Multilineage Differentiation Potential of Equine Adipose-Derived Stromal/Stem Cells from Different Sources
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection, Isolation and Cultivation of Equine ASCs
2.2. Proliferation Assay
2.3. Trilineage Differentiation Potential
2.3.1. Adipogenic Differentiation
Glycerol 3-Phosphat Dehydrogenase Assay
Nile Red Staining and Lipid/Nuclei-Ratio
2.3.2. Osteogenic Differentiation
Alkaline Phosphatase Activity Measurement
Von Kossa Staining and Index of Osteogenic Differentiation
2.3.3. Chondrogenic Differentiation
Hematoxylin–Eosin and Alcian Blue Staining
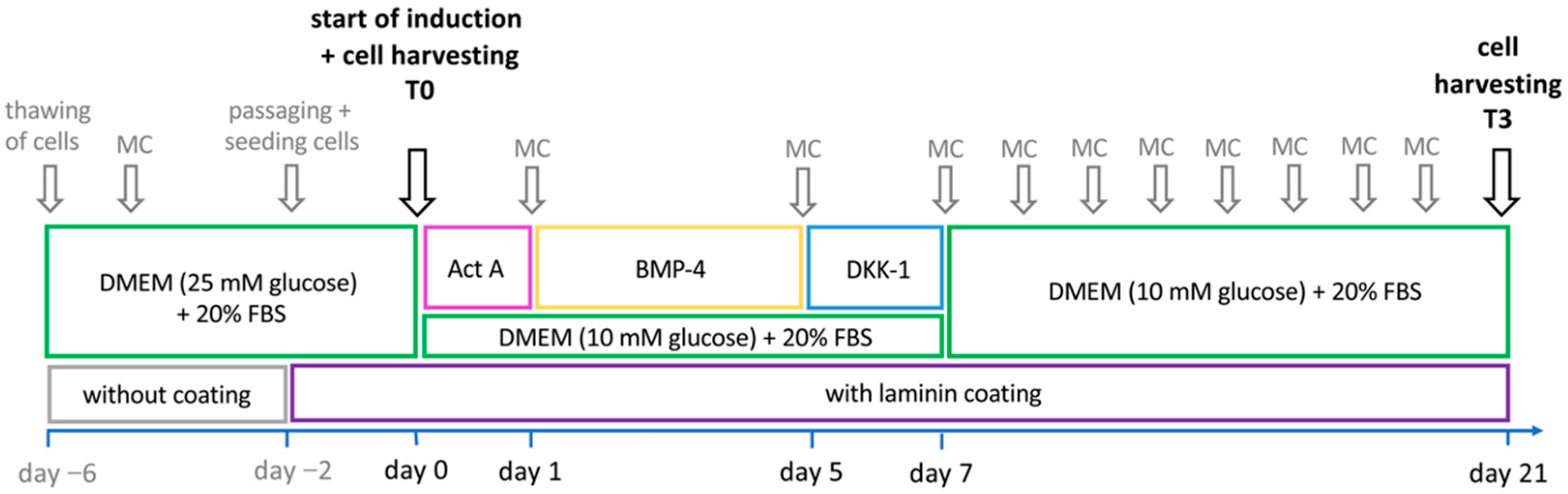
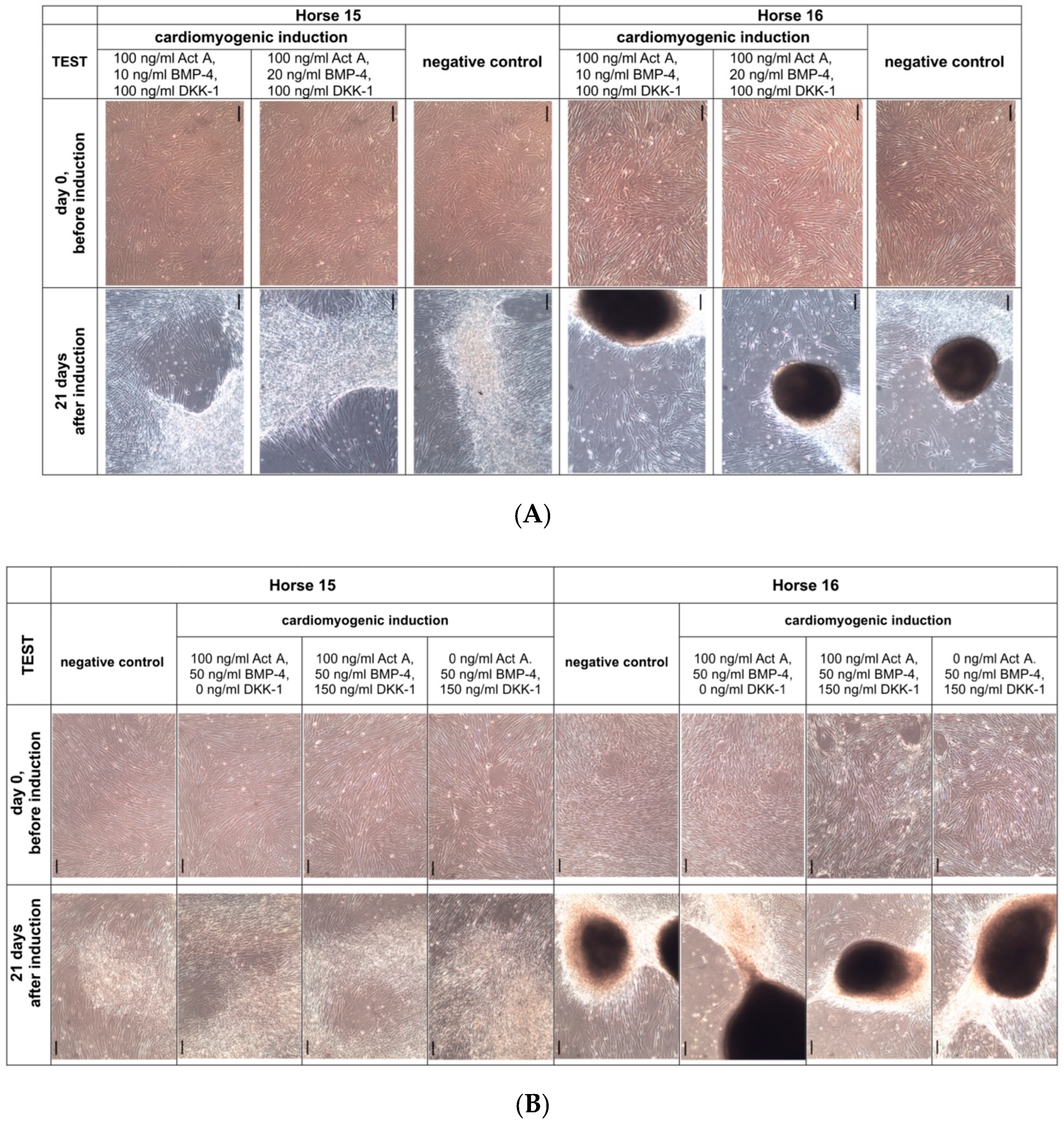
2.4. Cardiomyogenic Differentiation
2.5. Statistical Analysis
3. Results
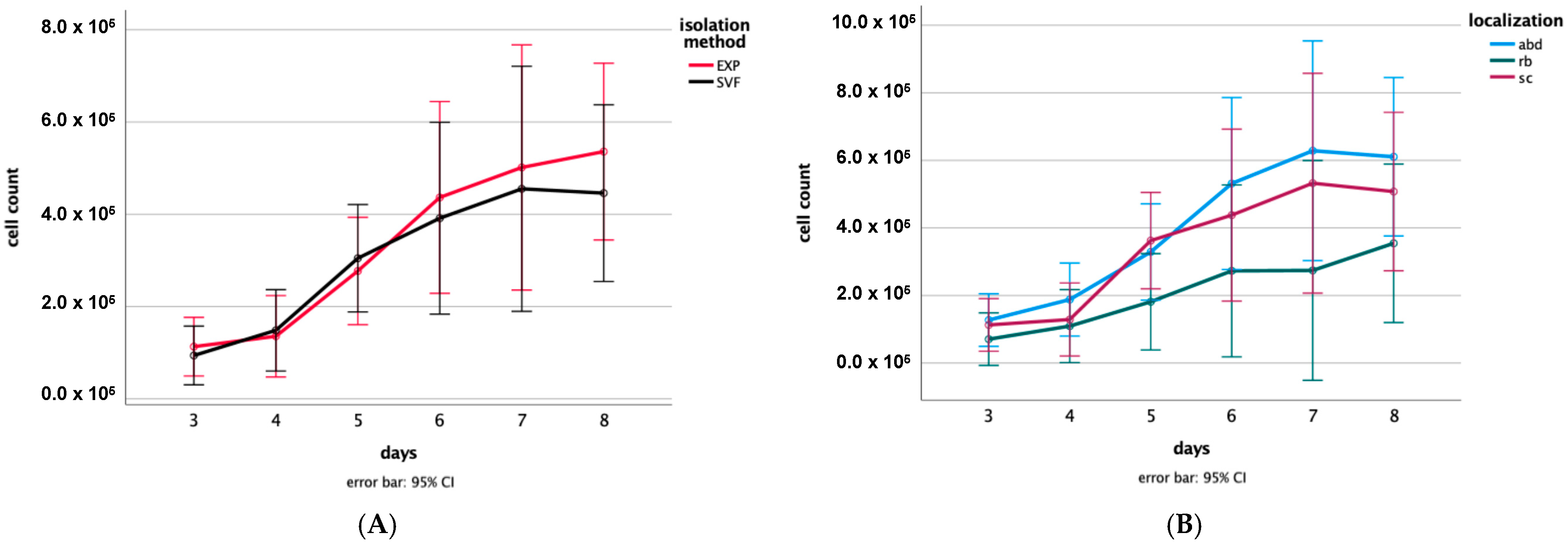
3.1. Proliferation Assay
3.2. Trilineage Differentiation Potential
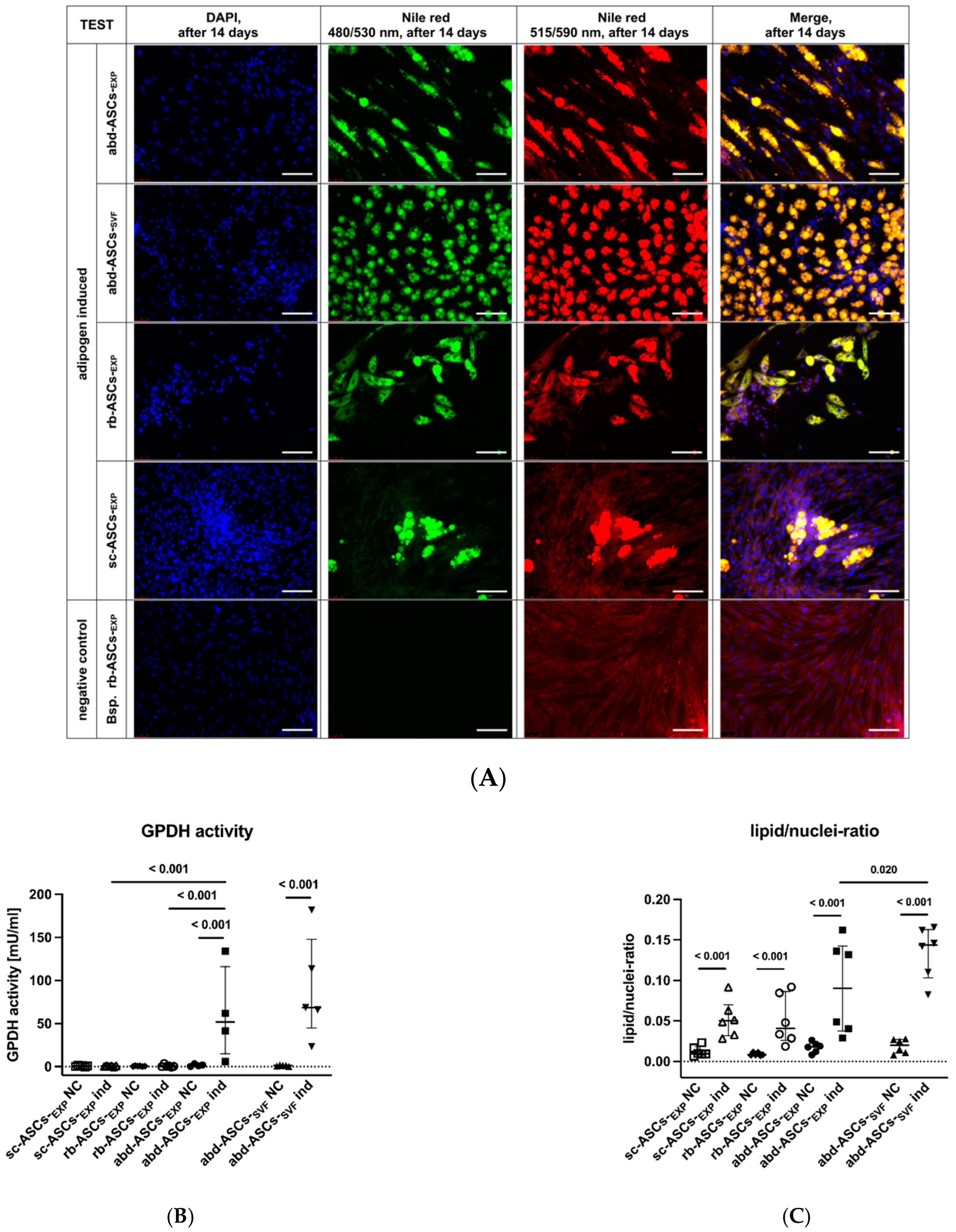
3.2.1. Adipogenic Differentiation
Glycerol-3-Phosphate Dehydrogenase Assay
Nile Red Staining and Lipid/Nuclei-Ratio
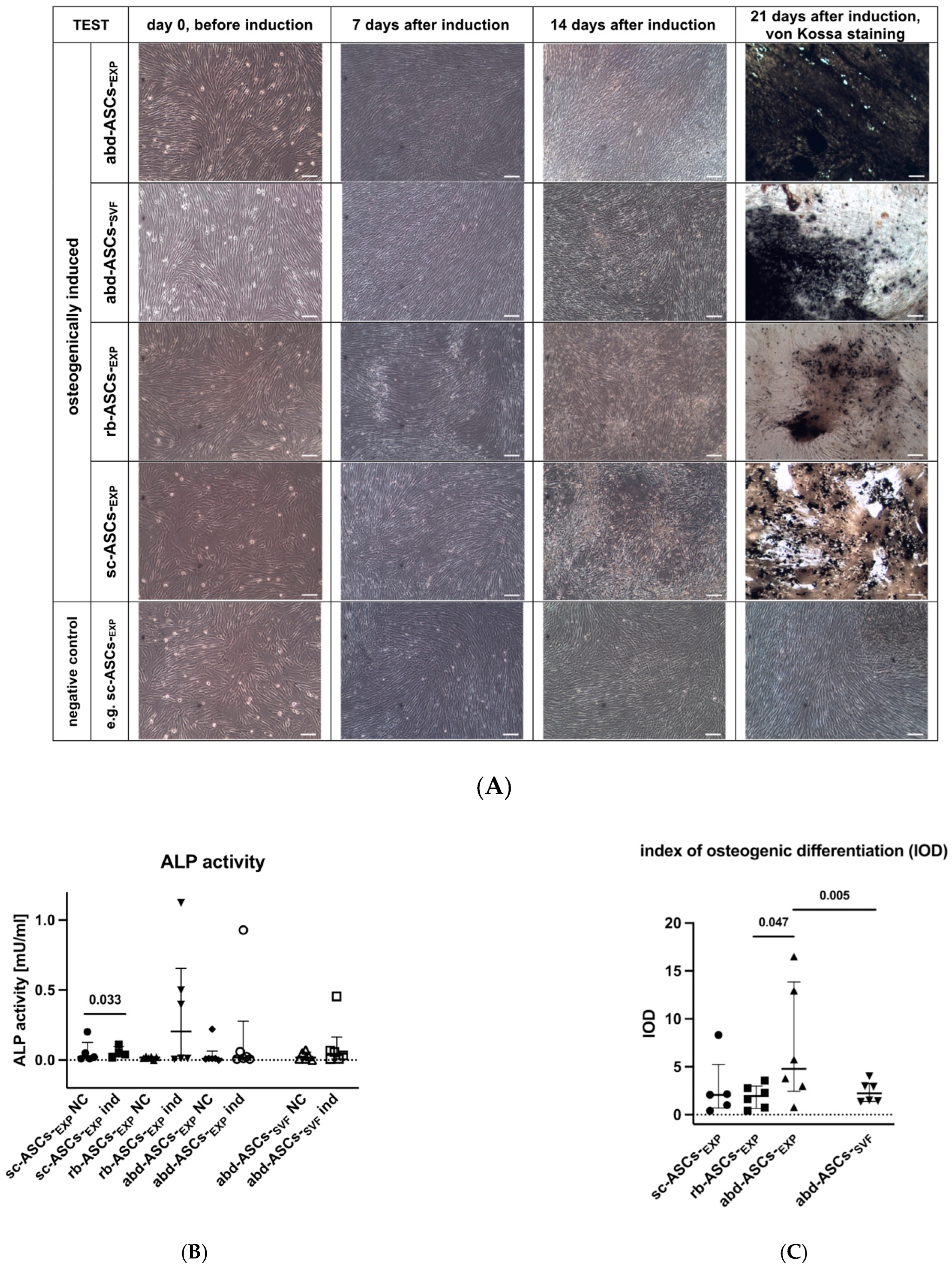
3.2.2. Osteogenic Differentiation
Alkaline Phosphatase Activity Measurement
Index of Osteogenic Differentiation
3.2.3. Chondrogenic Differentiation
3.3. Cardiomyogenic Differentiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friedenstein, A.; Chailakhjan, R.; Lalykina, K. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Prolif. 1970, 3, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Petrakova, K.V.; Kurolesova, A.I.; Frolova, G.P. Heterotopic transplants of bone marrow. Transplantation 1968, 6, 230–247. [Google Scholar] [CrossRef] [PubMed]
- Gang, E.J.; Jeong, J.A.; Hong, S.H.; Hwang, S.H.; Kim, S.W.; Yang, I.H.; Ahn, C.; Han, H.; Kim, H. Skeletal myogenic differentiation of mesenchymal stem cells isolated from human umbilical cord blood. Stem Cells 2004, 22, 617–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rangappa, S.; Fen, C.; Lee, E.H.; Bongso, A.; Wei, E.S.K. Transformation of adult mesenchymal stem cells isolated from the fatty tissue into cardiomyocytes. Ann. Thorac. Surg. 2003, 75, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Cho, H.H.; Cho, Y.B.; Park, J.S.; Jeong, H.S. Functional neural differentiation of human adipose tissue-derived stem cells using bFGF and forskolin. BMC Cell Biol. 2010, 11, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, K.; Laco, F.; Ramakrishna, S.; Liao, S.; Chan, C.K. Differentiation of bone marrow-derived mesenchymal stem cells into multi-layered epidermis-like cells in 3D organotypic coculture. Biomaterials 2009, 30, 3251–3258. [Google Scholar] [CrossRef] [PubMed]
- Choudhery, M.S.; Mahmood, R.; Harris, D.T.; Ahmad, F.J. Minimum criteria for defining induced mesenchymal stem cells. Cell Biol. Int. 2022, 46, 986–989. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yang, J.; Fang, J.; Zhou, Y.; Candi, E.; Wang, J.; Hua, D.; Shao, C.; Shi, Y. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal Transduct. Target. Ther. 2022, 7, 92. [Google Scholar] [CrossRef] [PubMed]
- English, K.; Mahon, B.P.; Wood, K.J. Mesenchymal stromal cells; role in tissue repair, drug discovery and immune modulation. Curr. Drug Deliv. 2014, 11, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Ryu, N.-E.; Lee, S.-H.; Park, H. Spheroid culture system methods and applications for mesenchymal stem cells. Cells 2019, 8, 1620. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Zhang, J.; Li, J.; Li, M.; Ge, J.; Wu, P.; You, B.; Qian, H. Roles of mesenchymal stem cell-derived exosomes in cancer development and targeted therapy. Stem Cells Int. 2021, 2021, 9962194. [Google Scholar] [CrossRef] [PubMed]
- Bobis, S.; Jarocha, D.; Majka, M. Mesenchymal stem cells: Characteristics and clinical applications. Folia Histochem. Cytobiol. 2006, 44, 215–230. [Google Scholar]
