Pre-Protection and Mechanism of Crude Extracts from Dioscorea alata L. on H2O2-Induced IPEC-J2 Cells Oxidative Damage
Abstract
:Simple Summary
Abstract
1. Introduction
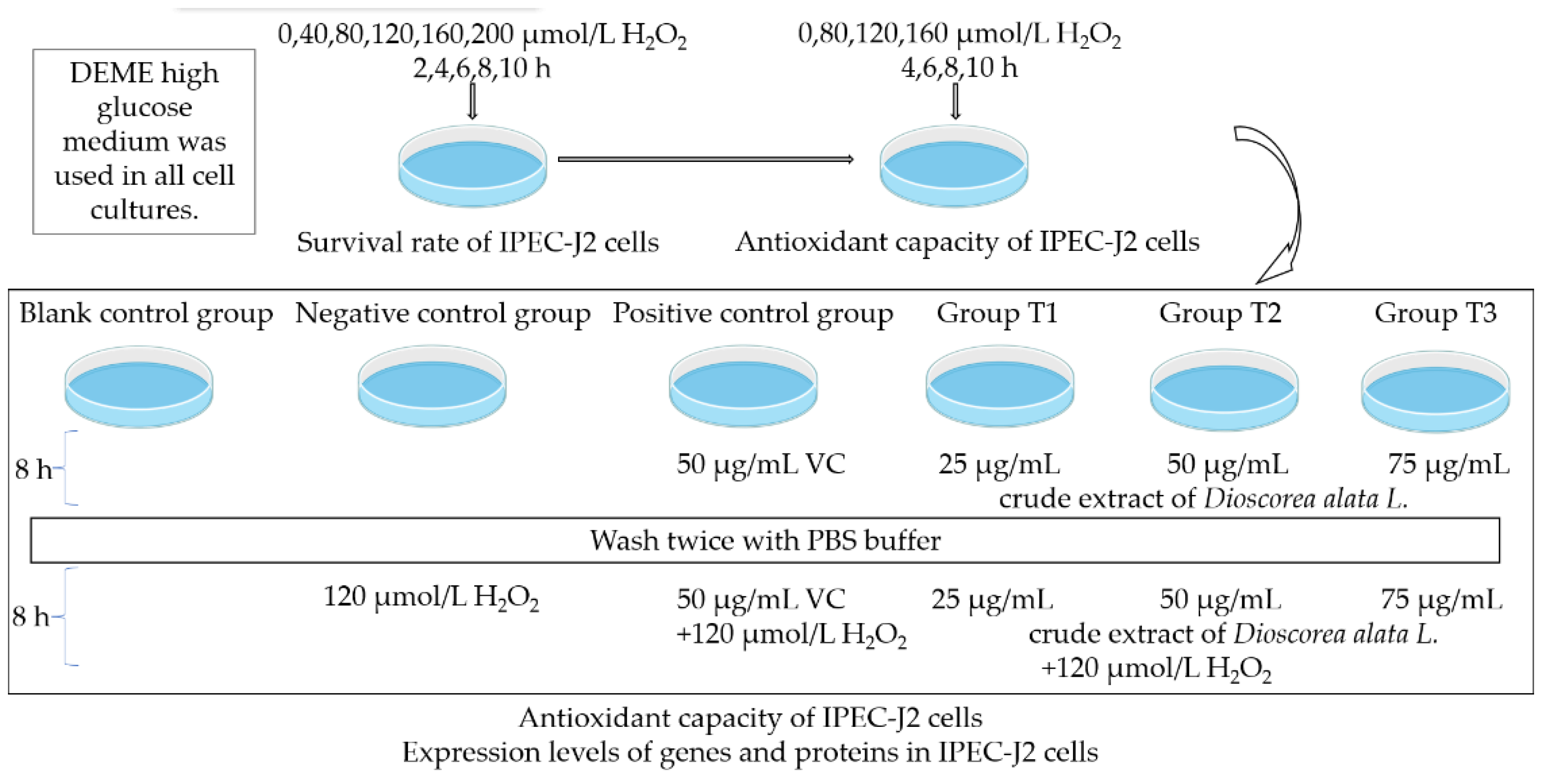
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Assessment of Cell Cytotoxicity
2.4. Enzyme Assays
2.5. Quantitative PCR
2.6. Western Blot
2.7. Statistical Analysis
3. Results
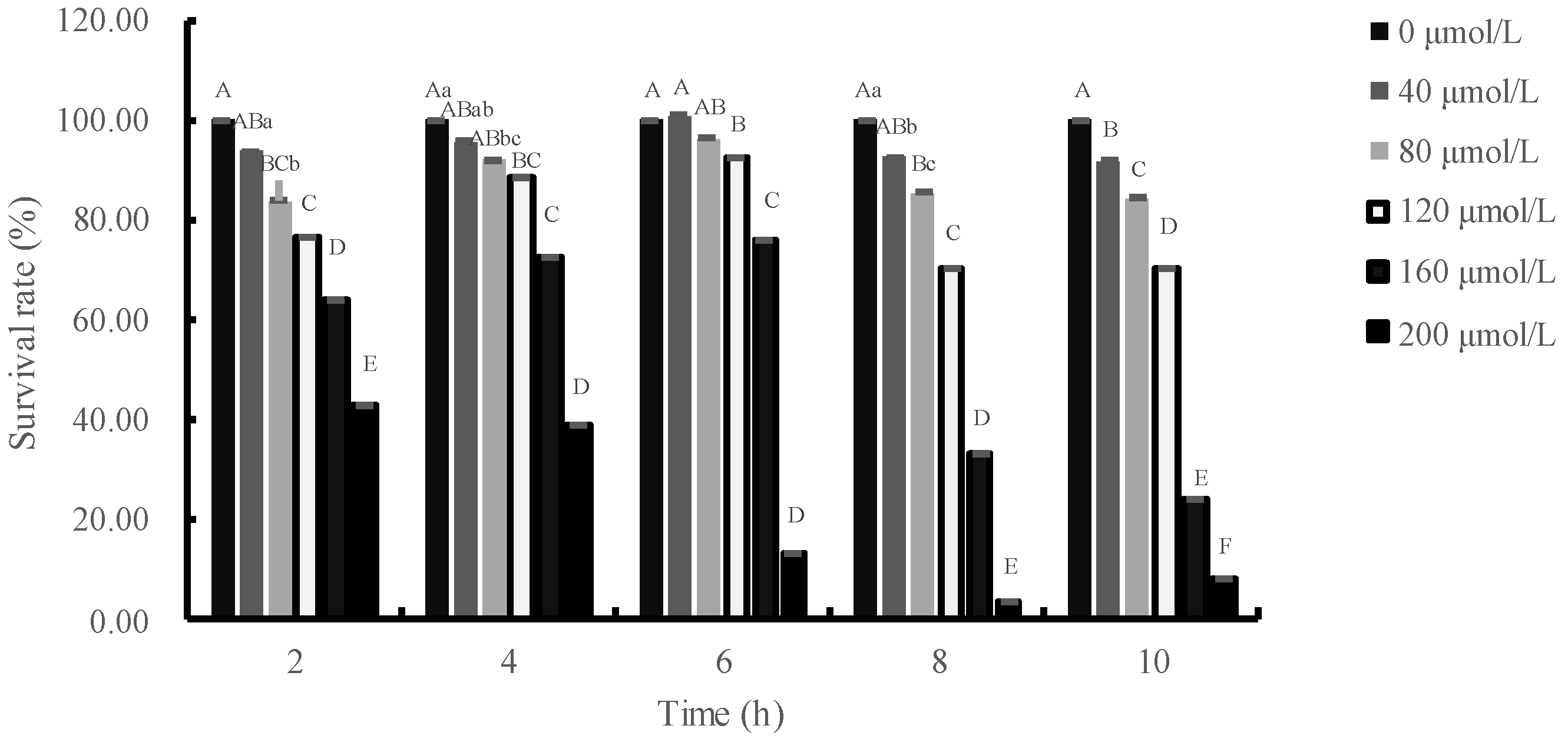
3.1. Effects of H2O2 Concentration and Treatment Time on Relative Survival Rate of IPEC-J2 Cells
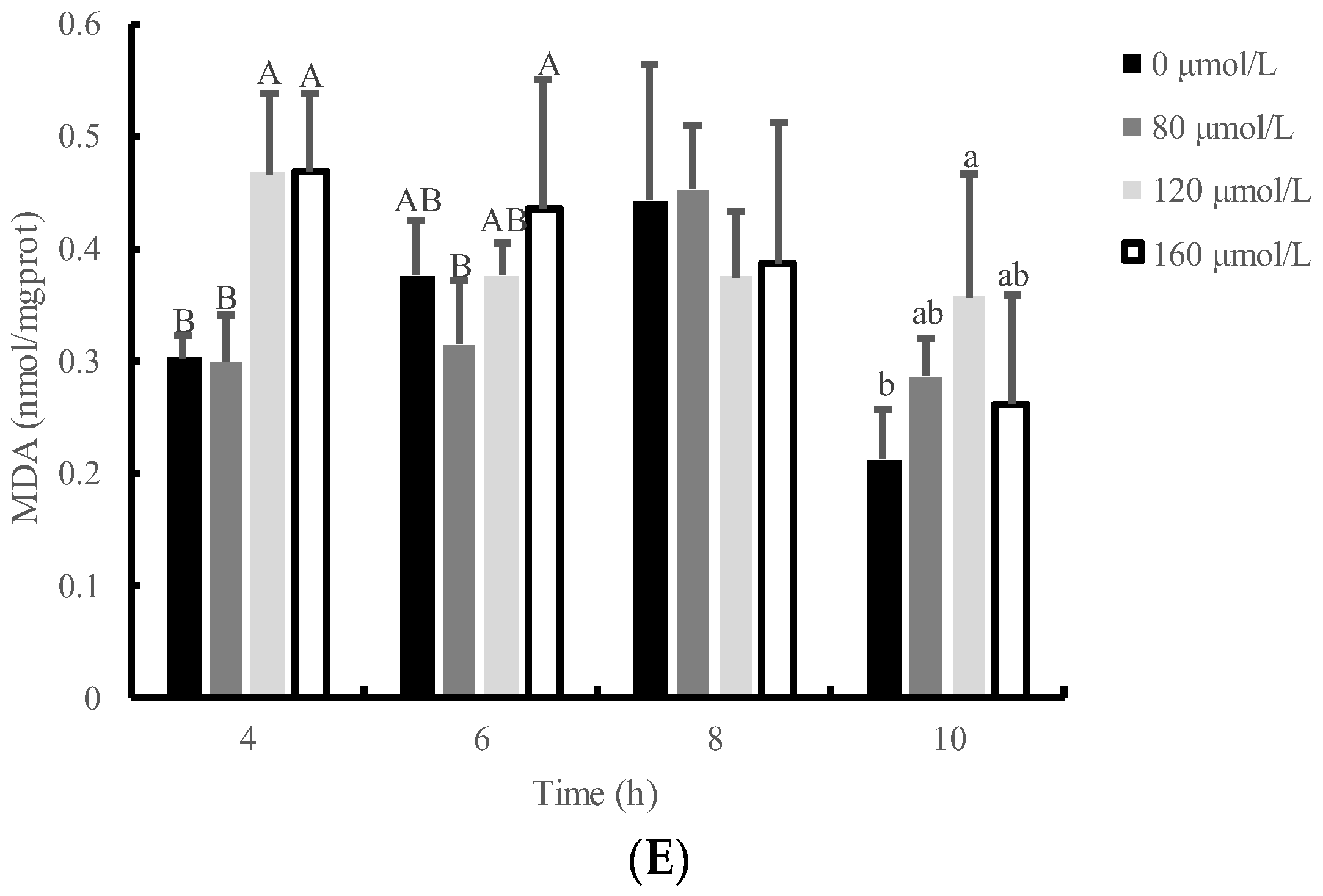
3.2. Effects of H2O2 Concentration and Time on Antioxidative Index of IPEC-J2 Cells
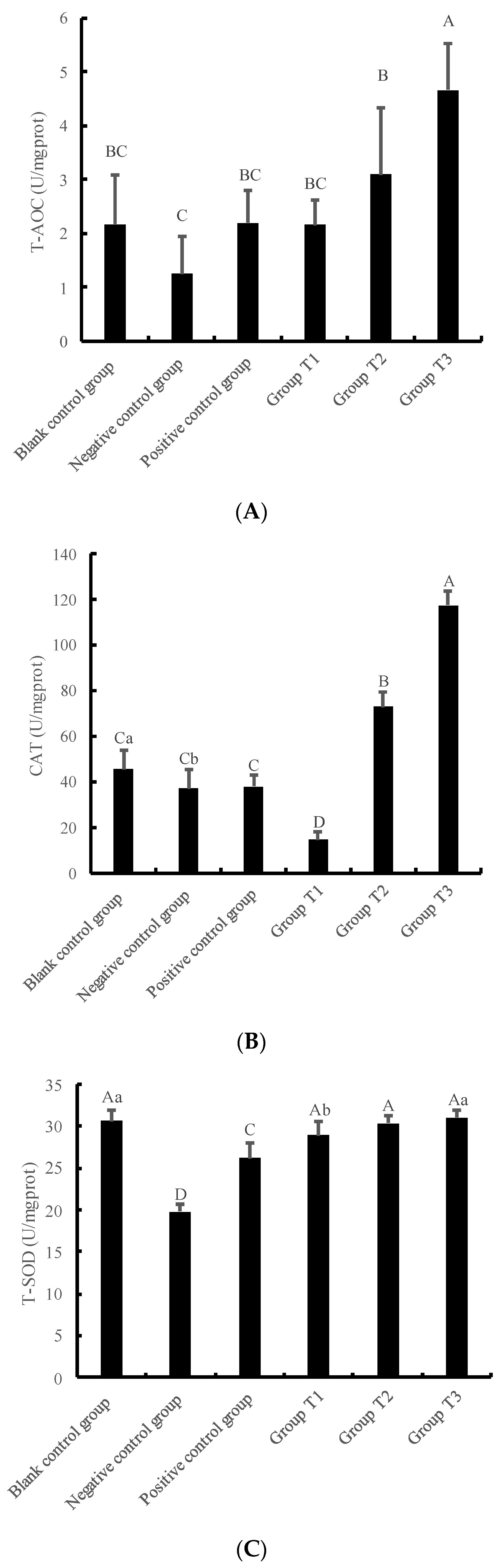
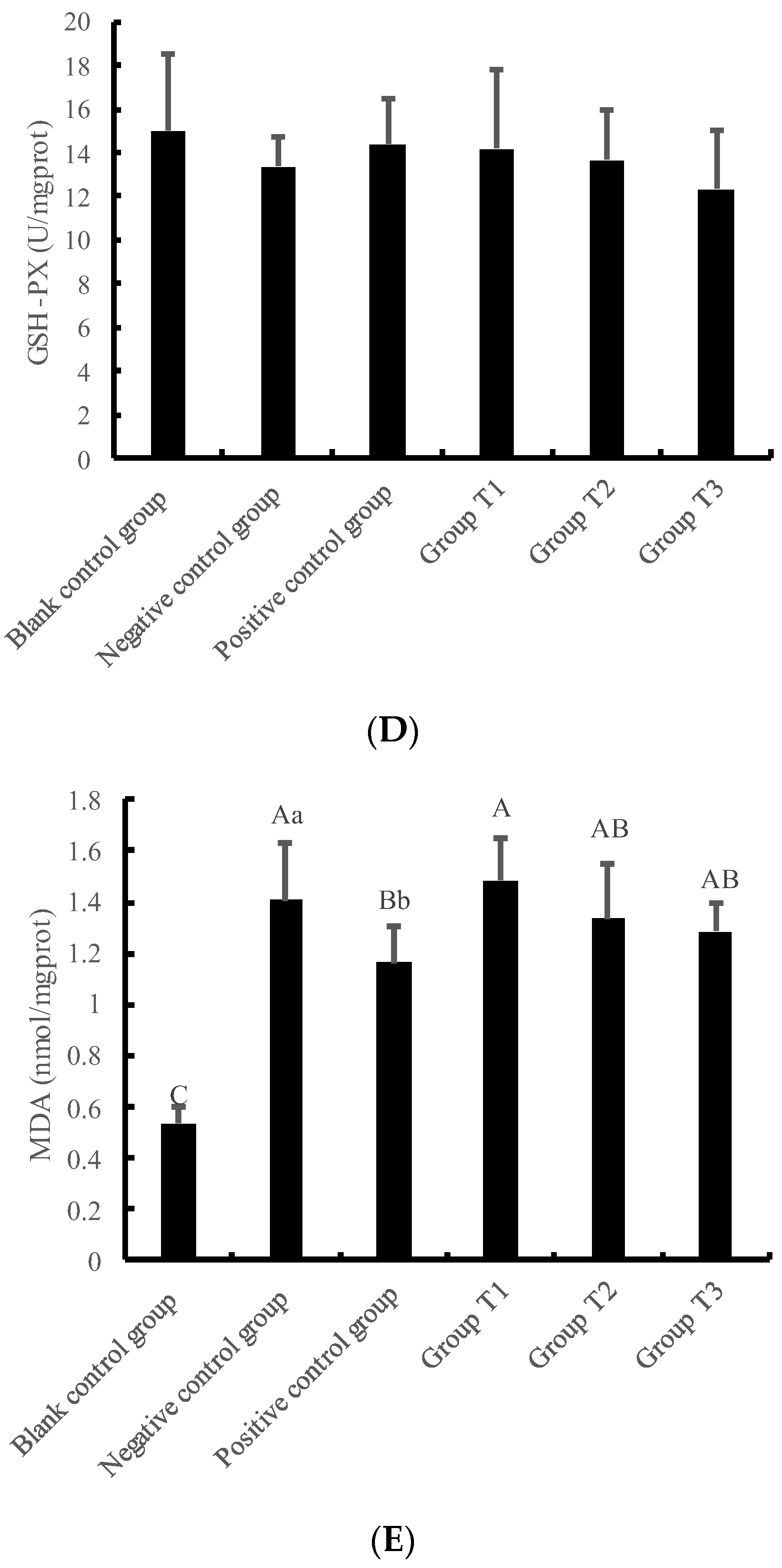
3.3. Changes in Antioxidant Function of IPEC-J2 Cells under Crude Extract Preprotection
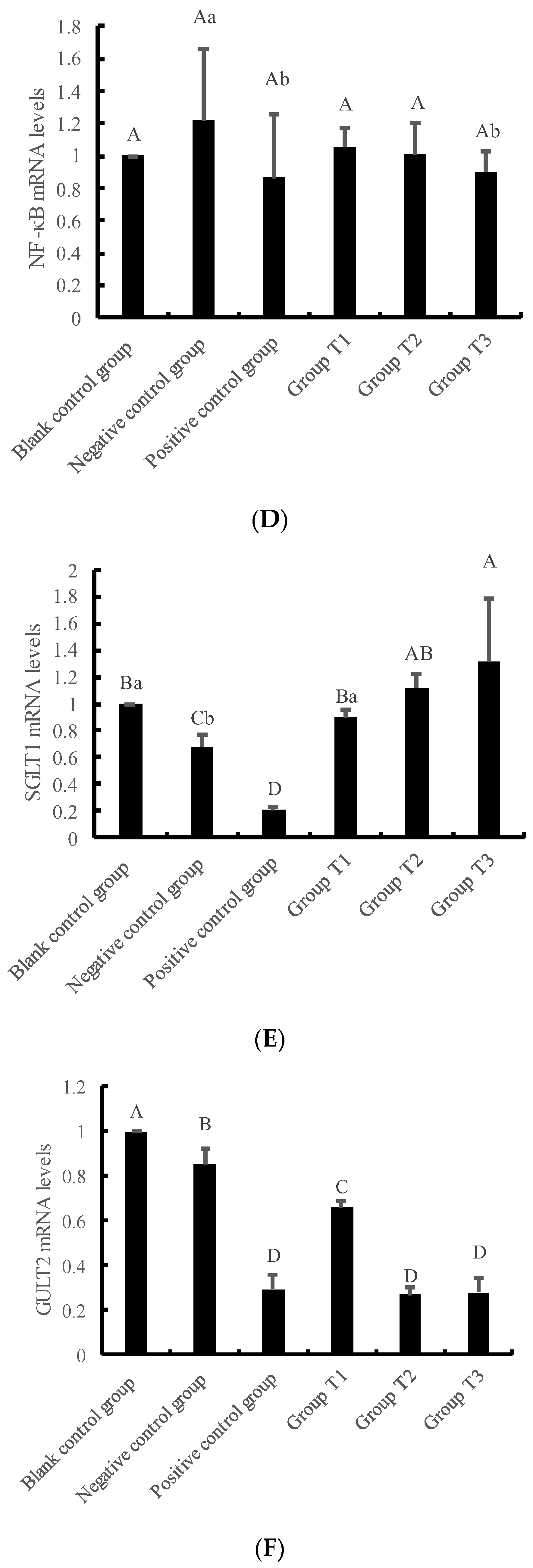
3.4. Effects of Crude Extracts on Gene Expression of IPEC-J2 Cells Pre-Protected by H2O2 Injury
3.5. Effects of Crude Extracts on Expression Level of Oxidative Damage Protein in IPEC-J2 Cells Induced by H2O2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Furbeyre, H.; van Milgen, J.; Mener, T.; Gloaguen, M.; Labussiere, E. Effects of oral supplementation with Spirulina and Chlorella on growth and digestive health in piglets around weaning. Anim. Int. J. Anim. Biosci. 2018, 12, 2264–2273. [Google Scholar] [CrossRef] [PubMed]
