemost. 1985, 54, 518–520. [Google Scholar] [CrossRef]
- Manetti, L.; Bogazzi, F.; Giovannetti, C.; Raffaelli, V.; Genovesi, M.; Pellegrini, G.; Ruocco, L.; Iannelli, A.; Martino, E. Changes in coagulation indexes and occurrence of venous thromboembolism in patients with Cushing’s syndrome: Results from a prospective study before and after surgery. Eur. J. Endocrinol. 2010, 163, 783. [Google Scholar] [CrossRef]
- Curnow, J. Managing and supporting surgery in patients with bleeding disorders. Semin Thromb Hemost. 2017, 43, 653–671. [Google Scholar] [CrossRef]
- Casella, S.; Giudice, E.; Giannetto, C.; Marafioti, S.; Piccione, G. Effects of hydrocortisone and aminophylline on the aggregation of equine platelets in vitro. J. Vet. Sci. 2011, 12, 215–219. [Google Scholar] [CrossRef]
- Casella, S.; Giudice, E.; Alberghina, D.; Giannetto, C.; Marafioti, S.; Piccione, G. Hydrocortisone inhibition of adenosine diphosphate (ADP)-induced platelet aggregation in horse. Comp. Clin. Pathol. 2011, 20, 327–331. [Google Scholar] [CrossRef]
- Liverani, E.; Banerjee, S.; Roberts, W.; Naseem, K.M.; Perretti, M. Prednisolone exerts exquisite inhibitory properties on platelet functions. Biochem. Pharmacol. 2012, 83, 1364–1373. [Google Scholar] [CrossRef]
- Kim, S.; Cipolla, L.; Guidetti, G.; Okigaki, M.; Jin, J.; Torti, M.; Kunapuli, S.P. Distinct role of Pyk2 in mediating thromboxane generation downstream of both G12/13 and integrin αIIbβ3 in platelets. J. Biol. Chem. 2013, 288, 18194–18203. [Google Scholar] [CrossRef]
- Jin, J.; Quinton, T.M.; Zhang, J.; Rittenhouse, S.E.; Kunapuli, S.P. Adenosine diphosphate (ADP)–induced thromboxane A2generation in human platelets requires coordinated signaling through integrin αIIbβ3 and ADP receptors. Blood 2002, 99, 193–198. [Google Scholar] [CrossRef]
- Garcia, A.; Kim, S.; Bhavaraju, K.; Schoenwaelder, S.M.; Kunapuli, S.P. Role of phosphoinositide 3-kinase β in platelet aggregation and thromboxane A2 generation mediated by Gi signalling pathways. Biochem. J. 2010, 429, 369–377. [Google Scholar] [CrossRef]
- Chung, A.W.; Jurasz, P.; Hollenberg, M.D.; Radomski, M.W. Mechanisms of action of proteinase-activated receptor agonists on human platelets. Br. J. Pharmacol. 2002, 135, 1123. [Google Scholar] [CrossRef]
- Braun, M.; Kramann, J.; Strobach, H.; Schrör, K. Incomplete inhibition of platelet secretion by low-dose aspirin. Platelets 1994, 5, 325–331. [Google Scholar] [CrossRef]
- Yacoub, D.; Théorêt, J.-F.; Villeneuve, L.; Abou-Saleh, H.; Mourad, W.; Allen, B.G.; Merhi, Y. Essential role of protein kinase Cδ in platelet signaling, αIIbβ3 activation, and thromboxane A2 release. J. Biol. Chem. 2006, 281, 30024–30035. [Google Scholar] [CrossRef]
- Ronchetti, S.; Ayroldi, E.; Ricci, E.; Gentili, M.; Migliorati, G.; Riccardi, C. A glance at the use of glucocorticoids in rare inflammatory and autoimmune diseases: Still an indispensable pharmacological tool? Front. Immunol. 2021, 11, 613435. [Google Scholar] [CrossRef]
- Williams, D.M. Clinical pharmacology of corticosteroids. Respir. Care 2018, 63, 655–670. [Google Scholar] [CrossRef]