- Burk, J.; Ribitsch, I.; Gittel, C.; Juelke, H.; Kasper, C.; Staszyk, C.; Brehm, W. Growth and differentiation characteristics of equine mesenchymal stromal cells derived from different sources. Vet. J. 2013, 195, 98–106. [Google Scholar] [CrossRef]
- Hillmann, A.; Ahrberg, A.B.; Brehm, W.; Heller, S.; Josten, C.; Paebst, F.; Burk, J. Comparative Characterization of Human and Equine Mesenchymal Stromal Cells: A Basis for Translational Studies in the Equine Model. Cell Transplant. 2016, 25, 109–124. [Google Scholar] [CrossRef] [Green Version]
- Paebst, F.; Piehler, D.; Brehm, W.; Heller, S.; Schroeck, C.; Tarnok, A.; Burk, J. Comparative immunophenotyping of equine multipotent mesenchymal stromal cells: An approach toward a standardized definition. Cytom. A 2014, 85, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Arévalo-Turrubiarte, M.; Olmeo, C.; Accornero, P.; Baratta, M.; Martignani, E. Analysis of mesenchymal cells (MSCs) from bone marrow, synovial fluid and mesenteric, neck and tail adipose tissue sources from equines. Stem Cell Res. 2019, 37, 101442. [Google Scholar] [CrossRef]
- Gale, A.L.; Linardi, R.L.; McClung, G.; Mammone, R.M.; Ortved, K.F. Comparison of the chondrogenic differentiation potential of equine synovial membrane-derived and bone marrow-derived mesenchymal stem cells. Front. Vet. Sci. 2019, 6, 178. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-H.; Joo, S.-D.; Han, S.-B.; Im, J.; Lee, S.-H.; Sonn, C.H.; Lee, K.-M. Isolation and expansion of synovial CD34− CD44+ CD90+ mesenchymal stem cells: Comparison of an enzymatic method and a direct explant technique. Connect. Tissue Res. 2011, 52, 226–234. [Google Scholar] [CrossRef]
- Dhar, M.; Neilsen, N.; Beatty, K.; Eaker, S.; Adair, H.; Geiser, D. Equine peripheral blood-derived mesenchymal stem cells: Isolation, identification, trilineage differentiation and effect of hyperbaric oxygen treatment. Equine Vet. J. 2012, 44, 600–605. [Google Scholar] [CrossRef]
- Koerner, J.; Nesic, D.; Romero, J.D.; Brehm, W.; Mainil-Varlet, P.; Grogan, S.P. Equine peripheral blood-derived progenitors in comparison to bone marrow-derived mesenchymal stem cells. Stem Cells 2006, 24, 1613–1619. [Google Scholar] [CrossRef] [PubMed]
- Si, Z.; Wang, X.; Sun, C.; Kang, Y.; Xu, J.; Wang, X.; Hui, Y. Adipose-derived stem cells: Sources, potency, and implications for regenerative therapies. Biomed. Pharmacother. 2019, 114, 108765. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.J.; Fu, R.H.; Shyu, W.C.; Liu, S.P.; Jong, G.P.; Chiu, Y.W.; Wu, H.S.; Tsou, Y.A.; Cheng, C.W.; Lin, S.Z. Adipose-derived stem cells: Isolation, characterization, and differentiation potential. Cell Transplant. 2013, 22, 701–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jumabay, M.; Zhang, R.; Yao, Y.; Goldhaber, J.I.; Bostrom, K.I. Spontaneously beating cardiomyocytes derived from white mature adipocytes. Cardiovasc. Res. 2010, 85, 17–27. [Google Scholar] [CrossRef] [Green Version]
- Khazaei, S.; Keshavarz, G.; Bozorgi, A.; Nazari, H.; Khazaei, M. Adipose tissue-derived stem cells: A comparative review on isolation, culture, and differentiation methods. Cell Tissue Bank. 2021, 23, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Arnhold, S.; Elashry, M.I.; Klymiuk, M.C.; Geburek, F. Investigation of stemness and multipotency of equine adipose-derived mesenchymal stem cells (ASCs) from different fat sources in comparison with lipoma. Stem Cell Res. Ther. 2019, 10, 309. [Google Scholar] [CrossRef]
- Braun, J.; Hack, A.; Weis-Klemm, M.; Conrad, S.; Treml, S.; Kohler, K.; Walliser, U.; Skutella, T.; Aicher, W.K. Evaluation of the osteogenic and chondrogenic differentiation capacities of equine adipose tissue-derived mesenchymal stem cells. Am. J. Vet. Res. 2010, 71, 1228–1236. [Google Scholar] [CrossRef]
- Gittel, C.; Brehm, W.; Burk, J.; Juelke, H.; Staszyk, C.; Ribitsch, I. Isolation of equine multipotent mesenchymal stromal cells by enzymatic tissue digestion or explant technique: Comparison of cellular properties. BMC Vet. Res. 2013, 9, 221. [Google Scholar] [CrossRef] [Green Version]