- Luppi, A. Swine enteric colibacillosis: Diagnosis, therapy and antimicrobial resistance. Porc. Health Manag. 2017, 3, 16. [Google Scholar] [CrossRef] [PubMed]
- Weber, H.; Hühns, S.; Jonas, L.; Sparmann, G.; Bastian, M.; Schuff-Werner, P. Hydrogen peroxide-induced activation of defense mechanisms against oxidative stress in rat pancreatic acinar AR42J cells. Free. Radic. Biol. Med. 2007, 42, 830–841. [Google Scholar] [CrossRef] [PubMed]
- Chen, T. Study on Antioxidant Capacity of Anthocyanins from Hainan Purple Sweet Potato In Vitro and Its Alleviating Effect on TNBS Induced Colitis in Mice; Hainan University: Haikou, China, 2016. [Google Scholar]
- Wang, Y. Study on the Protective Effect and Mechanism of Crude Extract of Dioscorea alata L. on Oxidative Damage Induced by H2O2 in IPEC-J2 Cells; Hainan University: Haikou, China, 2021. [Google Scholar]
- Xiao, K.; Liu, C.; Tu, Z.; Xu, Q.; Chen, S.; Zhang, Y.; Wang, X.; Zhang, J.; Hu, C.A.; Liu, Y. Activation of the NF-κB and MAPK Signaling Pathways Contributes to the Inflammatory Responses, but Not Cell Injury, in IPEC-1 Cells Challenged with Hydrogen Peroxide. Oxidative Med. Cell. Longev. 2020, 2020, 5803639. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yuan, Q.; Xu, G.; Chen, H.; Lei, H.; Su, J. Effects of Quercetin on Proliferation and H₂O₂-Induced Apoptosis of Intestinal Porcine Enterocyte Cells. Molecules 2018, 23, 2012. [Google Scholar] [CrossRef] [PubMed]