- Woolcock, A.; Keenan, A.; Cheung, C.; Christian, J.; Moore, G. Thrombocytosis in 715 Dogs (2011–2015). J. Vet. Intern. Med. 2017, 31, 1691–1699. [Google Scholar] [CrossRef]
- Filkova, A.A.; Martyanov, A.A.; Garzon Dasgupta, A.K.; Panteleev, M.A.; Sveshnikova, A.N. Quantitative dynamics of reversible platelet aggregation: Mathematical modelling and experiments. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Nakano, Y.; Oshima, T.; Matsuura, H.; Kajiyama, G.; Kambe, M. Effect of 17β-estradiol on inhibition of platelet aggregation in vitro is mediated by an increase in NO synthesis. Arter. Thromb. Vasc. Biol. 1998, 18, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Moro, L.; Reineri, S.; Piranda, D.; Pietrapiana, D.; Lova, P.; Bertoni, A.; Graziani, A.; Defilippi, P.; Canobbio, I.; Torti, M. Nongenomic effects of 17β-estradiol in human platelets: Potentiation of thrombin-induced aggregation through estrogen receptor β and Src kinase. Blood 2005, 105, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Blackmore, P.F. Progesterone metabolites rapidly stimulate calcium influx in human platelets by a src-dependent pathway. Steroids 2008, 73, 738–750. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J. Sex hormones and glucocorticoids: Interactions with the immune system. Ann. N. Y. Acad. Sci. 1999, 876, 102–118. [Google Scholar] [CrossRef]
- Bereshchenko, O.; Bruscoli, S.; Riccardi, C. Glucocorticoids, sex hormones, and immunity. Front. Immunol. 2018, 9, 1332. [Google Scholar] [CrossRef]
- Zucker, M.B.; Peterson, J. Inhibition of adenosine diphosphate-induced secondary aggregation and other platelet functions by acetylsalicylic acid ingestion. Proc. Soc. Exp. Biol. Med. 1968, 127, 547–551. [Google Scholar] [CrossRef]
- Savage, B.; Cattaneo, M.; Ruggeri, Z.M. Mechanisms of platelet aggregation. Curr. Opin. Hematol. 2001, 8, 270–276. [Google Scholar] [CrossRef]
- Kim, S.; Foster, C.; Lecchi, A.; Quinton, T.M.; Prosser, D.M.; Jin, J.; Cattaneo, M.; Kunapuli, S.P. Protease-activated receptors 1 and 4 do not stimulate Gi signaling pathways in the absence of secreted ADP and cause human platelet aggregation independently of Gisignaling. Blood 2002, 99, 3629–3636. [Google Scholar] [CrossRef]
- Dolan–O’Keefe, M.; Nick, H.S. Inhibition of cytoplasmic phospholipase A2 expression by glucocorticoids in rat intestinal epithelial cells. Gastroenterology 1999, 116, 855–864. [Google Scholar] [CrossRef]
- Goppelt-Struebe, M.; Wolter, D.; Resch, K. Glucocorticoids inhibit prostaglandin synthesis not only at the level of phospholipase A2 but also at the level of cyclo-oxygenase/PGE isomerase. Br. J. Pharmacol. 1989, 98, 1287–1295. [Google Scholar] [CrossRef]
- Liu, N.-K.; Xu, X.-M. Phospholipase A 2 and its Molecular Mechanism after Spinal Cord Injury. Mol. Neurobiol. 2010, 41, 197–205. [Google Scholar] [CrossRef]
- Qiu, J.; Wang, P.; Jing, Q.; Zhang, W.; Li, X.; Zhong, Y.; Sun, G.; Pei, G.; Chen, Y. Rapid activation of ERK1/2 mitogen-activated protein kinase by corticosterone in PC12 cells. Biochem. Biophys. Res. Commun. 2001, 287, 1017–1024. [Google Scholar] [CrossRef]