- Priya, N.; Sarcar, S.; Majumdar, A.S.; SundarRaj, S. Explant culture: A simple, reproducible, efficient and economic technique for isolation of mesenchymal stromal cells from human adipose tissue and lipoaspirate. J. Tissue Eng. Regen. Med. 2014, 8, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, M.A.; Jurek, S.; Trappe, S.; Kolisek, M.; Sponder, G.; Aschenbach, J.R. Influence of Bovine Serum Lipids and Fetal Bovine Serum on the Expression of Cell Surface Markers in Cultured Bovine Preadipocytes. Cells Tissues Organs 2017, 204, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.L.B.K.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Câmara, D.A.D.; Shibli, J.A.; Müller, E.A.; De-Sá-Junior, P.L.; Porcacchia, A.S.; Blay, A.; Lizier, N.F. Adipose tissue-derived stem cells: The biologic basis and future directions for tissue engineering. Materials 2020, 13, 3210. [Google Scholar] [CrossRef]
- Czapla, J.; Matuszczak, S.; Kulik, K.; Wiśniewska, E.; Pilny, E.; Jarosz-Biej, M.; Smolarczyk, R.; Sirek, T.; Zembala, M.O.; Zembala, M. The effect of culture media on large-scale expansion and characteristic of adipose tissue-derived mesenchymal stromal cells. Stem Cell Res. Ther. 2019, 10, 235. [Google Scholar] [CrossRef] [Green Version]
- Hua, J.; Gong, J.; Meng, H.; Xu, B.; Yao, L.; Qian, M.; He, Z.; Zou, S.; Zhou, B.; Song, Z. Comparison of different methods for the isolation of mesenchymal stem cells from umbilical cord matrix: Proliferation and multilineage differentiation as compared to mesenchymal stem cells from umbilical cord blood and bone marrow. Cell Biol. Int. 2013, 38, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Penny, J.; Harris, P.; Shakesheff, K.M.; Mobasheri, A. The biology of equine mesenchymal stem cells: Phenotypic characterization, cell surface markers and multilineage differentiation. Front. Biosci. 2012, 17, 892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, C.H.; Lee, Y.G.; Shin, W.H.; Kim, H.; Chai, J.W.; Jeong, E.C.; Kim, J.E.; Shim, H.; Shin, J.S.; Shin, I.S.; et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: A proof-of-concept clinical trial. Stem Cells 2014, 32, 1254–1266. [Google Scholar] [CrossRef] [PubMed]
- Latief, N.; Raza, F.A.; Bhatti, F.U.; Tarar, M.N.; Khan, S.N.; Riazuddin, S. Adipose stem cells differentiated chondrocytes regenerate damaged cartilage in rat model of osteoarthritis. Cell Biol. Int. 2016, 40, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.K.; Korda, M.; Blunn, G.W.; Goodship, A.E. Isolation and implantation of autologous equine mesenchymal stem cells from bone marrow into the superficial digital flexor tendon as a potential novel treatment. Equine Vet. J. 2003, 35, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, V.J.; Scheffer, C.J.; Genn, H.J.; Hoogendoorn, A.C.; Greve, J.W. Clinical follow-up of horses treated with allogeneic equine mesenchymal stem cells derived from umbilical cord blood for different tendon and ligament disorders. Vet. Q. 2014, 34, 92–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Govoni, K.E. HORSE SPECIES SYMPOSIUM: Use of mesenchymal stem cells in fracture repair in horses. J. Anim. Sci. 2015, 93, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Milner, P.I.; Clegg, P.D.; Stewart, M.C. Stem cell-based therapies for bone repair. Vet. Clin. N. Am. Equine Pract. 2011, 27, 299–314. [Google Scholar] [CrossRef]
- Ribitsch, I.; Oreff, G.L.; Jenner, F. Regenerative Medicine for Equine Musculoskeletal Diseases. Animals 2021, 11, 234. [Google Scholar] [CrossRef]