- Kaschubek, T.; Mayer, E.; Rzesnik, S.; Grenier, B.; Bachinger, D.; Schieder, C.; König, J.; Teichmann, K. Effects of phytogenic feed additives on cellular oxidative stress and inflammatory reactions in intestinal porcine epithelial cells1. J. Anim. Sci. 2018, 96, 3657–3669. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Mizugaki, A.; Inoue, Y.; Kato, H.; Murakami, H. Cystine reduces tight junction permeability and intestinal inflammation induced by oxidative stress in Caco-2 cells. Amino Acids 2021, 53, 1021–1032. [Google Scholar] [CrossRef]
- Zhang, Z.D.; Tao, Q.; Qin, Z.; Liu, X.W.; Li, S.H.; Bai, L.X.; Yang, Y.J.; Li, J.Y. Uptake and Transport of Naringenin and Its Antioxidant Effects in Human Intestinal Epithelial Caco-2 Cells. Front. Nutr. 2022, 9, 894117. [Google Scholar] [CrossRef]
- Zhou, N.; Tian, Y.; Wu, H.; Cao, Y.; Li, R.; Zou, K.; Xu, W.; Lu, L. Protective Effect of Resveratrol on Immortalized Duck Intestinal Epithelial Cells Exposed to H2O2. Molecules 2022, 27, 3542. [Google Scholar] [CrossRef]
- Wijeratne, S.S.; Cuppett, S.L.; Schlegel, V. Hydrogen peroxide induced oxidative stress damage and antioxidant enzyme response in Caco-2 human colon cells. J. Agric. Food Chem. 2005, 53, 8768–8774. [Google Scholar] [CrossRef]
- Roberfroid, M.B.; Bornet, F.; Bouley, C.; Cummings, J.H. Colonic microflora: Nutrition and health. Summary and conclusions of an International Life Sciences Institute (ILSI) [Europe] workshop held in Barcelona, Spain. Nutr. Rev. 