- Li, X.; Qiu, J.; Wang, J.; Zhong, Y.; Zhu, J.; Chen, Y. Corticosterone-induced rapid phosphorylation of p38 and JNK mitogen-activated protein kinases in PC12 cells. FEBS Lett. 2001, 492, 210–214. [Google Scholar] [CrossRef]
- Zhang, T.; Shi, W.L.; Tasker, J.G.; Zhou, J.R.; Peng, Y.L.; Miao, C.Y.; Yang, Y.J.; Jiang, C.L. Dexamethasone induces rapid promotion of norepinephrine-mediated vascular smooth muscle cell contraction. Mol. Med. Rep. 2013, 7, 549–554. [Google Scholar] [CrossRef]
- Rider, L.G.; Hirasawa, N.; Santini, F.; Beaven, M.A. Activation of the mitogen-activated protein kinase cascade is suppressed by low concentrations of dexamethasone in mast cells. J. Immunol. 1996, 157, 2374–2380. [Google Scholar] [CrossRef]
- Garcia, A.; Shankar, H.; Murugappan, S.; Kim, S.; Kunapuli, S.P. Regulation and functional consequences of ADP receptor-mediated ERK2 activation in platelets. Biochem. J. 2007, 404, 299–308. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, P.; Zhu, L.; Li, N.; Hu, H. P2X1-initiated p38 signalling enhances thromboxane A2-induced platelet secretion and aggregation. Thromb. Haemost. 2014, 112, 142–150. [Google Scholar]
- Luo, X.; Feng, H.; Jiang, L.; Chen, Q. Abnormal uterine bleeding induced by glucocorticoid treatment for pemphigus: Case series and proposed treatment algorithm. Saudi Med. J. 2016, 37, 1025. [Google Scholar] [CrossRef]
- Bauerle, K.T.; Harris, C. Glucocorticoids and diabetes. Mo. Med. 2016, 113, 378. [Google Scholar]
- Narum, S.; Westergren, T.; Klemp, M. Corticosteroids and risk of gastrointestinal bleeding: A systematic review and meta-analysis. BMJ Open 2014, 4, e004587. [Google Scholar] [CrossRef]
- Nagata, N.; Kojima, K.; Yuki, M. Comparison of survival times for dogs with pituitary-dependent hyperadrenocorticism in a primary-care hospital: Treated with trilostane versus untreated. J. Vet. Intern. Med. 2017, 31, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Mc Bride, M.; Crespo, I.; Webb, S.M.; Valassi, E. Quality of life in Cushing’s syndrome. Best Pr. Res. Clin. Endocrinol. Metab. 2021, 35, 101505. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Chaudhary, P.K.; Kim, S. Role of Prednisolone in Platelet Activation by Inhibiting TxA2 Generation through the Regulation of cPLA2 Phosphorylation. Animals 2023, 13, 1299. https://doi.org/10.3390/ani13081299
Kim S, Chaudhary PK, Kim S. Role of Prednisolone in Platelet Activation by Inhibiting TxA2 Generation through the Regulation of cPLA2 Phosphorylation. Animals. 2023; 13(8):1299. https://doi.org/10.3390/ani13081299
Chicago/Turabian StyleKim, Sanggu, Preeti Kumari Chaudhary, and Soochong Kim. 2023. "Role of Prednisolone in Platelet Activation by Inhibiting TxA2 Generation through the Regulation of cPLA2 Phosphorylation" Animals 13, no. 8: 1299. https://doi.org/10.3390/ani13081299
APA StyleKim, S., Chaudhary, P. K., & Kim, S. (2023). Role of Prednisolone in Platelet Activation by Inhibiting TxA2 Generation through the Regulation of cPLA2 Phosphorylation. Animals, 13(8), 1299. https://doi.org/10.3390/ani13081299