- Bahmad, H.F.; Daouk, R.; Azar, J.; Sapudom, J.; Teo, J.C.; Abou-Kheir, W.; Al-Sayegh, M. Modeling adipogenesis: Current and future perspective. Cells 2020, 9, 2326. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, P.H.; Daibert, A.P.F.; Monteiro, B.S.; Okano, B.S.; Carvalho, J.L.; Cunha, D.N.Q.d.; Favarato, L.S.C.; Pereira, V.G.; Augusto, L.E.F.; Carlo, R.J.D. Differentiation of adipose tissue-derived mesenchymal stem cells into cardiomyocytes. Arq. Bras. Cardiol. 2013, 100, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Hasani, S.; Javeri, A.; Asadi, A.; Taha, M.F. Cardiac Differentiation of Adipose Tissue-Derived Stem Cells Is Driven by BMP4 and bFGF but Counteracted by 5-Azacytidine and Valproic Acid. Cell J. 2020, 22, 273. [Google Scholar] [PubMed]
- Ibarra-Ibarra, B.R.; Franco, M.; Paez, A.; López, E.V.; Massó, F. Improved efficiency of cardiomyocyte-like cell differentiation from rat adipose tissue-derived mesenchymal stem cells with a directed differentiation protocol. Stem Cells Int. 2019, 2019, 8940365. [Google Scholar] [CrossRef]
- Jiang, A.; Chen, Y.; Shi, L.; Li, F. Differentiation of brown adipose-derived stem cells into cardiomyocyte-like cells is regulated by a combination of low 5-azacytidine concentration and bone morphogenetic protein 4. Int. J. Clin. Exp. Pathol. 2018, 11, 5514. [Google Scholar]
- Yang, J.; Song, T.; Wu, P.; Chen, Y.; Fan, X.; Chen, H.; Zhang, J.; Huang, C. Differentiation potential of human mesenchymal stem cells derived from adipose tissue and bone marrow to sinus node-like cells. Mol. Med. Rep. 2012, 5, 108–113. [Google Scholar] [CrossRef]
- Kattman, S.J.; Witty, A.D.; Gagliardi, M.; Dubois, N.C.; Niapour, M.; Hotta, A.; Ellis, J.; Keller, G. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell 2011, 8, 228–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Später, D.; Hansson, E.M.; Zangi, L.; Chien, K.R. How to make a cardiomyocyte. Development 2014, 141, 4418–4431. [Google Scholar] [CrossRef] [Green Version]
- Schultheiss, T.M.; Burch, J.B.; Lassar, A.B. A role for bone morphogenetic proteins in the induction of cardiac myogenesis. Genes Dev. 1997, 11, 451–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, E.D.; Wang, Z.; Lepore, J.J.; Lu, M.M.; Taketo, M.M.; Epstein, D.J.; Morrisey, E.E. Wnt/β-catenin signaling promotes expansion of Isl-1–positive cardiac progenitor cells through regulation of FGF signaling. J. Clin. Investig. 2007, 117, 1794–1804. [Google Scholar] [CrossRef] [Green Version]
- Paige, S.L.; Osugi, T.; Afanasiev, O.K.; Pabon, L.; Reinecke, H.; Murry, C.E. Endogenous Wnt/β-catenin signaling is required for cardiac differentiation in human embryonic stem cells. PLoS ONE 2010, 5, e11134. [Google Scholar] [CrossRef]
- Gupta, S.; Sharma, A.; Verma, R.S. Mesenchymal Stem Cells for Cardiac Regeneration: From Differentiation to Cell Delivery. Stem Cell Rev. Rep. 2021, 17, 1666–1694. [Google Scholar] [CrossRef] [PubMed]
- Léobon, B.; Roncalli, J.; Joffre, C.; Mazo, M.; Boisson, M.; Barreau, C.; Calise, D.; Arnaud, E.; André, M.; Pucéat, M. Adipose-derived cardiomyogenic cells: In vitro expansion and functional improvement in a mouse model of myocardial infarction. Cardiovasc. Res. 2009, 83, 757–767. [Google Scholar] [CrossRef] [Green Version]
- Miyahara, Y.; Nagaya, N.; Kataoka, M.; Yanagawa, B.; Tanaka, K.; Hao, H.; Ishino, K.; Ishida, H.; Shimizu, T.; Kangawa, K.; et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat. Med. 2006, 12, 459–465. [Google Scholar] [CrossRef] [Green Version]
- Paitazoglou, C.; Bergmann, M.W.; Vrtovec, B.; Chamuleau, S.A.J.; van Klarenbosch, B.; Wojakowski, W.; Michalewska-Włudarczyk, A.; Gyöngyösi, M.; Ekblond, A.; Haack-Sørensen, M.; et al. Rationale and design of the European multicentre study on Stem Cell therapy in IschEmic Non-treatable Cardiac diseasE (SCIENCE). Eur. J. Heart Fail. 2019, 21, 1032–1041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trachsel, D.S.; Stage, H.J.; Rausch, S.; Trappe, S.; Söllig, K.; Sponder, G.; Merle, R.; Aschenbach, J.R.; Gehlen, H. Comparison of Sources and Methods for the Isolation of Equine Adipose Tissue-Derived Stromal/Stem Cells and Preliminary Results on Their Reaction to Incubation with 5-Azacytidine. Animals 2022, 12, 2049. [Google Scholar] [CrossRef]