1995, 53, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Shoveller, A.K.; Stoll, B.; Ball, R.O.; Burrin, D.G. Nutritional and functional importance of intestinal sulfur amino acid metabolism. J. Nutr. 2005, 135, 1609–1612. [Google Scholar] [CrossRef] [PubMed]
- Soylu, E.M.; Soylu, S.; Kurt, S. Antimicrobial activities of the essential oils of various plants against tomato late blight disease agent Phytophthora infestans. Mycopathologia 2006, 161, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Suong, N.T.M.; Paengkoum, S.; Schonewille, J.T.; Purba, R.A.P.; Paengkoum, P. Growth Performance, Blood Biochemical Indices, Rumen Bacterial Community, and Carcass Characteristics in Goats Fed Anthocyanin-Rich Black Cane Silage. Front. Vet. Sci. 2022, 9, 880838. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Yu, K.; Shi, Y.; Wang, J.; Duan, C. Transcription Factor VviMYB86 Oppositely Regulates Proanthocyanidin and Anthocyanin Biosynthesis in Grape Berries. Front. Plant Sci. 2020, 11, 613677. [Google Scholar] [CrossRef]
- Jun, L.Z. Effects of Grape Seed Proanthocyanidins on Antioxidant Function of Weaned Piglets; Shanxi Agricultural University: Jinzhong, China, 2019. [Google Scholar]
- Passamonti, S.; Vanzo, A.; Vrhovsek, U.; Terdoslavich, M.; Cocolo, A.; Decorti, G.; Mattivi, F. Hepatic uptake of grape anthocyanins and the role of bilitranslocase. Food Res. Int. 2005, 38, 953–960. [Google Scholar] [CrossRef]
- Jin, X.; Yi, L.; Chen, M.L.; Chen, C.Y.; Chang, H.; Zhang, T.; Wang, L.; Zhu, J.D.; Zhang, Q.Y.; Mi, M.T. Delphinidin-3-glucoside protects against oxidized low-density lipoprotein-induced mitochondrial dysfunction in vascular endothelial cells via the sodium-dependent glucose transporter SGLT1. PLoS ONE 2013, 8, e68617. [Google Scholar] [CrossRef]
- Sigurdson, G.T.; Robbins, R.J.; Collins, T.M.; Giusti, M.M. Impact of location, type, and number of glycosidic substitutions on the color expression of o-dihydroxylated anthocyanidins. Food Chem. 2018, 268, 416–423. [Google Scholar] [CrossRef]
- Wood, I.S.; Trayhurn, P. Glucose transporters (GLUT and SGLT): Expanded families of sugar transport proteins. Br. J. Nutr. 2003, 89, 3–9. [Google Scholar] [CrossRef]
- Zou, T.B.; Feng, D.; Song, G.; Li, H.W.; Tang, H.W.; Ling, W.H. The role of sodium-dependent glucose transporter 1 and glucose transporter 2 in the absorption of cyanidin-3-o-β-glucoside in Caco-2 cells. Nutrients 2014, 6, 4165–4177. [Google Scholar] [CrossRef]
- Baron, G.; Altomare, A.; Regazzoni, L.; Redaelli, V.; Grandi, S.; Riva, A.; Morazzoni, P.; Mazzolari, A.; Carini, M.; Vistoli, G.; et al. Pharmacokinetic profile of bilberry anthocyanins in rats and the role of glucose transporters: LC–MS/MS and computational studies. J. Pharm. Biomed. Anal. 2017, 144, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Jing, Z. Studies on the Bioutilization and Metabolism of Anthocyanins from Purple Sweet Potato; Jiangsu Normal University: Xuzhou, China, 2018. [Google Scholar]