- Alipour, F.; Parham, A.; Mehrjerdi, H.K.; Dehghani, H. Equine adipose-derived mesenchymal stem cells: Phenotype and growth characteristics, gene expression profile and differentiation potentials. Cell J. 2015, 16, 456. [Google Scholar] [PubMed]
- Jurek, S.; Sandhu, M.A.; Trappe, S.; Bermúdez-Peña, M.C.; Kolisek, M.; Sponder, G.; Aschenbach, J.R. Optimizing adipogenic transdifferentiation of bovine mesenchymal stem cells: A prominent role of ascorbic acid in FABP4 induction. Adipocyte 2020, 9, 35–50. [Google Scholar] [CrossRef] [Green Version]
- Becker, S.K.; Sponder, G.; Sandhu, M.A.; Trappe, S.; Kolisek, M.; Aschenbach, J.R. The combined influence of magnesium and insulin on central metabolic functions and expression of genes involved in magnesium homeostasis of cultured bovine adipocytes. Int. J. Mol. Sci. 2021, 22, 5897. [Google Scholar] [CrossRef] [PubMed]
- Grogan, S.P.; Barbero, A.; Winkelmann, V.; Rieser, F.; Fitzsimmons, J.S.; O’driscoll, S.; Martin, I.; Mainil-Varlet, P. Visual histological grading system for the evaluation of in vitro–generated neocartilage. Tissue Eng. 2006, 12, 2141–2149. [Google Scholar] [CrossRef] [Green Version]
- Marvin, M.J.; Di Rocco, G.; Gardiner, A.; Bush, S.M.; Lassar, A.B. Inhibition of Wnt activity induces heart formation from posterior mesoderm. Genes Dev. 2001, 15, 316–327. [Google Scholar] [CrossRef] [Green Version]
- Samuel, L.J.; Latinkić, B.V. Early activation of FGF and nodal pathways mediates cardiac specification independently of Wnt/beta-catenin signaling. PLoS ONE 2009, 4, e7650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Katsev, S.; Cai, C.; Evans, S. BMP signaling is required for heart formation in vertebrates. Dev. Biol. 2000, 224, 226–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tirosh-Finkel, L.; Elhanany, H.; Rinon, A.; Tzahor, E. Mesoderm progenitor cells of common origin contribute to the head musculature and the cardiac outflow tract. Development 2006, 133, 1943–1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yatskievych, T.A.; Ladd, A.N.; Antin, P.B. Induction of cardiac myogenesis in avian pregastrula epiblast: The role of the hypoblast and activin. Development 1997, 124, 2561–2570. [Google Scholar] [CrossRef] [PubMed]
- Bender, R.; Lange, S.; Ziegler, A. Multiples testen. Dtsch. Med. Wochenschr. 2007, 132, e26–e29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metcalf, G.L.; McClure, S.R.; Hostetter, J.M.; Martinez, R.F.; Wang, C. Evaluation of adipose-derived stromal vascular fraction from the lateral tailhead, inguinal region, and mesentery of horses. Can. J. Vet. Res. 2016, 80, 294–301. [Google Scholar] [PubMed]
- Baglioni, S.; Cantini, G.; Poli, G.; Francalanci, M.; Squecco, R.; Di Franco, A.; Borgogni, E.; Frontera, S.; Nesi, G.; Liotta, F. Functional differences in visceral and subcutaneous fat pads originate from differences in the adipose stem cell. PLoS ONE 2012, 7, e36569. [Google Scholar] [CrossRef] [PubMed]
- Ntege, E.H.; Sunami, H.; Shimizu, Y. Advances in regenerative therapy: A review of the literature and future directions. Regen. Ther. 2020, 14, 136–153. [Google Scholar] [CrossRef] [PubMed]
- Patrikoski, M.; Mannerström, B.; Miettinen, S. Perspectives for clinical translation of adipose stromal/stem cells. Stem Cells Int. 2019, 2019, 5858247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sotiropoulou, P.A.; Perez, S.A.; Salagianni, M.; Baxevanis, C.N.; Papamichail, M. Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells 2006, 24, 462–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stolzing, A.; Coleman, N.; Scutt, A. Glucose-induced replicative senescence in mesenchymal stem cells. Rejuvenation Res. 2006, 9, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Merlo, B.; Teti, G.; Lanci, A.; Burk, J.; Mazzotti, E.; Falconi, M.; Iacono, E. Comparison between adult and foetal adnexa derived equine post-natal mesenchymal stem cells. BMC Vet. Res. 2019, 15, 277. [Google Scholar] [CrossRef] [PubMed]