- Yi, W.; Akoh, C.C.; Fischer, J.; Krewer, G. Absorption of anthocyanins from blueberry extracts by caco-2 human intestinal cell monolayers. J. Agric. Food Chem. 2006, 54, 5651–5658. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Wang, R.; Zhou, L.; Zhu, Y.; Gong, J.; Zhuang, S.M. MicroRNA-26b suppresses the NF-κB signaling and enhances the chemosensitivity of hepatocellular carcinoma cells by targeting TAK1 and TAB3. Mol. Cancer 2014, 13, 35. [Google Scholar] [CrossRef]
- Shamssain, M.H.; Thompson, J. A comparative study of pulmonary function and body composition in Scottish and Libyan schoolchildren aged 12-17 years. J. Trop. Pediatr. 1992, 38, 121–123. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.-J.; Middleton, J.; Kim, T.; Laganà, A.; Piovan, C.; Secchiero, P.; Nuovo, G.J.; Cui, R.; Joshi, P.; Romano, G.; et al. A set of NF-κB–regulated microRNAs induces acquired TRAIL resistance in Lung cancer. Proc. Natl. Acad. Sci. USA 2015, 112, E3355–E3364. [Google Scholar] [CrossRef]
- Schwartz, C.; Willebrand, R.; Huber, S.; Rupec, R.A.; Wu, D.; Locksley, R.; Voehringer, D. Eosinophil-specific deletion of IκBα in mice reveals a critical role of NF-κB-induced Bcl-xL for inhibition of apoptosis. Blood 2015, 125, 3896–3904. [Google Scholar] [CrossRef]
- Simões, A.E.; Pereira, D.M.; Gomes, S.E.; Brito, H.; Carvalho, T.; French, A.; Castro, R.E.; Steer, C.J.; Thibodeau, S.N.; Rodrigues, C.M.; et al. Aberrant MEK5/ERK5 signalling contributes to human colon cancer progression via NF-κB activation. Cell Death Dis. 2015, 6, e1718. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Dong, H.; Wu, L.; Feng, X.; Zhou, Z.; Zhao, C.; Liu, H.; Wu, H. Herb-Partitioned Moxibustion Regulates the TLR2/NF-κB Signaling Pathway in a Rat Model of Ulcerative Colitis. Evid.-Based Complement. Altern. Med. 2015, 2015, 949065. [Google Scholar] [CrossRef]
- Rothwarf, D.M.; Zandi, E.; Natoli, G.; Karin, M. IKK-gamma is an essential regulatory subunit of the IkappaB kinase complex. Nature 1998, 395, 297–300. [Google Scholar] [CrossRef]
- Xiao, G.; Harhaj, E.W.; Sun, S.C. NF-kappaB-inducing kinase regulates the processing of NF-kappaB2 p100. Mol. Cell 2001, 7, 401–409. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Gaynor, R.B. IkappaB kinases: Key regulators of the NF-kappaB pathway. Trends Biochem. Sci. 2004, 29, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Moynagh, P.N. The NF-κB pathway. J. Cell Sci. 2005, 118, 4589–4592. [Google Scholar] [CrossRef] [PubMed]
- Perkins, N.D.; Gilmore, T.D. Good cop, bad cop: The different faces of NF-kappaB. Cell Death Differ. 2006, 13, 759–772. [Google Scholar] [CrossRef] [PubMed]




| Gene | Serial Number | Forward Primer Sequence | Reverse Primer Sequence | Size (bp) |
|---|---|---|---|---|