- Colleoni, S.; Bottani, E.; Tessaro, I.; Mari, G.; Merlo, B.; Romagnoli, N.; Spadari, A.; Galli, C.; Lazzari, G. Isolation, growth and differentiation of equine mesenchymal stem cells: Effect of donor, source, amount of tissue and supplementation with basic fibroblast growth factor. Vet. Res. Commun. 2009, 33, 811–821. [Google Scholar] [CrossRef]
- Peng, L.; Jia, Z.; Yin, X.; Zhang, X.; Liu, Y.; Chen, P.; Ma, K.; Zhou, C. Comparative analysis of mesenchymal stem cells from bone marrow, cartilage, and adipose tissue. Stem Cells Dev. 2008, 17, 761–774. [Google Scholar] [CrossRef]
- Vidal, M.A.; Kilroy, G.E.; Johnson, J.R.; Lopez, M.J.; Moore, R.M.; Gimble, J.M. Cell growth characteristics and differentiation frequency of adherent equine bone marrow-derived mesenchymal stromal cells: Adipogenic and osteogenic capacity. Vet. Surg. 2006, 35, 601–610. [Google Scholar] [CrossRef]
- D’Alimonte, I.; Lannutti, A.; Pipino, C.; Di Tomo, P.; Pierdomenico, L.; Cianci, E.; Antonucci, I.; Marchisio, M.; Romano, M.; Stuppia, L.; et al. Wnt signaling behaves as a “master regulator” in the osteogenic and adipogenic commitment of human amniotic fluid mesenchymal stem cells. Stem Cell Rev. Rep. 2013, 9, 642–654. [Google Scholar] [CrossRef] [Green Version]
- Hoshiba, T.; Kawazoe, N.; Tateishi, T.; Chen, G. Development of extracellular matrices mimicking stepwise adipogenesis of mesenchymal stem cells. Adv. Mater. 2010, 22, 3042–3047. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, M.; Hacker, M.; Bauer-Kreisel, P.; Weiser, B.; Fischbach, C.; Schulz, M.B.; Goepferich, A.; Blunk, T. Adipose tissue engineering based on mesenchymal stem cells and basic fibroblast growth factor in vitro. Tissue Eng. 2005, 11, 1840–1851. [Google Scholar] [CrossRef] [PubMed]
- Marycz, K.; Weiss, C.; Śmieszek, A.; Kornicka, K. Evaluation of Oxidative Stress and Mitophagy during Adipogenic Differentiation of Adipose-Derived Stem Cells Isolated from Equine Metabolic Syndrome (EMS) Horses. Stem Cells Int. 2018, 2018, 5340756. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Lorente, R.; Bejar, M.T.; Badimon, L. Notch signaling pathway activation in normal and hyperglycemic rats differs in the stem cells of visceral and subcutaneous adipose tissue. Stem Cells Dev. 2014, 23, 3034–3048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Schauwer, C.; Goossens, K.; Piepers, S.; Hoogewijs, M.K.; Govaere, J.L.J.; Smits, K.; Meyer, E.; Van Soom, A.; Van de Walle, G.R. Characterization and profiling of immunomodulatory genes of equine mesenchymal stromal cells from non-invasive sources. Stem Cell Res. Ther. 2014, 5, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calle, A.; Zamora-Ceballos, M.; Bárcena, J.; Blanco, E.; Ramírez, M. Comparison of Biological Features of Wild European Rabbit Mesenchymal Stem Cells Derived from Different Tissues. Int. J. Mol. Sci. 2022, 23, 6420. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, C.; Leicht, U.; Rothe, C.; Drosse, I.; Luibl, V.; Röcken, M.; Schieker, M. Effects of different media on proliferation and differentiation capacity of canine, equine and porcine adipose derived stem cells. Res. Vet. Sci. 2012, 93, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Ostanin, A.; Petrovskiy, Y.L.; Shevela, E.Y.; Kurganova, E.; Drobinskaja, A.; Dobryakova, O.; Lisukova, E.; Chernykh, E. A new approach to evaluation of osteogenic potential of mesenchymal stromal cells. Bull. Exp. Biol. Med. 2008, 146, 534–539. [Google Scholar] [CrossRef]
- Trivedi, S.; Srivastava, K.; Gupta, A.; Saluja, T.S.; Kumar, S.; Mehrotra, D.; Singh, S.K. A quantitative method to determine osteogenic differentiation aptness of scaffold. J. Oral Biol. Craniofac. Res. 2020, 10, 158–160. [Google Scholar] [CrossRef]
- Westhauser, F.; Karadjian, M.; Essers, C.; Senger, A.-S.; Hagmann, S.; Schmidmaier, G.; Moghaddam, A. Osteogenic differentiation of mesenchymal stem cells is enhanced in a 45S5-supplemented β-TCP composite scaffold: An in-vitro comparison of Vitoss and Vitoss BA. PLoS ONE 2019, 14, e0212799. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, S.; Brehm, W.; Mainil-Varlet, P.; Nesic, D. Multilineage differentiation potential of equine blood-derived fibroblast-like cells. Differentiation 2008, 76, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, M.; Parham, A.; Dehghani, H.; Mehrjerdi, H.K. Stemness signature of equine marrow-derived mesenchymal stem cells. Int. J. Stem Cells 2017, 10, 93. [Google Scholar] [CrossRef] [Green Version]
- Kisiel, A.H.; McDuffee, L.A.; Masaoud, E.; Bailey, T.R.; Esparza Gonzalez, B.P.; Nino-Fong, R. Isolation, characterization, and in vitro proliferation of canine mesenchymal stem cells derived from bone marrow, adipose tissue, muscle, and periosteum. Am. J. Vet. Res. 2012, 73, 1305–1317. [Google Scholar] [CrossRef] [PubMed]
- Vidal, M.A.; Robinson, S.O.; Lopez, M.J.; Paulsen, D.B.; Borkhsenious, O.; Johnson, J.R.; Moore, R.M.; Gimble, J.M. Comparison of chondrogenic potential in equine mesenchymal stromal cells derived from adipose tissue and bone marrow. Vet. Surg. 2008, 37, 713–724. [Google Scholar] [CrossRef] [Green Version]
- Calle, A.; Gutiérrez-Reinoso, M.; Re, M.; Blanco, J.; De la Fuente, J.; Monguió-Tortajada, M.; Borràs, F.E.; Yáñez-Mó, M.; Ramírez, M. Bovine peripheral blood MSCs chemotax towards inflammation and embryo implantation stimuli. J. Cell. Physiol. 2021, 236, 1054–1067. [Google Scholar] [CrossRef] [PubMed]
- Calle, A.; López-Martín, S.; Monguió-Tortajada, M.; Borràs, F.E.; Yáñez-Mó, M.; Ramírez, M. Bovine endometrial MSC: Mesenchymal to epithelial transition during luteolysis and tropism to implantation niche for immunomodulation. Stem Cell Res. Ther. 2019, 10, 23. [Google Scholar] [CrossRef]
- Carrade, D.D.; Lame, M.W.; Kent, M.S.; Clark, K.C.; Walker, N.J.; Borjesson, D.L. Comparative Analysis of the Immunomodulatory Properties of Equine Adult-Derived Mesenchymal Stem Cells. Cell Med. 2012, 4, 1–11. [Google Scholar] [CrossRef]
- Kang, J.W.; Kang, K.S.; Koo, H.C.; Park, J.R.; Choi, E.W.; Park, Y.H. Soluble factors-mediated immunomodulatory effects of canine adipose tissue-derived mesenchymal stem cells. Stem Cells Dev. 2008, 17, 681–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broeckx, S.; Martens, A.; Bertone, A.; Van Brantegem, L.; Duchateau, L.; Van Hecke, L.; Dumoulin, M.; Oosterlinck, M.; Chiers, K.; Hussein, H. The use of equine chondrogenic-induced mesenchymal stem cells as a treatment for osteoarthritis: A randomised, double-blinded, placebo-controlled proof-of-concept study. Equine Vet. J. 2019, 51, 787–794. [Google Scholar] [CrossRef]
- Emmerich, I.U. Neue Arzneimittel für Pferde und landwirtschaftliche Nutztiere 2019. Tierarztl. Prax. Ausg. G Grosstiere Nutztiere 2020, 48, 118–123. [Google Scholar] [CrossRef]
- Waselau, M. Diagnose und Therapie von Kniegelenkerkrankungen–ein Update. Pferdespiegel Thieme 2021, 24, 99–105. [Google Scholar] [CrossRef]
- Choi, Y.S.; Dusting, G.J.; Stubbs, S.; Arunothayaraj, S.; Han, X.L.; Collas, P.; Morrison, W.A.; Dilley, R.J. Differentiation of human adipose-derived stem cells into beating cardiomyocytes. J. Cell. Mol. Med. 2010, 14, 878–889. [Google Scholar] [CrossRef] [Green Version]
- Van Dijk, A.; Niessen, H.W.M.; Doulabi, B.Z.; Visser, F.C.; Van Milligen, F.J. Differentiation of human adipose-derived stem cells towards cardiomyocytes is facilitated by laminin. Cell Tissue Res. 2008, 334, 457–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afflerbach, A.-K.; Kiri, M.D.; Detinis, T.; Maoz, B.M. Mesenchymal stem cells as a promising cell source for integration in novel in vitro models. Biomolecules 2020, 10, 1306. [Google Scholar] [CrossRef]
- Denning, C.; Borgdorff, V.; Crutchley, J.; Firth, K.S.A.; George, V.; Kalra, S.; Kondrashov, A.; Hoang, M.D.; Mosqueira, D.; Patel, A. Cardiomyocytes from human pluripotent stem cells: From laboratory curiosity to industrial biomedical platform. Biochim. Biophys. Acta Mol. Cell Res. 2015, 1863, 1728–1748. [Google Scholar] [CrossRef]