| GAPDH | NM 001206359.1 | CAAGGCTGTGGGCAAGGTCATC | TTCTCCAGGCGGCAGGTCAG | 111 |
| NF-κB | NM 001005150 | TGGTGTCGCTCTTGTTGAAGTGTG | GCTGCTGTATCCGAGTGCTTGG | 108 |
| GSH-PX1 | NM 214201.1 | CGCAATGACATCGCATGGAACTTC | CACTGCTAGGCTCCTGGGACAG | 139 |
| SOD1 | NM 001190422. | GAAGATTCTGTGATCGCCCTCTCG | TTCATTTCCACCTCTGCCCAAGTC | 99 |
| SOD2 | NM 214127.2 | TGTATCCGTCGGCGTCCAAGG | TCCTGGTTAGAACAAGCGGCAATC | 93 |
| SGLT1 | NM 001164021.1 | TCATCATCGTCCTGGTCGTCTCC | TGAATGTCCTCCTCCTCTGCATCC | 130 |
| GLUT2 | NM 00109741 | TGCTCTGGTCTCTGTCTGTGTCC | ATTCTTCCAAGCCGATCTCCAAGC | 91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, Y.; Shi, H.; Wang, Y.; Yang, F.; Zhang, Y.; Feng, H.; Chen, J.; Wang, X. Pre-Protection and Mechanism of Crude Extracts from Dioscorea alata L. on H2O2-Induced IPEC-J2 Cells Oxidative Damage. Animals 2023, 13, 1401. https://doi.org/10.3390/ani13081401
Yun Y, Shi H, Wang Y, Yang F, Zhang Y, Feng H, Chen J, Wang X. Pre-Protection and Mechanism of Crude Extracts from Dioscorea alata L. on H2O2-Induced IPEC-J2 Cells Oxidative Damage. Animals. 2023; 13(8):1401. https://doi.org/10.3390/ani13081401
Chicago/Turabian StyleYun, Yanhong, Huiyu Shi, Yanyu Wang, Fengyuan Yang, Yuanxin Zhang, Haibo Feng, Junpu Chen, and Xuemei Wang. 2023. "Pre-Protection and Mechanism of Crude Extracts from Dioscorea alata L. on H2O2-Induced IPEC-J2 Cells Oxidative Damage" Animals 13, no. 8: 1401. https://doi.org/10.3390/ani13081401