- Khaleghi, M.; Taha, M.F.; Jafarzadeh, N.; Javeri, A. Atrial and ventricular specification of ADSCs is stimulated by different doses of BMP4. Biotechnol. Lett. 2014, 36, 2581–2589. [Google Scholar] [CrossRef]
- Laflamme, M.A.; Chen, K.Y.; Naumova, A.V.; Muskheli, V.; Fugate, J.A.; Dupras, S.K.; Reinecke, H.; Xu, C.; Hassanipour, M.; Police, S. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat. Biotechnol. 2007, 25, 1015–1024. [Google Scholar] [CrossRef]
- Uosaki, H.; Fukushima, H.; Takeuchi, A.; Matsuoka, S.; Nakatsuji, N.; Yamanaka, S.; Yamashita, J.K. Efficient and scalable purification of cardiomyocytes from human embryonic and induced pluripotent stem cells by VCAM1 surface expression. PLoS ONE 2011, 6, e23657. [Google Scholar] [CrossRef] [PubMed]
- Calloni, R.; Cordero, E.A.A.; Henriques, J.A.P.; Bonatto, D. Reviewing and updating the major molecular markers for stem cells. Stem Cells Dev. 2013, 22, 1455–1476. [Google Scholar] [CrossRef] [Green Version]
- Safwani, W.K.Z.W.; Makpol, S.; Sathapan, S.; Chua, K.H. 5-Azacytidine is insufficient for cardiogenesis in human adipose-derived stem cells. J. Negat. Results Biomed. 2012, 11, 3. [Google Scholar] [CrossRef] [Green Version]
- Grajales, L.; García, J.; Geenen, D.L. Induction of cardiac myogenic lineage development differs between mesenchymal and satellite cells and is accelerated by bone morphogenetic protein-4. J. Mol. Cell. Cardiol. 2012, 53, 382–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takei, S.; Ichikawa, H.; Johkura, K.; Mogi, A.; No, H.; Yoshie, S.; Tomotsune, D.; Sasaki, K. Bone morphogenetic protein-4 promotes induction of cardiomyocytes from human embryonic stem cells in serum-based embryoid body development. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H1793–H1803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuasa, S.; Itabashi, Y.; Koshimizu, U.; Tanaka, T.; Sugimura, K.; Kinoshita, M.; Hattori, F.; Fukami, S.I.; Shimazaki, T.; Okano, H. Transient inhibition of BMP signaling by Noggin induces cardiomyocyte differentiation of mouse embryonic stem cells. Nat. Biotechnol. 2005, 23, 607–611. [Google Scholar] [CrossRef]
- Kakkar, A.; Nandy, S.B.; Gupta, S.; Bharagava, B.; Airan, B.; Mohanty, S. Adipose tissue derived mesenchymal stem cells are better respondents to TGFβ1 for in vitro generation of cardiomyocyte-like cells. Mol. Cell. Biochem. 2019, 460, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Wystrychowski, W.; Patlolla, B.; Zhuge, Y.; Neofytou, E.; Robbins, R.C.; Beygui, R.E. Multipotency and cardiomyogenic potential of human adipose-derived stem cells from epicardium, pericardium, and omentum. Stem Cell Res. Ther. 2016, 7, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soltani, L.; Mahdavi, A.H. Role of Signaling Pathways during Cardiomyocyte Differentiation of Mesenchymal Stem Cells. Cardiology 2022, 147, 216–224. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stage, H.J.; Trappe, S.; Söllig, K.; Trachsel, D.S.; Kirsch, K.; Zieger, C.; Merle, R.; Aschenbach, J.R.; Gehlen, H. Multilineage Differentiation Potential of Equine Adipose-Derived Stromal/Stem Cells from Different Sources. Animals 2023, 13, 1352. https://doi.org/10.3390/ani13081352
Stage HJ, Trappe S, Söllig K, Trachsel DS, Kirsch K, Zieger C, Merle R, Aschenbach JR, Gehlen H. Multilineage Differentiation Potential of Equine Adipose-Derived Stromal/Stem Cells from Different Sources. Animals. 2023; 13(8):1352. https://doi.org/10.3390/ani13081352
Chicago/Turabian StyleStage, Hannah J., Susanne Trappe, Katharina Söllig, Dagmar S. Trachsel, Katharina Kirsch, Cornelia Zieger, Roswitha Merle, Jörg R. Aschenbach, and Heidrun Gehlen. 2023. "Multilineage Differentiation Potential of Equine Adipose-Derived Stromal/Stem Cells from Different Sources" Animals 13, no. 8: 1352. https://doi.org/10.3390/ani13081352





